ABSTRACT
Purpose
Co-morbidities are common in chronic obstructive pulmonary disease (COPD) and are associated with increased morbidity and mortality. The aim of the present study was to explore the prevalence of several comorbid conditions in severe COPD, and to investigate and compare their associations with long-term mortality.
Methods
In May 2011 to March 2012, 241 patients with COPD stage 3 or 4 were included in the study. Information was collected on sex, age, smoking history, weight and height, current pharmacological treatment, number of exacerbations the recent year and comorbid conditions. At December 31st, 2019, mortality data (all-cause and cause specific) were collected from the National Cause of Death Register. Data were analyzed using Cox-regression analysis with gender, age, previously established predictors of mortality and comorbid conditions as independent variables, and all-cause mortality and cardiac and respiratory mortality, respectively, as dependent variables.
Results
Out of 241 patients, 155 (64%) were deceased at the end of the study period; 103 patients (66%) died of respiratory disease and 25 (16%) of cardiovascular disease. Impaired kidney function was the only comorbid condition independently associated with increased all-cause mortality (HR (95% CI) 3.41 (1.47-7.93) p=0.004) and respiratory mortality (HR (95%CI) 4.63 (1.61 to 13.4), p = 0.005). In addition, age ≥70, BMI <22 and lower FEV1 expressed as %predicted were significantly associated with increased all-cause and respiratory mortality.
Conclusion
In addition to the risk factors high age, low BMI and poor lung function; impaired kidney function appears to be an important risk factor for mortality in the long term, which should be taken into account in the medical care of patients with severe COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease and a major cause of morbidity and mortality worldwide [Citation1]. Examples of well-known predictors of mortality in COPD are high age, number of pack years smoking, exacerbation frequency, health status/symptoms, lower body mass index (BMI), the phenotype of chronic bronchitis [Citation2] and degree of lung function impairment [Citation3–6].
A majority of patients with COPD are diagnosed with more than one condition, and comorbidities are known to influence mortality as well as hospitalization risk and health-related quality of life [Citation7–12]. It has been suggested that chronic inflammation causes local pathology in the lungs and systemic pathology in other organs [Citation13,Citation14]. Cardiovascular disease, diabetes, osteoporosis, musculoskeletal symptoms, impaired kidney function, malnutrition, obesity/overweight, underweight and depression are all common comorbid conditions in COPD [Citation13,Citation15,Citation16]. Cardiovascular disease in particular is well-studied in COPD and is associated with poor clinical outcome and increased mortality [Citation13,Citation17–19]. However, there are few long-term studies comparing a large number of different comorbid conditions. The aim of the present study was to explore the frequency of several comorbid conditions in severe COPD, and to investigate and compare their associations with long-term mortality.
Methods
Data collection
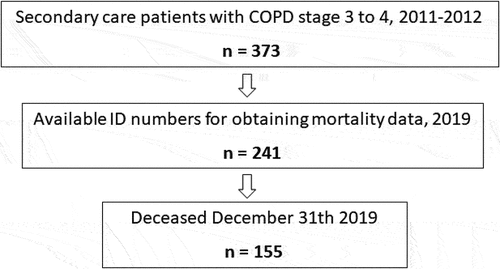
This was a retrospective follow-up of the study PROCEDUR, described elsewhere [Citation10,Citation20,Citation21]. The original study explored the association of comorbid conditions with health-related quality of life, and aimed to recruit 10–15 patients at all secondary care units in Sweden. In total, 373 patients with COPD stages 3 or 4 were consecutively enrolled at 27 secondary care respiratory units in Sweden from May 2011 to March 2012. At the time of the present study, identification numbers for 241 patients from 17 units were available and could be used for retrieving national mortality data in the present study (). Mortality data until last of December 2019 were obtained from the Swedish National Board of Health and Welfare. The only exclusion criterion was an inability to leave consent or to complete spirometry or questionnaires due to cognitive or linguistic barriers.
At baseline, information was collected by the responsible physician from history and medical record review on sex, age, smoking history, body weight and height, current pharmacological treatment, number of exacerbations the recent year, the phenotype of chronic bronchitis and comorbid conditions in terms of cardiovascular disease, diabetes, impaired kidney function, malnutrition, musculoskeletal symptoms, osteoporosis or depression. An exacerbation was defined as worsening of symptoms of dyspnea and sputum beyond normal day-to-day variation, requiring increased maintenance treatment, courses of antibiotics or oral steroids or an emergency visit or hospitalization [Citation22]. The phenotype of chronic bronchitis was defined as productive cough of more than three months occurring within the span of two years [Citation2]. All the comorbid conditions were defined as recorded doctor´s diagnoses with ongoing in need of pharmacological or non-pharmacological treatment. Cardiovascular disease included any of the diagnoses of ischemic heart disease, heart failure, atrial fibrillation or flutter or cerebrovascular disease. Impaired kidney function denoted chronical renal impairment and not transient renal failure with normalized kidney function. Musculoskeletal problems included any condition with symptoms of muscle weakness, pain or joint diseases including rheumatic diseases, osteoarthritis as well as arthrosis.
