ABSTRACT
Ankylosing spondylitis (AS) is associated with several unique pulmonary manifestations such as apical fibrobullous disease (AFBD), which is a rare extra-spinal complication, predominantly occurring in advanced disease. Infectious complications and differential diagnosis of cavitated lung lesions may be challenging, particularly in patients already submitted to immunosuppression. In this report, we present a low body-mass-index 47-year-old male patient, ex-smoker, with AS and severe joint involvement, medicated in the past with anti-TNF-α therapy, who was diagnosed with AFBD and developed pulmonary tuberculosis and later chronic cavitary pulmonary aspergillosis. The patient died due to lung cavity major bleeding.
Introduction
Ankylosing spondylitis (AS) is estimated to affect about 0.1% of the population [Citation1]. Although its etiology and pathogenesis are not fully understood, it is strongly associated with HLA-B27 (a class 1 histocompatibility antigen), and the prevalence of AS parallels the distribution of HLA-B27 in different populations [Citation2]. A strong familiar pattern is described and typically disease manifestations arise in the third decade of life, with a higher incidence in young men, 90% of which are HLA-B27 positive [Citation1,Citation3]. AS course involves the progression of inflammatory enthesopathy to ossification and ankylosis [Citation4]. It primarily affects the axial joints, mainly the sacroiliac joints, which occurs, in most patients, in early disease [Citation4]. Extra-articular manifestations vary widely in terms of frequency and severity, with descriptions of ocular, cardiovascular, renal, neurological, and pulmonary involvement, among others [Citation1,Citation4]. Pulmonary involvement in AS is frequently a result of chest wall restriction and/or parenchymal disease including some severe and unique lung manifestations such as apical fibrobullous disease (AFBD), with or without cavitation [Citation2]. Although AS is a common cause of AFBD, the latter is an infrequent extra-spinal complication of AS that requires careful differential evaluation. We report a case of a patient with 17-year evolution AS diagnosed with AFBD that developed tuberculosis (TB) and later chronic cavitary pulmonary aspergillosis (CCPA).
Case presentation
Forty-seven-year-old, male, ex-smoker (30 pack-year), diagnosed with AS HLA B27 + at the age of 30. He had, for several years, symptoms of cervical morning stiffness and axial pain of inflammatory rhythm, conditioning frequent use of non-steroidal anti-inflammatory medication, bilateral sacroiliitis grade IV, lumbar spine ankylosis and syndesmophytes as well as a restrictive ventilatory pattern. He had been medicated with Infliximab for almost 2 years, after negative screening for latent TB (4.5 years prior to first contact with the Pulmonary Department), was no longer under biological therapy and had abandoned ambulatory follow-up in Rheumatology 3 years ago.
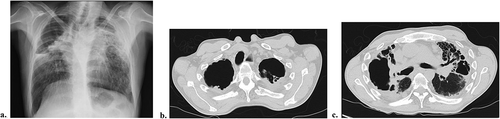
He was admitted at the Pulmonary Department of a District Hospital with a 3-month evolution worsening dyspnea, productive cough with mucopurulent sputum, evening sweating and fever, anorexia, and unquantified weight loss. On initial physical examination, there was low body mass index (BMI <18.5 kg/m2), muscle wasting, pronounced thoracic kyphosis, decreased chest wall expansion, and crepitant rales over the upper thirds of both hemithorax. Laboratory study revealed elevated erythrocyte sedimentation rate (120 mm/h) and C-reactive protein (CRP 23 mg/dL), anemia (hemoglobin (Hb) 10.3 g/dL), and hypoalbuminemia (albumin 3.1 g/dL). Chest x-ray () showed hypotransparency of the upper thirds of both pulmonary hemifields with retractile findings and lung volume loss as well as a cavity in the right upper lobe (RUL). Chest CT confirmed RUL consolidation with cavitation; left upper lobe (LUL) peripheral consolidation with air bronchogram; important upper lobe (UL) volume loss and multiple cylindric bronchiectasis ( B and C). Sputum molecular tests detected Mycobacterium tuberculosis complex (MTC) and first-line anti-bacillary therapy was initiated. Since fever and cough persisted, a flexible bronchoscopy was performed, and a methicillin-resistant Staphylococcus aureus (MRSA) was isolated on bronchial biopsies. The patient was treated according to drug-susceptibility test, with improvement. On the seventh month of anti-bacillary therapy, he developed persistent dry cough. A new chest CT was performed, which revealed retractile AFBD, with significant lung volume loss and bronchiectasis as well as a bulky RUL-cavitated lesion with mediastinal contact.
