ABSTRACT
Background
Persistent air leak (PAL) is common in secondary spontaneous pneumothorax (SSP), with risk factors only been determined for post-pulmonary resection PAL. Information about its risk factors and long-term outcome is, however, necessary to enable selection of treatment modalities for elderly SSP patients with comorbid conditions.
Methods
A retrospective observational study was performed on chest drain-treated SSP patients from 2009 to 2018. The risk factors, long-term recurrent pneumothorax, and mortality rates of those with and without PAL were evaluated.
Results
Of 180 non-surgical SSP patients, PAL prevalence for >2 days and >7 days were 81.1% and 43.3%, respectively. Bulla was associated with PAL >7 days (OR: 2.32; P: 0.027) and serum albumin negatively associated (OR: 0.94; P: 0.028). PAL resulted in longer hospitalization in the index episode (P: <0.01). PAL >7 days was associated with a higher pneumothorax recurrence rate in three months (HR: 2.65; P: 0.041), one year (HR: 2.50; P: 0.040) and two-year post-discharge (HR: 2.40; P: 0.029). Patients treated with medical pleurodesis were significantly older (P: <0.01), had higher Charlson Co-morbidity index scores (P: <0.01), and 77.8% of those who had PAL >7 days were considered unfit for surgery. Of these, pneumothorax had not recurred in 69.4% after two years (HR: 0.47; P: 0.044).
Conclusion
Bulla was positively associated with PAL over seven days in SSP patients while albumin was negatively associated. PAL over seven days increased future recurrent pneumothorax risks, while elderly SSP patients unfit for surgery had acceptable recurrence rates after medical pleurodesis.
Introduction
Secondary spontaneous pneumothorax (SSP) is a common complication of underlying lung conditions. Chronic obstructive pulmonary disease (COPD) accounted for 50–70% of SSP, followed by prior pulmonary tuberculosis (40%) and malignancy (8%) [Citation1–3], while 32% had more than one respiratory condition [Citation2]. Compared to primary spontaneous pneumothorax (PSP), pneumothorax recurrence is more prevalent after SSP [Citation3].
Persistent air leak (PAL) is common in SSP, frequently lasting longer than that in PSP [Citation4] and prolonging hospitalization [Citation5–8]. Studies on PAL risk factors have been mostly on patients post-lung resection [Citation9–13] or PSP [Citation14], with only very few on SSP [Citation4,Citation7,Citation15]. Although early recognition may allow timely institution of appropriate treatment and improve outcome, predictors for PAL in SSP have not been reported [Citation4,Citation8]. It is also not clear whether PAL during the index pneumothorax episode is associated with long term consequences post-discharge.
Methods
Objectives
We aimed to evaluate the prevalence of PAL in SSP patients in a Hong Kong hospital to identify its risk factors and two-year clinical outcome.
Study design
A retrospective observational study on SSP patients was conducted from 1 January, 2009 to 31 December, 2018 in Pamela Youde Nethersole Eastern Hospital (PYNEH), an acute general Hong Kong hospital serving 0.6 million population. The study was approved by the Research Ethics Committee of Hong Kong East Cluster (Ref. No.: HKECREC-2021-076) of the Hong Kong Hospital Authority (HA).
All patients who had diagnostic coding of SSP were abstracted from the HA Clinical Management System. Inclusion criteria were patients: 1) older than 18 years; and 2) who suffered from SSP diagnosed on chest X-ray; and 3) who had been treated with chest drains.
Exclusion criteria included: 1) Younger than 18 years; or had any one or more of the following: 2) Initial diagnosis of pneumothorax made by CT scan of the thorax and not visible by chest X-ray on the same day; 3) Primary spontaneous pneumothorax; 4) Trauma-related or iatrogenic pneumothorax; 5) Pneumothorax not treated with chest drain insertion; 6) Air leak duration uncertain; 7) Chest tube accidentally dislodged before pneumothorax resolution and not replaced; 8) Received mechanical ventilation (invasive or non-invasive) during the index episode; 9) Trapped lung; 10) COPD documented as diagnosis but not confirmed by lung function; 11) Diagnosis of underlying lung condition uncertain and 12) Wrong coding.
PAL was defined according to the 2010 British Thoracic Society (BTS) guideline, as air leak for more than 2 days after chest tube insertion [Citation16]. The prevalence of PAL at 2 days and 7 days were calculated. Air leak duration in days was determined by doctors’ documentation supplemented by nursing record on air leak. If air leak had stopped and recurred, the day the last air leak documented was used to determine the air leak duration. Residual pneumothorax after a planned chest tube removal was not counted as PAL for the purpose of this study.
