ABSTRACT
Objectives
For patients admitted with an acute exacerbation of COPD (AECOPD) and a need for supplementary oxygen therapy, to determine if peripheral oxygen saturation < 88% (hypoxemia) or >92% (hyperoxemia), within first 24 hours of admission, is associated with ‘treatment failure’ or fewer days alive and out of hospital within 14 days after admission.
Design
A retrospective multicenter observational study, reviewing consecutive data on SpO2, oxygen, and drug administration at three predefined time points, on adverse events in patients admitted with COPD between December 2019 and June 2020. Multivariable logistic regression analysis, Mann Whitney U- and Chi-square-test were used.
Setting
Acute hospital setting, across four different hospitals in the capital region of Denmark.
Participants
Patients with a confirmed diagnosis of COPD admitted with an acute exacerbation and an oxygen need within the first 24 hours admission.
Results
In total 289 COPD patients were included. The median age was 74.8 years [interquartile range (IQR):69.6 to 81.8], 191 were female and 132 patients experienced ‘treatment failure’. A minimum of one episode of hypoxemia (SpO2 < 88%) within first 24 hours was associated with having a low number (≤4) of days alive and out of hospital within 14 days after admission: OR 2.4 (95%CI 1.2 to 4.8), p = 0.02, absolute risk 44% vs. 26% p = 0.01, Chi-square. Comparable results were observed after 30 days of follow-up: OR 2.6 (95% CI 1.0 to7.1), p = 0.05. A minimum of one measurement of hyperoxemia (SpO2 > 92%), within first 24 hours of admission was not associated with low number of days alive and out of hospital within 14 days OR 1.0 (95% CI 0.5 to 2.1) nor at 30 days.
Conclusion
For admitted patients with AECOPD, being hypoxemic ever within the first 24 hours after admission is associated with a substantially increased risk of a poor prognosis.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is highly prevalent and associated with high morbidity and mortality [Citation1–5]. Respiratory failure or arterial hypoxemia defined as an oxygen saturation (SpO2) < 88% is not uncommon in acute exacerbations of COPD (AECOPD) and often leads to hospital admissions [Citation6]. Oxygen supplementation, bronchodilators and steroids are often used in the first-line treatment of respiratory failure. The associated dyspnea is sometimes alleviated by administration of opioids and/or benzodiazepines (BZD) and I associated with inpatient mortality [Citation7].
Oxygen therapy may impair alveolar ventilation and CO2 elimination through different mechanisms, and thus induce hypercapnia and subsequent respiratory failure in susceptible patients [Citation8–10]. Survival rates in patients with COPD who experience their first episode of hypercapnic respiratory failure requiring Non-Invasive Ventilation (NIV) are low and have been estimated to be 72% at one year, 52% at two years, and 26% at five years respectively [Citation11]. To reduce adverse effects in the acute phase of an exacerbation, it is suggested that oxygen therapy can be titrated according to guidelines to achieve a SpO2 between 88–92% [Citation6,Citation12]. Also, guidelines advocate that opioids and BZD suppress respiration and can be titrated individually to avoid respiratory adverse effects [Citation13,Citation14].
It is known that hypoxemia induces pulmonary hypertension, secondary polycythemia, systemic inflammation, skeletal muscle dysfunction and, ultimately death if the hypoxemia is severe and untreated [Citation10,Citation15]. It has also been shown that high-flow or liberal oxygen therapy increases the risk of hypercapnia, respiratory acidosis and mortality in prehospital settings [Citation15,Citation16]. Few studies have examined associations between initial therapy of hypoxemia and dyspnea, incorporating oxygen, opioids and/or BZD administration, in patients with COPD and outcome in terms of treatment failure (initiation of mechanical ventilation after at least 24 hours of admission) or death in a hospital setting. Considerable unclarity remains of whether hypoxemia (<SpO2 < 88%) or hyperoxemia (SpO2 > 92%) is associated with the worst prognosis. Also, the potential risk attributed to administration of opioids and/or BZD, within first 24 hours of admission, remains unclear in the literature [Citation17,Citation18].
The primary aim of the current study was to determine, for patients admitted with COPD exacerbation and a need for supplementary oxygen therapy if peripheral oxygen saturation < 88% (hypoxemia) or >92% (hyperoxemia), within first 24 hours of admission, is associated with fewer days alive and out of hospital within 14 days after admission.
