ABSTRACT
In this case report, we describe the novel use of a permanent indwelling catheter (PiC) in the management of refractory malignant pericardial effusion (PE). The patient had disseminated lung cancer and was hospitalised repeatedly with circulatory collapse due to malignant PE despite treatments with pericardiocentesis (PCC) and a pericardial window (PW). The PiC was inserted as a last resort with no complications and was a mediator of pericardiodesis (PCD), resulting in the cease of PE. The PiC could subsequently be removed, and there was no relapse of PE.
Summary
In this case report, we describe the novel use of a permanent indwelling catheter (PiC) in the management of refractory malignant pericardial effusion (MPcE). The patient had disseminated lung cancer and was hospitalised repeatedly with circulatory collapse due to MPcE despite treatments with pericardiocentesis (PCC) and a pericardial window (PW). The PiC was inserted as a last resort with no complications and was properly a mediator of pericardiodesis (PCD), resulting in the cessation of MPcE. The PiC could subsequently be removed, and there was no relapse of MPcE.
Learning points
PiC can be used in patients with refractory MPcE as a permanent treatment
PiC may increase the likelihood of PCD, just as in the pleural cavity, diminishing the risk of the PcE relapsing.
PiC placement in the pericardial sack seems safe with few complications and high degree of patient satisfaction, but more studies are necessary to evaluate the risk of pericardial constriction.
Background
Permanent indwelling catheters are primarily used in cases with malignant refractory pleural effusion [Citation1] or ascites [Citation2] and has proven to be a safe procedure associated with relatively few complications [Citation1,Citation2]. In addition to avoiding unnecessary admissions, spontaneous pleurodesis occurs in 41.1% of patients with malignant pleural effusions treated with PiC [Citation1]. Despite being a well-known treatment of recurrent ascites and pleural effusion, PiC has only been described once in the literature for treating PcE [Citation3]. Development of MPcE in cancer patients is a well-known complication. There are no large studies supporting either of the treatment options including PCC including prolonged drainage [Citation4], pericardial sclerosis (PCS), subxiphoid PW, and percutaneous balloon pericardiectomy or pericardiotomy either by thoracotomy or video-assisted thoracoscopy (VATS). The treatment is therefore chosen in the perspective of the patient and in cooperation between highly specialised health professionals in the relevant specialties [Citation5,Citation6]. In the acute situation, an ultrasound-guided PCC is recommended [Citation7,Citation8], but in 21% [Citation9] to 50% [Citation5,Citation8] of the cases, the PcE is refractory. In this case report, we present the novel use of PiC for treating refractory PcE due to malignancy.
Case presentation
A 69-year-old male suspected of disseminated lung cancer underwent diagnostic extirpation of a positron emission tomography–computer tomography (PET–CT)-positive lymph node on the neck. During the procedure, the patient became circulatory unstable after initiation of anesthesia. An acute postoperative transthoracic echocardiography (TTE) revealed a very large, circumscript PcE with compression of the right ventricle and dilatation of the inferior vena cava. Due to tachycardia, hypotension, and tachypnea, an ultrasound-guided pericardiocentesis (PCC) using Seldinger technique was performed. After removing 600 mL pericardial fluid, the patient hemodynamically stabilised.
The lymph node was subsequently successfully removed for diagnostic evaluation, and histological examination was consistent with adenocarcinoma from the lung. PD-21 was positive >50% and ALK, ROS-1, EGFR, RET and MET were negative. The lunge cancer was staged T3N3M1C. The PcE was positive with adenocarcinoma, and CK7 and TTF-1 were also positive. A PET-CT revealed increased FDG uptake in the right lung hilus and nodule in the middle lobe. Furthermore, an increase was also found in lymph nodes on the neck, mediastinum, and the epigastrium. Magnetic resonance imaging of cerebrum did not reveal any metastases. A multidisciplinary team conference was held, and immunotherapy with pembrolizumab was found to be the best suitable treatment for the patient.
A control TTE 14 days after the PCC showed recurrence of very large MPcE with swinging heart, compression of the right ventricle and dilated vena cava. Due to recurrent MPcE, the patient was treated with pericardiectomy and establishment of a pericardial window (PW). The procedure was performed thoracoscopic. Nine days after the PW was established, there was no MPcE on TTE, and the treatment with immunotherapy was initiated. The patient was admitted at the Department of Cardiology 18 days after the PW due to shortness of breath. Acute TTE revealed recurrence of MPcE with compression of the right ventricle. An acute PCC with removal of 500 mL fluid was performed. The catheter was left in the pericardial sac. The case study was discussed at a multidisciplinary team conference with attendance of cardiologists and pulmonologists from the lung cancer unit. Due to recurrent MPcE predominantly over the right ventricle, there was an increased risk of complications during repeated PCC and lack of efficiency of the PW. A decision to attempt to place a PiC (PleurX) in the pericardial sac for palliation and possible PCD was made. The patient was informed about the experimental nature of the procedure and a lack of other effective treatment methods. He accepted the PiC treatment.