The following data were collected:
Patient’s demographics;
Underlying lung condition as defined in the supplementary file (Supplementary Appendix A);
For the purpose of this study, bulla(e) regardless of underlying lung disease was defined as: Bulla(e) reported by radiologists to be present on the same side as the pneumothorax on CT thorax, HRCT or chest X-ray taken before the pneumothorax episode, and bulla(e) reported to be the reason for the pneumothorax during thoracic surgical procedure(s).
Patient’s co-morbidities and Charlson Co-morbidity Index
Data of the index admission including: i) Initial pneumothorax size; ii) History of prior pneumothorax and laterality; iii) Loculation in the pneumothorax; iv) Data related to PAL occurrence including date of onset and date of resolution; v) Serum albumin level closest to onset of pneumothorax; vi) Hospitalization duration; vii) All-cause mortality and pneumothorax-related mortality during the same hospitalization; viii) Any form of treatment for PAL including medical (Talc/minocycline) or surgical pleurodesis and ix) initial pneumothorax size measured using the Collin’s method [Citation17] as follows:
Percentage of pneumothorax = 4.2 + [4.7 × (A + B + C)], where:
A: Maximum apical inter-pleural distance
B: Inter-pleural distance at the midpoint of the upper half of the lung
C: Inter-pleural distance at the midpoint of the lower half of the lung
Differentiation between a large (>2 cm between chest wall and lung margin) from small pneumothorax (<2 cm between chest wall and lung margin) was also estimated according to the BTS guideline [Citation16].
5) Two-year clinical outcomes including recurrent pneumothorax and pneumothorax-related mortality (defined as death due to tension pneumothorax) following discharge from the index admission.
The primary objective was to evaluate the prevalence and risk factors of PAL among our SSP patients. Secondary objectives included evaluation of the effect of PAL on 1) hospitalization duration, all-cause mortality and pneumothorax-related mortality during the index episode; and 2) 2-year risks of recurrent pneumothorax and pneumothorax-related mortality post-discharge.
Statistical analysis
Descriptive statistics were used to summarize the baseline patient demographic characteristics. Comparison of continuous variables was performed using Student’s T-test/ANOVA or Mann-Whitney/Kruskal Wallis test as appropriate. Categorical variables between groups were compared by the Chi-square test or Fisher’s exact test as appropriate. Missing data would be handled by mean imputation if necessary.
Multiple logistic regression analysis was used to assess the risk factors of PAL. Sample size estimation was based on the formula N(sample size) > 50 + 8 × (number of independent variables) [Citation18]. Following initial univariate correlation, possible risk factors with P value <0.2 were recruited into the multiple logistic regression analysis, in which P value of <0.05 was considered to be statistically significant. Hosmer & Lemeshow test and C statistic were used to assess the quality of the results of logistic regression.
Cox regression analysis was used to evaluate the effect of PAL on recurrent pneumothorax in 2 years. Factors that show P value <0.2 in initial univariate analysis and important potential confounding factors (e.g. pleurodesis treatment) were recruited into the Cox regression analysis. P value of <0.05 was considered to be statistically significant. Results were calculated using the computer software Statistical Package for Social Sciences (SPSS, Version 22, for Windows).
Result
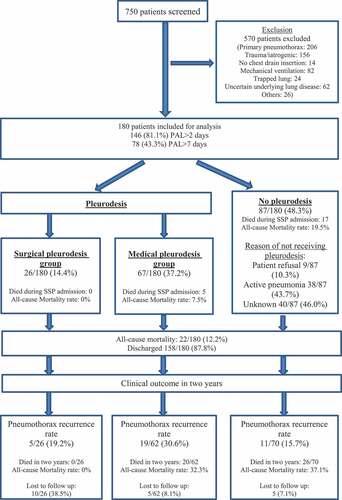
Data collection was complete apart from one missing data, being the reasons for patients not receiving pleurodesis in 40/87 patients as reflected in , and imputation was therefore not necessary. Of 750 patients suffering from pneumothorax in the 10-year period, 570 met the exclusion criteria ( and Appendix Table A). The medical records of the remaining 180 SSP patients were retrieved for analysis. Of these, 157 (87.2%) were male. Mean age was 63.6 years, mean air leak duration was 10.4 days (median 5 days; IQR 2.0–12.8 days) (Appendix Figure B), and air leak resolution occurred within 14 days in 77.8%. The mean pneumothorax size as measured by Collin’s method was 40.2%. Prior pneumothorax was diagnosed in 29.4% (53/180 patients), of whom 21.1% (38/180) had ipsilateral recurrence.