The secondary aim was to find out if peripheral oxygen saturation < 88% (hypoxemia) or >92% (hyperoxemia) within first 24 hours of admission is associated with a higher risk of the combined outcomed measures; ‘treatment failure’* or ‘late hypercapnic respiratory failure’**.
Further we wanted to explore whether administration of either opioids or BZD *** within the first 24 hours of admission is associated with fewer days alive and out of hospital within 30 days after admission.
* defined as NIV-treatment, ICU admission and/or invasive mechanical ventilation initiated after first 24 hours of admission, hospital admission length >10 days or death within 30 days after admission.
**defined as NIV-treatment, ICU admission and/or invasive mechanical ventilation initiated after first 24 hours of admission
*** opioids and/or BZD, but not midazolam.
Materials and methods
A multicenter observational study performed by, reviewing consecutive data on patients admitted in respiratory wards in four different hospitals in the capital region of Denmark between December 2019 and June 2020. Patients were identified through electronic medical records (EMR), contained in retrospective reports of admissions from each ward.
Patients with a confirmed diagnosis of COPD admitted with an acute exacerbation (ICD code J440, J441, J448 and J449 in possible combination with J18.X or J96.X) and a minimum of one episode of documented oxygen therapy in the Early Warning Score (EWS) [Citation19] within the first 24 hours admission, were included.
Ethics
According to the national Committee on Health Research Ethics in Denmark non-interventional studies are not covered (www.nvk.dk).The study has been approved by the Danish Data Protection agency (31–1521-436) and is conducted in accordance with the Declaration of Helsinki [Citation20]. The authors will share all study data to other scientists. However, data sharing should always comply with national and international data regulations to protect patient´s right to own data. Thus, other scientists can approach the authors with a plan within this legal frame. If this plan does not comply to the regulations, the authors will do every effort to help formulating the request within legislative frames.
Data collection and exposure definitions
As a daily routine, data on the vital parameters of the patients are collected by nursing staff and documented according to modified Early Warning Score EWS guidelines in the EMR [Citation21]. Data on oxygen delivery, SpO2, respiratory rate, blood pressure, pulse rate, awareness and temperature were obtained on included patients at three different points; at arrival and by intervals of 8 hours until 24 hours. Thus, first measurement was documented at arrival or up to 8 hours after (8 hours measurement), second measurement was documented between 8–16 hours after arrival (16 hours measurement) and third measurement was documented between 16–24 hours after arrival (24-hours measurement). A total of three EWS-measurements were obtained on each patient. In case of missing value, because the patient had died or was discharged, the ‘last observations carried forward’ approach was used. Patients who had no EWS-measurements before admission in ICU or death were excluded. Oxygen delivery method was primarily by nasal canula unless patients were mechanically ventilated. Data on administration of opioids or BZD within first 24 hours/ 3 shifts were obtained, as were data on events of Non-Invasive Ventilation (NIV) or invasive ventilation, ICU during admission or death within 30 days from admission. Additional data on Long Term Oxygen therapy (LTOT), Long Term NIV and demographic factors were obtained.
To describe the exposure of oxygen therapy within first 24 hours, patients were categorized according to SpO2 in the following categories: SpO2 between 88–92% (saturation within range), SpO2 > 92% (hyperoxemic event) or SpO2 < 88% (hypoxemic event). Thus, it was possible to determine if patients had received ‘perfectly controlled oxygen therapy’ (all three measurements within saturation range), had been ‘ever hyperoxemic’ (minimum of one episode of SpO2 > 92%) or ‘ever hypoxemic’ (minimum of one episode of SpO2 < 88%).
We obtained data on administration of opioids and/or BZD within first 24 hours of admission. Midazolam is often used in the context of terminal care and may as such have contributed with bias by indication, hence administration of midazolam did not contribute to data. Exposure of opioids or BZD was categorized into administration of ‘opioids or BZD ever’ versus never.
Statistics
Sample size was estimated for the primary outcome ‘Days Alive and Out of Hospital within 14 days’. The standard deviation has been found in other studies to be approximately 3.8 days for patients admitted with COPD [Citation22]. Conventional type II error limit of 0.2 (corresponding to a power of 80%) and an alfa of 0.05 were used, and a 1.3 day difference as detection limit, established in an earlier AECOPD study [Citation22,Citation23]. This would require at least 272 patients. We decided to include 270–300 patients.