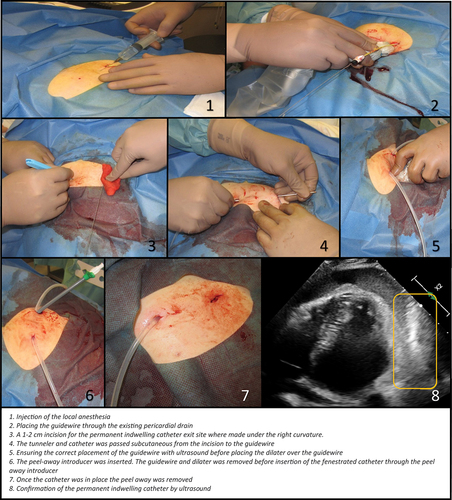
The procedure was performed under local anesthesia and analgesics in sterile settings, in collaboration between a pulmonologist who has experience in the placement of PiC in the pleural cavity and a cardiologist with experience in PCC. Using ultrasound guidance, a guidewire was placed through the existing pericardial drain. The existing drain was removed. The PiC was placed subcutaneous from under the right curvature to the left and then placed in the pericardial sac over the guidewire. The location of PiC in the pericardial sac was confirmed by ultrasound contrast (SonoVue; see .
Outcome and follow-up
The patient was closely monitored with repeated TTE after placement of the PiC. At day 9 following PiC placement, 200 mL MPcE was drained through the PiC. There was still MPcE around the apex of the heart. Due to the lack of symptoms and no affection of the hemodynamic parameters, it was decided not to remove or alter the position of the PiC. A TTE performed 14 days after PiC placement showed hyperechoic MPcE with septation. The placement of the PiC in the pericardial sac was confirmed by infusion of ultrasound contrast (SonoVue) into the PiC. An additional control TTE on day 30 still showed MPcE over the apex but no increase and no signs of recurrence of MPcE along the right ventricle. It was the same after day 59. The PiC was removed after 93 days in situ.
At follow-up, the patient had good response on the immunotherapy and was generally in good condition. The patient had some pain in relation to the drain but had no signs of infection during or after the PiC treatment. Four months after the drain was discontinued, the patient still felt some rigidity in the chest specifically when turning the upper body and weight lifting, but no treatment was needed.
Patient’s perspective
The patient is very satisfied with the treatment. Prior to the treatment with PiC, our patient had several hospitalisations, where the patient had a high risk of mortality and was told that there were no further treatments. The PiC was a lifeline for the patient, who was willing to try anything to treat the refractory MPcE. It was agreed on a multidisciplinary team discussion to offer the patient the PiC. The patient was willing to try the PiC after both being thoroughly informed of the risks and that the procedure had never been done before. After successful completion, the only complaint was pain in the chest when turning the upper body. After the PiC was discontinued, there was still some rigidity in the chest when moving but without need of medical treatment. The patient feels a significant increase in quality of life and is responding well to the immunotherapy.
Discussion
This case illustrates that there might be an additional opportunity in the pallet of treatments for refractory PcE with the use of PiC. There can only be found one other case report [Citation3] that describes the usages of a PleurX catheter used to drain PcE; however in that case, the PcE was in relation to a heart transplantation and not malignancy. Furthermore, it had the same outcome as the present case: PCD and the cessation of the PcE 37 days after the PIC was inserted. In both cases, the PiC was most likely the mediator of PCD and in the cessation of PcE production. However, it must be taken into consideration that the treatment with immunotherapy could also have been the mediator for the cessation of PcE production in the present case. Yet the fact that the case with the heart transplant also experienced cessation in PcE production after inserting the PiC suggests the explanation that the PiC was the mediator of PCD.
In both cases, the treatment with PiC was a ‘back-against-the-wall’ treatment. The use of the PleurX system for MPcE is off-label and would require approval to use in a larger scale. The PleurX design must be adjusted firstly because it cannot be seen on ultrasound and secondly because the trochar is quite large, considering that it is inserted in such close proximity to the heart. If the trochar penetrates the right ventricle, it would most likely be fatal. This treatment should be considered for patients where the cessation of MPcE would significantly increase the quality of life, and the only acute life-threatening condition is the MPcE.
PiC can be seen as a combination of PCC and PCS. A study shows that there is no difference between PCS and surgical intervention in relation to complication, recurrence and survival [Citation10]. PiC is most likely less expensive than any surgical intervention due to a reduction of the length of hospital stay [Citation5]. The same is true for PCS, where the patient must be given at least three treatments before effect [Citation7], which requires more days in admission than the PleurX. This can probably be done with only 1 day of admission. Perhaps with some more experience, it can be performed in an outpatient setting similar to the use of PiC for pleural effusion and ascites. After insertion of the PiC, it is suggested to follow up with a TTE after 7 days and thereafter only when the MPcE has ceased or if there is a worsening of the condition. This set-up will reduce the costs compared to PCS or surgery, and will likely increase the quality of life for the patient. The PiC provides continued drainage which minimises the risk of acute PCC.