Figure 1. Chest CT and x-ray during first admission to Pulmonary Department. Chest x-ray depicting right upper lobe (RUL) cavity, retractile findings, and bilateral lung volume loss (a). Axial chest CT image evidencing important upper lobe volume loss, apical cavitary lesions and multiple cylindric bronchiectasis (b and c).
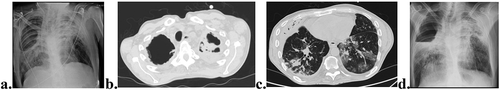
Flexible bronchoscopy with bronchial wash and biopsies was repeated, with no microbiological isolation or characteristic histopathological findings. The diagnoses of progressive AFBD secondary to AS aggravated by TB sequelae were assumed. The patient was referred for physical and pulmonary rehabilitation, re-evaluation by a rheumatologist (after patients’ consent and willingness) and evaluation by a thoracic surgeon. However, 9 months later (16 months after first admission), the patient worsened, and was admitted again at the Pulmonology Department, presenting a 1-month evolution worsening of productive cough, increased purulent sputum, hemoptysis, and weight loss. On physical examination, he had worsening muscle wasting, low oxygen saturation on pulse oximetry and bilateral crepitant rales. Blood tests revealed microcytic hypochromic anemia (Hb 9.2 g/dL), leukocytosis 18.100 with neutrophilia 93% and elevated CRP 18.7 mg/dL. He had worsened chest x-ray findings with a significant wider cavitation in the RUL and bilateral reticular infiltrates at the right lower lobe and LUL (). Empirical antibiotic therapy was initiated. Revaluation by chest CT revealed size increase of RUL cavitation, condensation with air bronchogram particularly at the ULs, mass-like lesion inside LUL cavitation, diffuse multifocal ground-glass opacities, sub-pleural micronodules, and tree-in-bud ( B and C). Sputum microbiology was inconclusive. A new flexible bronchoscopy was performed and Aspergillus fumigatus (AF) was isolated in the bronchial wash with an elevated bronchial wash Galactomannan antigen test and elevated serum AF IgG levels. He initiated voriconazole for the treatment of CCPA. During hospitalization, he presented fast worsening of respiratory failure and severe acute hemoptysis with a sudden 3 g/dL drop of Hb and the evidence of a new chest x-ray finding of an air-fluid level at the RUL cavity (). Poor performance status conditioned by low BMI, sarcopenia and extent of pulmonary and extra-pulmonary disease determined unfitness for surgical intervention and other major invasive procedures. The patient worsened fast and died despite optimized medical treatment before arterial embolization could be attempted.
Figure 2. Chest CT and x-ray, 16 months after first Pulmonary Department admission. Significant wider cavitation in the right upper lobe and bilateral reticular infiltrates at the right lower lobe and left upper lobe (LUL) (a). Mass-like lesion (aspergilloma) inside LUL cavitation (b). Diffuse multifocal ground-glass opacities, sub-pleural micronodules, and tree-in-bud (c). Air fluid level at the RUL cavity on X-ray (d).
Discussion
A broad spectrum of pulmonary manifestations is described in AS and, in medical literature, the prevalence of this involvement varies widely (0–30%) [Citation1,Citation5]. High-resolution chest CT (HRCCT) has been frequently used to evaluate patients with AS and it is known that evaluations in early AS may already show alterations [Citation6,Citation7]. These may include pleuro-parenchymal changes in 40–90% of patients with AS in early phases (less than 10 years of disease) [Citation7,Citation8]. Besides AFBD, with or without cavitation, these may include findings of pleural thickening, bronchiectasis, emphysema, small airways disease, ground-glass opacities and pleural effusion [Citation5,Citation7,Citation9]. Non-specific parenchymal linear opacities have also been described in patients with AS with no respiratory symptoms, probably translating early lung disease [Citation10]. These opacities have been found to have a statistically significant association with the involvement of the dorsal spine, development of a restrictive ventilatory pattern and existence of smoking habits [Citation10]. Very few studies have analyzed lung changes prevalence according to AS duration [Citation5]. In general, a subclinical AS lung involvement is considered frequent [Citation10].