Days Alive and Out of Hospital within 14 days (DAOH-14) is a continuous outcome, used as our primary outcome and is known for its sensitivity [Citation22,Citation24]. Date of admission, discharge from hospital and death within 14 days from admission were used to calculate number of Days Alive and Out of Hospital (DAOH), hence avoiding lead-time bias due to death during admission. If patients had more than one admission within a period of 14 days, the second admission did not contribute to data. DAOH-30 was used as third outcome and followed same methodology as given for DAOH-14.
Treatment failure, defined as NIV-treatment initiated after first 24 hours of admission, ICU and/or mechanical ventilation initiated after first 24 hours of admission, hospital admission length >10 days or death within 30 days after admission was calculated as a composite outcome based on dates and time of all of the above and dichotomized, as we anticipated that each single outcome would not provide sufficient power to detect associations. Hospital length >10 days was added to the combined outcome treatment failure as it was decided that 10 days without sufficient recovery, was a proxy for treatment failure.
Logistic regression was used to calculate Odds Ratio (OR) for associations between peripheral oxygen saturation and the dichotomized versions of treatment failure and DAOH-14 and DAOH-30. Treatment failure was categorized into ‘no event’ or ‘event’, with ‘no event’ being reference category. DAOH-14 was categorized into less than or equal to 4 days alive and out of hospital (DAOH-14 ≤ 4 days) [Citation24] vs. more than 4 days alive and out of hospital from admission (DAOH-14 > 4 days). DAOH-30 was categorized into less than or equal to 10 days alive and out of hospital within 30 days from admission (DAOH-30 ≤ 10 days) [Citation24] and more than 10 days alive and out of hospital within 30 days from admission (DAOH-30 > 10 days). All regression analyses were adjusted for covariates (sex, LTOT and Home-based NIV) and results are presented as differences in means with 95%CI, significance P ≤ .05.
For sensitivity testing, we used a non-parametric two-tailed Mann Whitney U-test to determine if oxygen therapy or administration of opioids or BZD had significant effects on number of days alive and out of hospital.
We present results by medians and Inter Quartile Range (IQR). P-values < 0.05 were considered significant. IBM SPSS statistical package version 25 was used for analysis.
Results
Patients with several admissions could only enter once (unique patients), thus we finally included 289 consecutive patients in the analysis, which complied with our sample size calculation.
Patient characteristics
Patient characteristics are described in .
Table 1. Patient characteristics; demographic data, clinical exposures and total number of events.
Days Alive and Out of Hospital within 14 days (DAOH-14) was median 8.6 [IQR 2.2–10.8]. Within 14 days from admission 28.7% of patients had less than 4 days alive and out of hospital (DAOH-14 < 4).
Patient’s Days Alive and Out of Hospital within 30 days (DAOH-30) was median 24.4 [IQR 17.2–26.7] and 17.6% of these patients had less than 10 days alive and out of hospital (DAOH-30 < 10).
Treatment failure occurred in 45.6% of admissions shown in .
Figure 1. Shows patient flow from screening, to inclusion and through first 24 hours treatment and into Days Alive and Out of Hospital up until 30 days from admission (DAOH-30). The flowchart illustrates the composites of ‘Treatment failure’ and ‘late hypercapnic respiratory failure’ by showing a) the number of events within first 24 hours of treatment, B) number of events after 24 hours of treatment given by numbers (%); ‘treatment failure’ 132 (45.6%) and ‘late hypercapnic respiratory failure’ 28(9%) and C) total number of events.
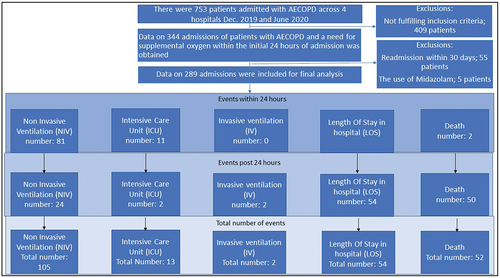
‘Number of admissions; potentially eligible for inclusion, excluded, confirmed eligible and number of events within 24 hours exposure and after exposure but within 30 days from admission’:
A total of 48 patients experienced being ”hypoxemic ever”, with 36 patients experiencing just ‘one hypoxemic episode’ in three measurements. From these 48 ‘hypoxemic ever’ patients, 21 patients (44%) experienced the outcome of ‘less than 4 days alive and out of hospital within 14 days’, whereas for ‘never-hypoxemic’ patients, 62 of 241 (26%) experienced less than 4 days alive and out of hospital, p = 0.01, Chi-square. For further analysis of patients with moderate or severe hypxemia, please see supplemental materials
Logistic regression analysis showed a strong association between ‘hypoxemic ever’ and low number of days alive and out of hospital (DAOH-14 < 4) with OR 2.4 (95%CI 1.2–4.8), p = 0.02. ( and below).