Data on forced expiratory volume in one second in percentage of predicted value (FEV1%pred) from the most recently performed spirometry was used. At inclusion, 59% of the included patients performed a new spirometry. In the remaining cases, data on previous spirometries were accepted due to stable functional status. In total 24% of all patients had a spirometry from less than one year before inclusion, 11% 1–2 years before inclusion and 9% more than 2 years before being included in the study. Post-bronchodilator values were used but substituted with pre-bronchodilator values if post-bronchodilator values were missing. In addition, each patient completed the Swedish version of COPD Assessment Test (CAT) [Citation23].
At the end of the study, 31 December 2019, mortality data (all-cause and cause specific) were obtained from the National Cause of Death Register.
Statistical analyses
Statistical analyses were performed using IBM SPSS version 25 (IBM Corporation, Armonk, NY). Body mass index (BMI) was calculated and categorized as <22 or ≥22 kg/m2. The cut-off value was chosen based on the previous knowledge about prognostic value of BMI <22 in COPD [Citation24]. Age was classified into three groups, <70 years, 70–74 years and ≥75 years.
Patient characteristics were cross-tabulated distributed over vital status (dead or alive) at censoring of the study. Student´s t-test was used to investigate differences in continuous variables and χ2 test was used to investigate differences in categorical variables. Multivariate Cox-regression analyses were used to study associations between all-cause mortality and sex, age, number of pack years, BMI <22, CAT score, number of exacerbations recent year, FEV1%pred and comorbidities. Potential confounders were chosen a priori based on previous knowledge [Citation3–6]. The multivariable Cox-regression model was also performed with cause-specific mortality (cardiovascular- and respiratory death, respectively) as dependent variables. Furthermore, as it is known that mortality differs between men and women with COPD [Citation25] the Cox-regression model with all-cause mortality was repeated with stratification and interaction analysis by sex.
In all analyses p < 0.05 was considered statistically significant.
Ethics
The study was conducted as a non-interventional trial, in accordance with EU directive 2001/20/EC and the Declaration of Helsinki. The study was reviewed and approved by the Regional Ethical Review Board of Umeå University (Dnr 2011–10-31 M and 2016–139-32 M). Informed oral and written consent was obtained from all patients.
Results
Out of 241 patients, 155 (64,3%) were deceased at the end of the study period (December 31st, 2019). Of these, 103 patients (66%) died due to respiratory disease and 25 patients (16%) due to cardiovascular disease. For 27 patients (17%) death was due to other causes. The mean follow-up time for the included patients was 5.45 ± 2.76 years.
Patient characteristics distributed over vital status at the end of the study period are presented in and the prevalence of the studied co-morbidities are presented in . In summary, the most common comorbid conditions/phenotypes were cardiovascular disease (n = 137) and chronic bronchitis (n = 88), and number of deaths was increased with high age, impaired lung function, more frequent exacerbations and the presence of cardiovascular disease.
Figure 2. Prevalence of co-morbid conditions.
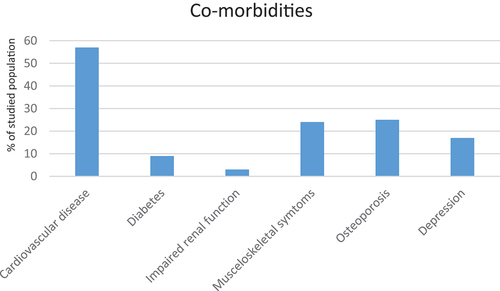
Table 1. Patient characteristics.
The results of the Cox-regression analysis with all-cause mortality and cardiac and respiratory mortality, respectively, as dependent variables are presented in . When analyzing each comorbid condition with adjustment for potential confounders, impaired kidney function was the only co-morbidity associated with increased all-cause mortality (HR (95% CI) 3.41 (1.47–7.93) p = 0.004) and respiratory mortality (HR (95%CI) 4.63 (1.61–13.34) p = 0.005). None of the investigated co-morbidities were associated with increased cardiovascular mortality (). In addition, age > = 70 and lower FEV1%pred were independently associated with increased all-cause and respiratory mortality, BMI <22 with all-cause mortality and number of exacerbations with respiratory mortality ().
Table 2. Predictors of all-cause mortality.
Table 3. Predictors of respiratory and cardiovascular mortality.