Baseline characteristics of included patients and prevalence of PAL
The prevalence of PAL >2 days and that for PAL >7 days were 81.1% (146/180) and 43.3% (78/180) respectively. COPD (35.0%) and bulla (27.2%) were the most frequent underlying respiratory conditions, while 28.9% had more than one such condition (). Baseline characteristics of patients with and without PAL are shown in .
Table 1. Frequency of underlying lung condition in 180 secondary spontaneous pneumothorax (SSP) patients.
Table 2. Baseline characteristics of patients with or without persistent air leak.
*Among 49 patients who had bullae, 15/49 (30.6%) had coexisting COPD, 6/49 (12.2%) had prior PTB, 2/49 (4.1%) had bronchiectasis and 1/49 (2%) had asthma, while the other 25/49 (51%) had no underlying lung disease.
Risk factors of PAL
Potential predictors for PAL >2 days with P value <0.2 identified in the univariate analysis and included in the multiple logistic regression analysis were: Ex-smoker, bulla, asthma, initial pneumothorax size (%), large pneumothorax as defined by the BTS guideline and history of ipsilateral pneumothorax. The regression model did not identify any risk factors of PAL >2 days. ( and Appendix Table E)
Table 3. Logistic regression for risk factors on PAL >2 days and >7 days (SE: standard error; df: degree of freedom; OR: odds ratio: CI: confidence interval).
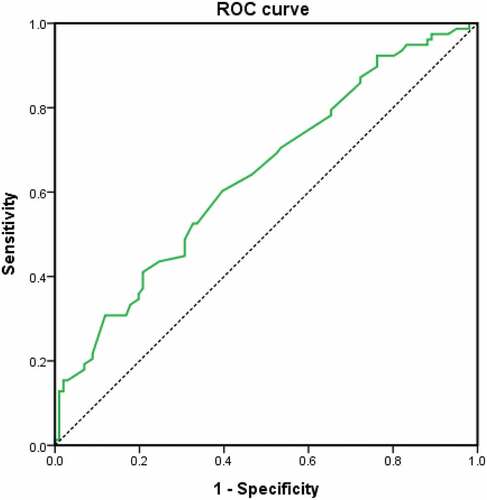
Potential predictors for PAL >7 days with P value <0.2 identified in the univariate analysis and included in the multiple logistic regression analysis were: Bulla, lung tumour, large pneumothorax as defined by the BTS guideline, previous ipsilateral pneumothorax and albumin (g/L). The presence of bulla was found to be independently associated with PAL >7 days (OR: 2.32; P: 0.027) while albumin level was negatively associated (OR 0.94; P: 0.028) (Hosmer and Lemeshow test: Chi-square: 4.82, P: 0.776) (C statistics: AUC: 0.64; 95% CI: 0.56–0.72; P: 0.001) (, and Appendix Table G).
Clinical outcome and long-term pneumothorax recurrence risks
Compared to patients without PAL, those with PAL had longer hospitalization in the index pneumothorax episode (No PAL: 7.5 days; PAL 2–6 days: 10.5 days; PAL >7 days: 25.5 days; P: <0.01) but there were no statistically significant differences in all-cause or pneumothorax-related mortality between the two groups (Appendix Table H).
Ninety-three PAL patients (93/180; 51.7%) were treated with either medical (67/93; 72.0%) or surgical pleurodesis (26/93; 28.0%) in the index episode (Appendix Table I). The median time from pneumothorax onset to pleurodesis was 8 days (IQR: 4.3–19.5) in the medical group and 13.5 days (IQR: 8.0–19.3) in the surgical group. In the medical pleurodesis group with PAL >7 days (n = 27), 77.8% (21/27) were considered unfit for surgery.