Figure 2. Forest plot showing risks of exposures as Odds Ratios [95% Confindence Intervals] in relation to low numbers of days alive and out of hospital within 14 days and treatment failure.
![Figure 2. Forest plot showing risks of exposures as Odds Ratios [95% Confindence Intervals] in relation to low numbers of days alive and out of hospital within 14 days and treatment failure.](/cms/asset/5894e72e-87c3-415b-b5b4-073d4c5288ab/zecr_a_2153644_f0002_b.gif)
Table 2. Associations between exposures and outcomes NB: Only powered for the oxygenation variables (marked with #).
Male gender was also strongly associated with (DAOH-14 < 4) with OR 1.8 (95%CI 1.0–3.1), p = 0.034. There was no significant association between ‘hyperoxemic ever’, and (DAOH-14 < 4) ( and below). Administration of opioids or BZD ‘opioids or BZD ever’ showed a trend towards an increased risk of low DAOH-14 < 4, with OR 1.6 95%CI (0.9–2.7). However, this increased risk was not significant (p = 0.12). There were no associations between DAOH-14 < 4 and LTOT or Long Term-NIV.
Sensitivity analysis, testing ‘hypoxemic ever’ versus patients who never experienced hypoxemic measurements, revealed a clinically meaningful difference in DAOH-14: median 7.1 days [IQR 0.0–10.2]) vs. median 8.8 days [IQR 4.0–10.9]), p = 0.0477.
Being “hyperoxemic ever “compared to patients who never experienced hyperoxemic measurements did not seem to influence the DAOH-14 outcome: median 8.8 [IQR 0.3–10.2] vs. 8.1 [IQR 3–10.9], p = 0.08
A minimum of one administration of ‘opioids or BZD ever’ Vs. ‘opioids or BZD never’ on DAOH-14 showed no significant difference between groups median 8.9 [IQR 4.3–11 .1] Vs. median 8.6 [IQR 1.2–10.6], p = 0.23
As shown in and , the logistic regression analysis adjusted for age, gender, LTOT and Long Term NIV did not show any association between a minimum of one measurement of hypoxemia ‘hypoxemic ever’ and treatment failure or late hypercapnic respiratory failure. Male gender showed a strong and significant association with treatment failure. There was no association between ‘hyperoxemic ever’ and treatment failure or late hypercapnic respiratory failure. Administration of ‘opioids or BZD ever’, showed a nonsignificant trend towards an increased risk of treatment failure and late hypercapnic respiratory failure.
Logistic regression showed a strong and significant association between ‘opioids or BZD ever’ and (DAOH-30 < 10). Strong associations were also shown between ‘hypoxemic ever’ and DAOH-30 < 10 as well as gender.
We did not find any differences on the DAOH30 outcome between the groups ‘opioids or BZD ever’ vs. ‘opioids or BZD never’: median DAOH30: 24.8[IQR 19.0–27.4 vs. median 24.3 [IQR 15.0–26.5], Mann-Withney U-test on p = 0.37
Discussion
Among patients admitted for AECOPD, being ‘hypoxemic ever’, within first 24 hours of admission was associated with a substantially increased risk of having fewer days alive and out of hospital within 14 days from admission. Opposite, and surprisingly, being hyperoxemic ever” did not increase the risk of fewer days alive and out of hospital within 14 days.
We found numerically substantially more patients who progressed to treatment failure after being hypoxemic, however this was not statistically significant.
Further, we observed a signal of increased risk of fewer days alive and out of hospital within 30 days, if patients had had a minimum of one administration of opioids or benzodiazepines.