AFBD is one of the most recognized pulmonary complications although rare. It was described for the first time in 1941 and better characterized since 1980, with the advent of chest CT [Citation2,Citation5]. It affects predominantly men (ratio 50:1), can be uni-/bilateral, is classically described as having a slow progression, may cavitate and is frequently confounded with pulmonary TB [Citation2]. AFBD prevalence varies between published revisions. A retrospective evaluation of 2080 patients with AS who performed HRCCT reported apical fibrosis or fibro-cavitary disease in 28 (1.3%) patients [Citation10]. In another prospective study over a 10-year period, including 1028 patients with AS, 2.1% developed AFBD [Citation2]. In a systematic review, the prevalence of chest CT anomalies in patients with AS was of 61%, for a mean disease length of 11.7 ± 5.2 years. UL fibrosis, with or without cavitation, was a rare finding (6.9%) [Citation5].
More than 50 years after the first description of AS lung involvement, its pathophysiology remains unclear [Citation4]. Proposed mechanisms include, among others, mechanical theories of reduced UL ventilation due to the increased chest wall rigidity and deformity, recurrent respiratory infections and augmented systemic pro-inflammatory state [Citation1,Citation4,Citation11]. Some have proposed that extra-articular AS manifestations parallel the inflammatory process responsible for the articular ones [Citation4]. In lung biopsies, cellular inflammatory infiltrates and interstitial fibrosis have been found [Citation9]. A rough joint inflammation control conditioning daily symptoms and frequent use of rescue medication, as in the described case report, may have contributed to the AFBD development.
Classically, AFBD is described as occurring in advanced AS, with an average 15-year disease evolution between first articular and lung manifestations. It usually shows slow progression. However, a great variability exists, and pulmonary lesions may occur 6–35 years after first joint manifestations, in asymptomatic patients as well as in early AS [Citation1,Citation8,Citation9]. In the presented case, there was a 17-year evolution AS, but the lack of a chest CT before first respiratory evaluation at our department unenabled time estimation of lung involvement. Considering that tTB screening was negative before anti-TNF-α therapy was started and that screening usually requires a chest x-ray, we assumed that no significant structural lung disease was present at that point, but we had no access to this data.
Lung affection by AFBD, in its large majority, consists of bilateral and apical fibrobullous lesions, mainly progressive with nodular coalescency, cysts, cavities, bronchiectasis, and fibrosis [Citation1]. The usual silent progression and rarity of this disease, unless infection develops, explains, in part, its sub-diagnosis. In more advanced stages, symptoms like cough, dyspnea, and sputum production may be present. In the reported case, AFBD was diagnosed after respiratory infection developed.
The higher risk of pulmonary infection is, in part, related to lung structural disease and several authors have evaluated the most frequently involved pathogens. In one study including 26 patientswith AFBD, the most frequently isolated agent was Aspergillus fumigatus, followed by various mycobacterial species (including Mycobacterium kansasii, M. avium, M. fortuitum, and M. scrofulaceum); other studies found other Aspergillus and Candida species [Citation1,Citation3,Citation12]. Chronic colonization by Aspergillus species occurs in 50–60% of the patients with AS having lung structural disease, and 10–30% of these will develop disease [Citation13,Citation14].
In the case report, the patient developed several lung infections by different microorganisms, namely MTC, MRSA, and AF. Anti-bacillary treatment was performed for 9 months and concluded after confirmation of negative cultures in bronchial wash. Later, voriconazole was started to treat CCPA based on clinical, laboratory, and bronchial wash findings. AFBD, especially with cavitation features, can be erroneously diagnosed as pulmonary TB, particularly in patients under or previously submitted to AS biological treatment [Citation14]. In the reported case, the patient had been under anti-TNF-α therapy for almost 2 years after negative TB screening and was no longer under that medication at the time of first observation. However, low BMI and structural lung disease are also well-known risk factors for the development of active TB. Hemoptysis is the most frequent sign of aspergillus infection. In our medical literature review, the description of hemoptysis in patients with AS and aspergillosis was frequently encountered in the presence of aspergilloma [Citation11,Citation14]. In the reported case, the patient evidenced a mass-like lesion inside the LUL cavity most likely representing an aspergilloma and developed severe bleeding with massive hemoptysis.