Outcomes of the index episode included: 22/180 died (mean age: 75.4 ± 2.8 years) and 158/180 (87.8%) (mean age: 61.9 ± 20.1) were discharged from hospital. Common causes of death were pneumonia (81.8%) and malignancy (13.6%). Cox regression analysis after adjustment of covariates including treatment with pleurodesis showed that PAL >7 days resulted in a higher rate of pneumothorax recurrence at 3 months (HR: 2.65; P: 0.041), 1 year (HR: 2.50; P: 0.040) and 2 years (HR: 2.40; P: 0.029) (; Appendix Table J – Table O), but did not confer significant differences in pneumothorax-related mortality (PAL 1.6% vs non-PAL 0%; P: 1.000) in 2 years. A single 87-year-old male COPD patients treated with medical pleurodesis died of tension pneumothorax one-month post-discharge.
Table 4. Cox regression analysis of the effect of PAL on recurrent pneumothorax in 3 months, 1 year and two-year post discharge (SE: standard error; df: degree of freedom; HR: hazard ratio: CI: confidence interval; PAL: persistent air leak).
A total of 88 patients treated with pleurodesis were discharged from the index episode. Medical pleurodesis-treated patients were significantly older (71.1 vs 33.6 years; P < 0.01) than those who had surgical pleurodesis, and more likely to have more than one respiratory condition (38.8% vs 15.4%; P: 0.048) as well as a higher co-morbidity score (Appendix Table I). The unadjusted 2-year pneumothorax recurrence rates, which did not show statistical significance, were 30.6% (medical group), 19.2% (surgical group) and 15.7% (No-pleurodesis) respectively (Appendix Table P). After adjustment of covariates, medical pleurodesis was associated with lower recurrence rate two-year post-discharge (HR: 0.47; P: 0.044) (Appendix Table O).
Discussion
Pneumothorax commonly affects patients with underlying lung condition and SSP is known to be a risk factor of PAL >2 days [Citation4,Citation7]. Since SSP patients are older, have more co-morbidities [Citation7,Citation19], and higher operative risks [Citation20] compared with PSP patients, evaluation of PAL risk factors and long-term outcome could enable the correct selection of treatment modalities for PAL, which could also prevent pneumothorax recurrence.
Risk factor of PAL
Our study showed that bulla was associated with PAL >7 days and albumin level was negatively associated. Because 49% of the bullae had ruptured during surgical pleurodesis, size measurement was not feasible for evaluation of its relationship with PAL. While a study of 492 post-pulmonary surgery patients had correlated bullae diameter with PAL >7 days (OR 1.261; P: <0.001) [Citation21], a 2013 Japanese study of a mixed group of 333 PSP and 108 SSP patients did not find any association between the presence of blebs and need for invasive treatment (P: 0.09), nor did it study its correlation with air leak duration [Citation15]. Based on our findings, early definitive treatment for PAL may be considered in the presence of bulla(e) and hypoalbuminemia.
Despite its high accuracy, it is impractical to apply CT-scan enabled three-dimensional volume measurement of pneumothorax size in the real-world situation [Citation16,Citation22]. Of the different chest X-ray methods proposed to estimate the pneumothorax size [Citation23–26], Collin’s method, a mathematical equation based on volumetric measurement of pneumothorax size by helical CT scan, demonstrated a good correlation with the estimated percentage pneumothorax size on an erect postero-anterior chest radiography (r = 0.98, P: <0.0001) [Citation17]. Previous studies on the relationship between PAL and initial pneumothorax size [Citation4,Citation7,Citation14] was limited by inaccurate size measurement and small sample size [Citation4,Citation7,Citation14]. More recently, a Japanese study found that interpleural distance predicted PAL for over 72 h in PSP patients using three different methods: Interpleural distance at the hilum according to the BTS guideline, apex-cupola distance of the ACCP guideline, and the Light’s index calculated from the ratio of the diameter of the collapsed lung to that of the hemithorax [Citation14]. In contrast to previous studies [Citation14], initial pneumothorax size as measured by Collin’s method was not found to have a significant impact on PAL prevalence in our study.
Recurrence risk of PAL
Similar to previous studies [Citation5–8], our study found that PAL prolonged hospitalization during the index episode. Few studies evaluated the effect of PAL on pneumothorax recurrence rate [Citation14]. After adjustment of covariates in our study, PAL >7 days was found to increase pneumothorax recurrence risks in our patients two-year post-discharge. Since patients with pneumothorax secondary to trauma, iatrogenic cause, and mechanical ventilation had been excluded, underlying respiratory conditions with abnormal lung parenchyma likely affected the healing process after pneumothorax occurred, as reflected by the presence of PAL and higher rates of recurrence.