Relation to other studies
Our results are surprising and contradict previous evidence that has primarily focused on harm from hyperoxemia. However, most studies on oxygen administration are based on prehospital- or emergency settings [Citation15,Citation16,Citation25–27], and these results cannot be extrapolated into a hospital environment of admitted patients. Our findings could not, in a hospital setting, confirm the high risk associated with hyperoxemia.
The high risk of adverse events associated with organ hypoxemia and tissue ischemia in patients admitted with AECOPD is highlighted by our results, but data also indicates, how high oxygen flows and not solely high SpO2, may be an attention point in future oxygen therapy. This speculation emerges, as oxygen flows represent the most substantial deviation between our observations and the observations contributing to the risks described in pre-hospital literature, since our mean oxygen delivery was 2.7 L/min (range 0–30) compared to Austin et. al.’s liberal oxygen delivery of 8–10 L/min [Citation16].
Furthermore, gas exchange in the lungs is complex with hypoxemia, hypercapnia and acidosis influenced by a number of processes such as hypoxic pulmonary vasoconstriction, increased ventilation/perfusion-mismatch, the Haldane effect, resorption atelectasis, and inhibition of hypoxic drive, making therapy is equally complex [Citation28]. It is known that hypercapnia and/or acidosis develops at a slower rate than hypoxemia. Despite this, literature indicates that between 10–20% of AECOPD admitted in hospitals present with type 2 respiratory failure by arrival [Citation29,Citation30], these patients are not included in our study, making it hard to compare our risk estimates with previous literature. However, it has been shown, that patients who develop acidosis during admission and initiate NIV after 24 hours of admission, have a higher mortality rate than those who arrive at the hospital with acidosis [Citation31,Citation32]. These patients, susceptible for hypercapnia and known for their need of close monitoring, are presented in our data. However, the high rate of hyperoxemic episodes reported in our study is associated with a relatively low risk of hypercapnia. This indicates that the complex clinical procedure of monitoring oxygen administration via observations, algorithms, and Arteria Blood Gas (ABG) values have better conditions in hospital settings as opposed to pre-hospital settings and stress the importance of it.
Another explanation why our results might be that some of the included patients may quite rapidly be able to oxygenate spontaneously. These may have a better prognosis than those who continue to have a need of oxygen supplementation.
Also the differences in risks (OR) between the observations by others [Citation15,Citation26] and our results may be explained by the fact that both previous studies reflect emergency arrivals detected by a single EWS measurement or Arterial Blood Gases (ABG) within 4 hours, and thus they include most sick subjects eligible for direct NIV, ICU or death within first 24 hours of admission regardless the specific underlying pathophysiology. This part of the AECOPD-population is also excluded from our analysis.
Thus, our data does not confirm the high risk of hyperoxemia described by most literature, but underlines the high risks associated with hypoxemia in admitted patients with AECOPD. This especially so, since most of the patients who experienced hypoxemia, only experienced a single episode. Moreover, based on explorative analysis, it seemed that the number of hypoxemic episodes could be of essence for the prognosis rather than the degree of hypoxemia. Our results pinpoint the need for interventional studies on the correct oxygen target among admitted patients with COPD exacerbation, and they raise considerable doubt on whether results from pre-hospital settings can be extrapolated into hospital settings. This is a serious concern, since oxygen saturation targets of 88%-92% have been adapted in hospital-settings, despite them being based on pre-hospital studies.
Patients qualifying for LTOT or Long Term-NIV are a severely sick subgroup, but none the less and interestingly so, no associations between DAOH-14 < 4 and LTOT or Long Term-NIV was found I our study. We believe our results are reflections of optimized therapies and a confirmation of the already reported effects of these on readmissions and mortality [Citation33,Citation34]
Hypoxemia seems to be highly associated with an increased risk of a poor prognosis and certainty on this crucial part of COPD exacerbation therapy should be obtained shortly.