Besides pain in relation to the PiC, as seen in our case, some other complications have been registered when using permanent indwelling catheters in malignant refractory pleural effusion and may be a risk when using PiC for MPcE in a large scale. Infection was seen in <5% and treated with antibiotics with no need of catheter removal or surgery. Catheter tract metastasis can occur, and fibrin clots within the catheter lumen is a possibility [Citation11]. Other complications in relation to PiC are arrhythmia and that the PCD could cause constrictio cordis. It is uncertain how high the risk of developing constrictio cordis is; it could potentially occur regardless in the case of auto-pericardiodesis, but fortunately, it is treatable. In this case, the patient was submitted in an acute setting several times with a relatively short period of time in between the events and with a high risk of mortality. Therefore, a ‘back-against-the-wall’ treatment was initiated despite the risk that the PCD could cause pericardial constriction. It could not be predicted when or if an auto-pericardiodesis would happen; therefore, it was decided to try alternative methods to prevent another life-threatening event.
This case shows that PiC is a method that could be considered in future treatment of refractory PcE. It provides a less expensive treatment option and possibly a better quality of life for the patient compared to other interventions. Further studies with focus on outcomes and patient experience in patients treated with PiC are needed. An alteration of the equipment must be considered before using on a larger scale. We believe that the treatment should initially be aimed at patients with recurrent MPcE with relatively few comorbidity and with a potential for increasing quality of life due to the risk of the procedure and developing pericardial constriction.
Source and support
Self-finance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Frederik Schultz Pustelnik
Frederik Schultz Pustelnik, MD at the Department of Respiratory Medicine, Odense University Hospital, Odense, Denmark from juni 2019 - juni 2020. Now in the process of specializing as a general practitioner (started march 2021- finishing september 2025).
Christian B. Laursen
Christian B. Laursen, PhD, is a Professor and Senior Consultant at the Department of Respiratory Medicine, Odense University Hospital, Odense, Denmark. He is Head of Research at Odense Respiratory Research Unit (ODIN), Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Arman Arshad
Arman Arshad, Senior Consultant MD. Interventional pulmonologist. Department of Respiratory Medicine, OUH Odense University Hospital. Head of the Unit at Center For Lung Cancer (CFL). Heserve as head of the unit for the lung cancer and the diagnostic work up throughout the Funen area.
Ahmed Aziz
Ahmed Aziz, MD, PhD was senior consultant in cardiology at Odense University Hospital at the time when the patient was admitted in Odense. Today he is senior consultant at Esbjerg University Hospital and associated professor at University of Southern Denmark.
References
- Bertolaccini L, Viti A, Gorla A, et al. Home-management of malignant pleural effusion with an indwelling pleural catheter: ten years experience. Eur J Surg Oncol. 2012;38(12):1161–5. published Online First: 2012/09/11.
- Meier M, Mortensen FV, Madsen HH. Malignant ascites in patients with terminal cancer is effectively treated with permanent peritoneal catheter. Acta Radiol Open. 2015;4(7):2058460115579934. published Online First: 2015/09/09.
- Louka B, Yang E, Lanza L, et al. Recurrent pericardial effusion in a heart transplant recipient managed with a pleurx catheter. Conference: International Academy of Cardiology 21st World Congress on Heart Disease Annual Scientific Sessions 2016 Boston, MA USA Cardiology (Switzerland) 134Supplement 1 (2016): 104 Embase Web 17 May 2022 July 2016.
- Flannery EP, Gregoratos G, Corder MP. Pericardial effusions in patients with malignant diseases. Arch Internal Med. 1975;135(7):976–977.
- Anderson TM, Ray CW, Nwogu CE, et al. Pericardial catheter sclerosis versus surgical procedures for pericardial effusions in cancer patients. J Cardiovasc Surg (Torino). 2001;42(3):415–419. published Online First: 2001/06/09.
- Supportive PDQ, Palliative Care Editorial B. Cardiopulmonary syndromes (PDQ®): health professional version. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US) 2002.
- Fiocco M, Krasna MJ. The management of malignant pleural and pericardial effusions. Hematol Oncol Clin North Am. 1997;11(2):253–265. published Online First: 1997/04/01.
- Martinoni A, Cipolla CM, Civelli M, et al. Intrapericardial treatment of neoplastic pericardial effusions. Herz. 2000;25(8):787–793. published Online First: 2001/02/24.
- Tsang TS, Seward JB, Barnes ME, et al. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc. 2000;75(3):248–253. published Online First: 2000/03/22.
- Girardi LN, Ginsberg RJ, Burt ME. Pericardiocentesis and intrapericardial sclerosis: effective therapy for malignant pericardial effusions. Ann Thorac Surg. 1997;64(5):1422–7; discussion 27–8. published Online First: 1997/12/05.
- Lui MMS, Thomas R, Lee YCG. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res. 2016;3(1):e000123.