Pneumothorax treatment for prevention of recurrence in SSP patients
Early recognition of PAL followed by prompt treatment, through surgical referral by 48-h post-admission, has been reported to be beneficial in prevention of pneumothorax recurrence [Citation16], and delayed referral may reduce the success rate of video-assisted thoracoscopic surgery (VATS) [Citation27]. Although surgical pleurodesis is the recommended definitive treatment [Citation16], only a few studies evaluated the effectiveness of surgery among SSP patients [Citation20,Citation28,Citation29]. Recurrence rates of 9.3% to 12.5% have been reported among those aged 66.8–70 years, in contrast to much lower rates (Video assisted surgery: 5.4%; open surgery 1.1%) among mixed younger PSP and SSP patients averaging 20–50 years old [Citation30]. The safety and efficacy of surgical pleurodesis need to be considered in older SSP patients with multiple comorbidities. A study reported post-operative morbidity and mortality rates among 94 such patients to be 20.6% and 4.1%, with poor performance status, old age, significant emphysematous change on preoperative CT thorax and pulmonary fibrosis identified as risk factors for pneumothorax recurrence at a rate of 9.3%, being nearly double that for PSP patients post-surgical pleurodesis [Citation20]. While another single-centre retrospective study found low post-surgical pleurodesis recurrence rate of 4.8% in 104 elderly (aged >70 years) PSP patients with either massive air leak or bullae or failed medical pleurodesis, poor performance status was also correlated with higher risks of post-operative complication [Citation31]. Nearly 80% of our patients who had PAL >7 days and treated with medical pleurodesis had been considered unfit for surgery. Of all our medical pleurodesis patients (Mean age: 71.1 years, median Charlson co-morbidity index: 4) who were spared from postoperative morbidity and mortality risks, 70% did not have recurrence in 2 years (HR: 0.47; P: 0.044). Based on the limited data on surgical pleurodesis efficacy in older SSP patients with multiple comorbidities, our experience suggests that medical pleurodesis may be a reasonable choice of treatment to prevent pneumothorax recurrence.
Strength and limitation
There are several limitations in this study. Firstly, this is a single-centre retrospective study. Secondly, the original study of the Collin’s method required erect chest X-ray films [Citation17] but there was no control on our patients’ positions when they had their chest X-rays taken. Thirdly, the timing of definitive treatment with pleurodesis was not controlled and surgical referral might be delayed because of institutional (e.g. availability of thoracic surgery service on site) and patient factors (e.g. time required to optimize medical condition).
The strengths of this study include the following: The initial pneumothorax size was more accurately measured than in previous studies. This is one of the few studies that defined underlying lung conditions in detail to investigate their effects on air leak duration. Only SSP patients were studied and the sample size is large enough to reflect real-world practice in managing unselected SSP patients.
Conclusion
In our study of non-surgical SSP patients, PAL was significantly associated with the presence of bulla and suboptimal nutritional status as reflected by serum albumin level, prolonged hospitalization, and predicted higher 2-year pneumothorax recurrence rates. Early recognition of PAL risk factors and timely institution of appropriate treatment may improve patient outcome. Elderly SSP patients unfit for surgery had acceptable recurrence rate after medical pleurodesis. In view of the preponderance of these characteristics among SSP patients in general, the optimal method of pleurodesis should be evaluated in future studies.
Abbreviations
| ACCP American College of Chest Physicians | = | |
| AUC Area under curve | = | |
| BTS British Thoracic Society | = | |
| COPD Chronic obstructive pulmonary disease | = | |
| CT scan Computed tomography scan | = | |
| FEV1 Forced expiratory volume in one second | = | |
| FVC Forced vital capacity | = | |
| HRCT High resolution computed tomography scan | = | |
| PAL Persistent air leak | = | |
| PSP Primary spontaneous pneumothorax | = | |
| ROC Receiver operator characteristics | = | |
| SSP Secondary spontaneous pneumothorax | = | |
| PTB Pulmonary Tuberculosis | = |
CRediT author statement
Hei-Shun CHENG: Methodology, Formal analysis, Writing-Original Draft.
Yi-Tat LO: Validation, Formal analysis.
Flora Pui-Ling MIU: Investigation, Data curation.
Loletta Kit-Ying SO: Conceptualization, Methodology, Supervision
Loretta Yin-Chun YAM: Writing-Review & Editing, Visualization, Supervision
Supplemental Material
Download MS Word (143.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2023.2168345.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev. 2010;19(117):217–9.