Strengths and weaknesses of this study
Our study was a multicenter design, and we collected substantial and systematic data across four different hospitals and 8 different wards (4 Emergency wards and 4 respiratory wards) in a highly selected population. We believe our study differentiates from previous studies [Citation15,Citation16,Citation25,Citation26,Citation35], due to the in-hospital setting, the extended timeframe of data collection, with three times repeated measurements within a 24 hours and at clearly predefined time points and not upon indication. Further, we followed patients for 30 days and used fixed-time follow-up, not hospital admission lengths, that may vary in different patient groups and be prolonged by oxygen needs, circumstances associated with a higher risk of death. By using fixed-time follow-up, we included data on potential readmissions and death within the fixed period, while at the same time avoiding lead-time bias, which would have been the case if we only described length of stay and time to death separated from a fixed-time follow-up. Our study describes a high rate of anxiolytic usage (58%) that may differ from international practice. We believe this rate to be comparable to Scandinavian standards of a selected population with severe COPD and an oxygen need, since Ekström et al. describe a prescription rate of opioids to be 46% in a Swedish national cohort of end-stage COPD patients starting LTOT. Our somewhat higher rate could be interpreted as an consequence of the acute setting, that in combination with the disease severity and weakness of the patients, induces a high risk of delirium. According to literature the crude prevalence estimate of delirium amongst patients age>60 in emergency departments is 15.2% 95% CI (12.5–18.0) [Citation36]. Since guidelines often recommend BZD in the clinical management of delirium [Citation37], the significant association between ‘opioids or BZD ever’ and (DAOH-30 < 10) in our findings could potentially be proxy for delirium. Further, our results related to use of ‘opioids or BZD’ should be interpreted as explorative, since our study only have sufficient power to conclude on our primary analysis.
Limitations include lacking data on ABG’s as they may have contributed with validation of the effects of oxygen therapy. We decided not to include ABG’s as it may have impacted our statistical power or exceed the frame of this observational study. In addition no overall statistically significant bias in paired SpO2/SaO2 measurements have been detected in a previous observational study, supporting the use of pulse oximetry for titration of oxygen therapy [Citation26,Citation38]. Although our observational study had a high degree of data completeness, used fixed time outcome and our analysis was adjusted, there may still be a risk of residual confounding and the retrospective analysis does not allow for any conclusions regarding causality of the findings. However, though the lack of ability to conclude on causality is a considerable limitation it is a well-known condition of the design.
Conclusions and implications for clinicians treating patients admitted with AECOPD and need of oxygen therapy
For admitted patients with AECOPD, being ‘hypoxemic ever’ seems to be associated with a highly increased risk of a poor prognosis. Randomized trials should be conducted to find the correct and most safe target interval for in-hospital oxygen therapy in admitted COPD patients with exacerbation.
Supplemental Material
Download MS Word (19 KB)Acknowledgments
The authors kindly thank the contributing hospital wards. There, has been no direct patient involvement in the current study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2022.2153644.
References
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. 2004;364(Aug 14):4–9.
- Murray SA, Kendall M, Boyd K, et al. Clinical review Illness trajectories and palliative care. Lancet. 1997 may;24:1007–1011.
- Decramer M, Janssens W.Chronic obstructive pulmonary disease and comorbidities.Lancet Respir [Internet]. 2021;1(1):73–83.
- Chronic-obstructive-pulmonary-disease-(COPD) [Internet]. World Health Organization. 2020 Oct 24. [cited 2020 Oct 24]. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- Andreassen H, Vestbo J. Chronic obstructive pulmonary disease as a systemic disease: an epidemiological perspective. Eur Respir J. 2003;22(Supplement 46):2s–4s.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of. COPD GOLD REPORT 2021. 2021.
- Stevens JP, Dechen T, Schwartzstein RM, et al. Association of dyspnoea, mortality and resource use in hospitalised patients. Eur Respir J. 2021;58(3):online.
- Brill SE, Wedzicha JA. Oxygen therapy in acute exacerbations of chronic obstructive pulmonary disease. Int J COPD. 2014;9:1241–1252.
- Aubier M, Murciano D, Milic-Emili J, et al. Effects of the administration of O2 on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. Am Rev Respir Dis. 1980;122(5):747–754.
- Kent BD, Mitchell PD, Mcnicholas WT. Hypoxemia in patients with COPD : cause, effects, and disease progression. Int J COPD. 2011;6:199–208.
- Chung LP, Winship P, Phung S, et al. Five-year outcome in COPD patients after their first episode of acute exacerbation treated with non-invasive ventilation. Respirology. 2010;15(7):1084–1091.
- Guideline BTS, Oxygen FOR, Adults IN, et al., BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax an Int J Respir Med. 2017;72(June):1–100.
- Parshall MB, Schwartzstein RM, Adams L, et al. American Thoracic society documents an official American thoracic society statement : update on the mechanisms, assessment, and management of dyspnea. Amarican J Respir Crit care Med. 2012;185(4):435–452.