Imaging findings in AFBD can also mimic neoplasms and this differential diagnosis should be considered, particularly in an ex-smoker. It is also believed that smoking habits can have a role in AFBD, increasing the macrophages and neutrophils in lung parenchyma [Citation14]. However, in a systematic review, no differences were found in HRCCT anomalies prevalence between smokers and non-smokers AS patients [Citation5]. Smoking habits contribution to AFBD needs still better comprehension.
Since pathophysiology of lung destruction in AS-associated AFBD is not well known, therapeutic options are also limited. Smoking cessation and pulmonary rehabilitation are recommended [Citation14]. Anti-TNF-α effects in AFBD are not yet well stablished. There are descriptions of its benefit on lung manifestations control in rheumatoid arthritis, but it is not clear if it has some benefit in AS lung manifestations [Citation1]. Besides, there is an increased risk of TB reactivation that should be cautioned [Citation1,Citation15]. Surgical intervention in AS-associated AFBD is poorly described.
In the reported case, the lack of effective and specific medical treatment conditioned disease progression and poor prognosis.
Conclusion
It is essential to early investigate lung involvement in patients with AS, particularly recognizing rare and unique manifestations such as AFBD. AFBD requires careful differential diagnosis since infection can lead to worsening structural lung disease and lung function loss with increased morbidity and mortality. Further studies are needed to understand what may delay, cease or prevent pleuro-pulmonary involvement in patients with AS, since no treatment has yet managed to alter the clinical course of AFBD.
Author contribution
AA and VD conceptualized, designed, and wrote the manuscript. All authors revised the manuscript critically and approved final version.
Disclosure statement
The authors report no conflict of interest.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Kanathur N, Lee-Chiong T. Pulmonary manifestations of ankylosing spondylitis, Clin Chest Med. 2010;31:547–5.
- Trofimenko IN, Nashatyreva MS, Chernyak BA. Pulmonary involvement in ankylosing spondylitis. Pulmonologiya. 2017;27:97–102.
- Franquet T, Müller NL, Flint JD. A patient with ankylosing spondylitis and recurrent haemoptysis. Eur Respir J. 2004;23:488–491.
- El Maghraoui A. Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur J Intern Med. 2011;22:554–560.
- El Maghraoui A, Dehhaoui M. Prevalence and characteristics of lung involvement on high resolution computed tomography in patients with ankylosing spondylitis: a systematic review. Pulm Med. 2012;2012. doi:10.1155/2012/965956.
- Rumancik M, Leitman S, Mccauley I, et al. Fibrobullous disease of the upper lobes: an extraskeletal manifestation of ankylosing spondylitis. J Comput Tomogr. 1984;8:225–229.
- Kiris A, Ozgocmen S, Kocakoc E, et al. Lung findings on high resolution CT in early ankylosing spondylitis. Eur J Radiol. 2003;47:71–76.
- Hasiloglu ZI. Lung parenchymal changes in patients with ankylosing spondylitis. World J Radiol. 2012;4:215.
- Ulusoy H, Tuna NT, Tanrivermis Sayit A. Rapidly progressive pulmonary apical fibrosis and parenchymal destruction in a patient with ankylosing spondylitis. Case Rep Rheumatol. 2020;2020:1–4.
- Sampaio-Barros PD, Cerqueira EMFP, Rezende SM, et al. Pulmonary involvement in ankylosing spondylitis. Clin Rheumatol. 2007;26:225–230.
- Pamuk ÖN, Harmandar O, Tosun B, et al. A patient with ankylosing spondylitis who presented with chronic necrotising aspergillosis. Clin Rheumatol. 2005;24:415–419.
- Levy H, Hurwitz MD, Strimling M, et al. Spondylitis lung disease and mycobacterium scrofulaceum. Br J Dis Chest. 1988;82:84–87.
- Van der Horst-Bruinsma Irene E. Clinical aspects of ankylosing spondylitis Van Royen, BJ, Dijkmans, BAC. In: Ankylosing Spondylitis. Diagnosis and Management. CRC Press; 2006:45–70.
- Kim DY, Lee SJ, Ryu YJ, et al. Progressive pulmonary fibrocystic changes of both upper lungs in a patient with ankylosing spondylitis. Tuberc Respir Dis. 2015;78:459–462.
- De Keyser F, Van den Bosch F, Mielants H. Anti-TNF-alpha therapy in ankylosing spondylitis. Cytokine. 2006;33:294–298.