- Chan JW, Ko FW, Ng CK, et al. Management of patients admitted with pneumothorax: a multi-centre study of the practice and outcomes in Hong Kong. Hong Kong Med J. 2009;15(6):427–433.
- Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology. 2005;10(3):378–384.
- Mathur R, Cullen J, Kinnear WJ, et al. Time course of resolution of persistent air leak in spontaneous pneumothorax. Respir med. 1995;89(2):129–132.
- Travaline JM, McKenna RJ Jr., De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. 2009;136(2):355–360. DOI:10.1378/chest.08-2389
- Cerfolio RJ, Tummala RP, Holman WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg. 1998;66(5):1726–1731.
- Chee CB, Abisheganaden J, Yeo JK, et al. Persistent air-leak in spontaneous pneumothorax–clinical course and outcome. Respir med. 1998;92(5):757–761.
- Schoenenberger RA, Haefeli WE, Weiss P, et al. Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126(6):764–766.
- Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis. 2014;6(3):271–284.
- Stolz AJ, Schutzner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27(2):334–336.
- Cerfolio RJ, Bass CS, Pask AH, et al. Predictors and treatment of persistent air leaks. discussion 30-1 Ann Thorac Surg. 2002;731:1727–1730. 10.1016/s0003-4975(02)03531-2
- Isowa N, Hasegawa S, Bando T, et al. Preoperative risk factors for prolonged air leak following lobectomy or segmentectomy for primary lung cancer. Eur J Cardiothorac Surg. 2002;21(5):951.
- Abolhoda A, Liu D, Brooks A, et al. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest. 1998;113(6):1507–1510.
- Akamine T, Kometani T, Hashinokuchi A, et al. Interpleural distance predicts persistent air leak after initial primary spontaneous pneumothorax. J Thorac Dis. 2020;12(5):2228–2235.
- Haga T, Kurihara M, Kataoka H. Spontaneous pneumothorax with persistent air leakage and invasive procedures. Intern Med. 2013;52(19):2189–2192.
- MacDuff A, Arnold A, Harvey J. Group BTSPDG. Management of spontaneous pneumothorax: british thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii18–31.
- Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR. 1995;165(5):1127–1130.
- Tabachnick BG, Fidell LS, Osterlind SJ. Using multivariate statistics. Boston: Allyn and Bacon; 2001.
- Bobbio A, Dechartres A, Bouam S, et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax. 2015;70(7):653–658.
- Isaka M, Asai K, Urabe N. Surgery for secondary spontaneous pneumothorax: risk factors for recurrence and morbidity. Interact Cardiovasc Thorac Surg. 2013;17(2):247–252.
- Jiang L, Jiang G, Zhu Y, et al. Risk factors predisposing to prolonged air leak after video-assisted thoracoscopic surgery for spontaneous pneumothorax. Ann Thorac Surg. 2014;97(3):1008–1013.
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American college of chest physicians delphi consensus statement. Chest. 2001;119(2):590–602. DOI:10.1378/chest.119.2.590
- Rhea JT, DeLuca SA, Greene RE. Determining the size of pneumothorax in the upright patient. Radiology. 1982;144(4):733–736.
- Kovach JC, Avedian V, Morales G, et al. Lung compartment determination. J Thorac Surg. 1956;31(4):452–457.
- Engdahl O, Toft T, Boe J. Chest radiograph–a poor method for determining the size of a pneumothorax. Chest. 1993;103(1):26–29.
- Axel L. A simple way to estimate the size of a pneumothorax. Invest Radiol. 1981;16(2):165–166.
- Waller DA, McConnell SA, Rajesh PB. Delayed referral reduces the success of video-assisted thoracoscopic surgery for spontaneous pneumothorax. Respir med. 1998;92(2):246–249.
- Waller DA, Forty J, Soni AK, et al. Videothoracoscopic operation for secondary spontaneous pneumothorax. Ann Thorac Surg. 1994;57(6):1612–1615.
- Tanaka F, Itoh M, Esaki H, et al. Secondary spontaneous pneumothorax. Ann Thorac Surg. 1993;55(2):372–376.
- Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet (London, England). 2007;370(9584):329–335.
- Nagata S, Omasa M, Tokushige K, et al. Efficacy and safety of surgery for spontaneous pneumothorax in elderly patients. Interact Cardiovasc Thorac Surg. 2020;30(2):263–268.