- Mahler DA, Selecky PA, Harrod CG. Management of dyspnea in patients with advanced lung or heart disease. Practical guidance from the American college of chest physicians consensus statement. chest journal, Am Coll chest physicians [Internet]. 2010;120(5):160–166.
- Cameron L, Pilcher J, Weatherall M, et al. The risk of serious adverse outcomes associated with hypoxaemia and hyperoxaemia in acute exacerbations of COPD. Postgr Med J. 2012;88(september):684–689.
- Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive randomised controlled trial. BMJ Res. 2010;34(online):1–8.
- Verberkt CA, Everdingen MHJVDB, Schols JMGA, et al. Respiratory adverse effects of opioids for breathlessness : a systematic review and meta-analysis. (836031012. Eur Respir J. 2017;50(5):1701153.
- Boon M, Dorp E, Van, Broens S, et al. Combining opioids and benzodiazepines : effects on mortality and severe adverse respiratory events. Ann Palliat Med. 2020;9(2):542–557.
- Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified early warning score in medical admissions. QJM - Mon J Assoc Physicians. 2001;94(10):521–526.
- WHO. World medical association declaration of helsinki ethical principles for medical research involving human subjects Clinical Review and Education. 2019;310(30): 2191–2193 .
- O’Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63(SUPPL. 6):vi1–68.
- Sivapalan P, Lapperre TS, Janner J, et al. Articles Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled. Lancet Respir. 2019;20:6–30176.
- Walters J, White C, Gibson P, et al. Systematic corticosteoids for acute exacerbations og chronic obstructive pulmonary disease (review). Cochrane Database Syst Rev. 2014;9:391–405.
- Sivapalan P, Ulrik CS, Lappere TS, et al. Proactive prophylaxis with azithromycin and hydroxychloroquine in hospitalized patients with COVID-19 (ProPAC-COVID): a statistical analysis plan. BMC Trails. 2020;1(8): 1–9
- Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet [Internet]. 2021;391(10131):1693–1705.
- Echevarria C, Steer J, Wason J, et al. Oxygen therapy and inpatient mortality in COPD exacerbation. Emerg Med J. 2021;38(3):170–177.
- Ringbaek TJ, Terkelsen J, Lange P. Outcomes of acute exacerbations in COPD in relation to pre-hospital oxygen therapy. European Clinical Respiratory Journal. 2015;1:1–6.
- Cornet AD, Kooter AJ, Peters MJL, et al. The potential harm of oxygen therapy in medical emergencies. Crit Care. 2013;17(2):313.
- Plant PK, Owen JL, Elliott MW, et al. One year period prevalence study of respiratory acidosis in acute exacerbations of COPD : implications for the provision of non-invasive ventilation and oxygen administration. Thorax. 2000;55(7):550–554
- Roberts CM, Stone RA, Buckingham RJ, et al. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66(1):43–48
- Jayadev A, Stone R, Steiner MC, et al. Time to NIV and mortality in AECOPD hospital admissions : an observational study into real world insights from national COPD audits. BMJ Open Respir Res. 2019;6(1):1–7.
- Trethewey SP, Edgar RG, Morlet J, et al.Late presentation of acute hypercapnic respiratory failure carries a high mortality risk in COPD patients treated with ward-based NIV.Respir Med [Internet]. 2021;151(January 2019):128–132.
- Cranston JM, Crockett A, Moss J, et al. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2008(4).
- Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. J Am Med Assoc. 2017;317(21):2177–2186.
- Wijesinghe M, Perrin K, Healy B, et al. Pre-hospital oxygen therapy in acute exacerbations of chronic obstructive pulmonary disease. Intern Med J. 2009;39(december):1–18.
- Chen F, Liu L, Wang Y, et al. Delirium prevalence in geriatric emergency department patients: a systematic review and meta-analysis. Am J Emerg Med Internet]. 2022;59:121–128.
- Grover S, Avasthi A. Clinical practice guidelines for management of delirium in elderly. Indian J Psychiatry. 2018;60(7):S329–40.
- Ebmeier SJ, Barker M, Bacon M, et al. A two centre observational study of simultaneous pulse oximetry and arterial oxygen saturation recordings in intensive care unit patients. Anaesth Intensive Care. 2018;46(3):297–303.