In the analyses stratified by sex, musculoskeletal problems were associated with lower mortality in men (HR (95%CI) 0.37 (0.15–0.95)) but not in women, p for interaction 0.015. No other statistically significant associations were found (data not shown).
Discussion
We think that the most important finding of the present study is that, in long-term follow-up, impaired kidney function is independently associated with increased all-cause and respiratory mortality for patients with severe COPD as defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria. We also observed that high age, decreased lung function and low BMI predicted increased risk for mortality in long-term follow-up of severe COPD, thereby extending the understanding of these particular risk factors.
In several previous studies, impaired kidney function is reported as a co-morbidity more common in COPD than in the general population [Citation7,Citation26,Citation27]. Chronic kidney failure is associated with increased levels of inflammatory biomarkers such as IL-6, IL-8 and TNF-α and protrombine molecules that may be involved in systemic inflammation among patients with COPD and thereby contribute to damage of lung parenchyma leading to disease progression [Citation27,Citation28]. Furthermore, chronic hypercapnia in COPD not fully compensated metabolically in patients with kidney failure may lead to chronic respiratory acidosis, that is associated with poor outcome in COPD [Citation28,Citation29]. The disturbed acid-base balance can also induce vasoconstriction in the kidneys and increased activation of the renal-angiotensin-aldosterone system affecting sodium- and fluid balance with subsequent fluid-retention and pulmonary edema [Citation28,Citation29]. Conversely, the disease burden of COPD per se can lead to impaired kidney function. Chronic hypoxia in COPD may induce kidney-damage, because the kidneys are sensitive to low oxygen levels and the autoregulation directing the blood flow to the kidneys may not be sufficient to compensate for this [Citation28]. Moreover, the metal cadmium, which is present in high levels in tobacco and cigarettes may contribute to pulmonary emphysema in smokers and possibly to impaired kidney function as well [Citation30,Citation31]. Thus, our current results on impaired kidney function and mortality provide a rationale to examine cadmium levels in relation to survival in future studies of smokers with COPD.
In a study by Antonelli et al., both cardiovascular disease and impairment kidney function were reported to be predictors of mortality in COPD after a more modest observation time of 3.4 years [Citation7]. However, while the relationship between cardiovascular disease and short-term mortality in COPD has been demonstrated in several studies [Citation13,Citation17,Citation19], the relationship between impaired kidney function and long-term mortality in COPD has not been studied elsewhere. Thus, our study is the first to forward evidence for a relatively strong association of impaired kidney function with long-term follow-up of mortality in COPD.
In our study, only a minority of the study population reported impaired kidney function in the beginning of the observation period. Because of this, it is reasonable to presume that the impaired kidney function was pronounced and thereby more likely to contribute to increased mortality risk. On the other hand, impaired kidney function may be underdiagnosed in COPD, given that the most common way of measuring kidney function is by estimating glomerular filtration rate (GFR) using serum creatinine [Citation27,Citation29] or cystatin C. Many patients with COPD are elderly and often suffer from muscle wasting. Therefore, their kidney function may be impaired despite serum creatinine within the normal range or slightly elevated. Moreover, given that impaired kidney function proved to be important for mortality in the long run, our findings motivate further study to address whether even modest kidney failure should be monitored during the early course of COPD.
It is well established that cardiovascular disease is associated with poor outcomes in COPD. Our data showed that cardiovascular disease at baseline was associated with mortality in unadjusted analyses, but somewhat surprisingly the results did not remain after adjustment for confounders. A factor that may influence our results is that our study population only included patients with severe COPD (GOLD stage 3 and 4). It has previously been reported that this sub-group of patients most often die due to respiratory failure whereas cardiovascular disease has been shown to be the most common cause of death in patients with mild- and moderate COPD [Citation32]. According to Soriano et al., [Citation33] patients may even have co-existing cardiovascular disease already at the time of diagnosis and it can be speculated that this co-morbidity plays a larger roll and influences mortality to a greater extent in earlier stages of disease. This hypothesis is strengthened by our finding that the most common cause of death in the studied patient group was respiratory disease and not cardiovascular disease.
Our results are consistent with a recent Canadian study reporting that cardiovascular disease, as a cause of mortality in COPD, decreases over time [Citation34]. We speculate that this may be due to generally improved prevention, treatment and prognosis of ischemic heart disease [Citation35]. Another potential contributing cause may be maintenance triple therapy with inhaled corticosteroids, long acting beta-2-agonists and long acting muscarinic antagonists, which has recently been shown to impact cardiac death [Citation36].
Importantly, the results from our study demonstrate that high age, BMI <22 and reduced FEV1% pred are important predictors of long-term mortality in COPD [Citation3–6], thereby extending previous knowledge about these risk factors.
Previous studies have also shown that diabetes and depression are important risk factor for death in COPD.3.13,15,16 In our cohort, there was a trend towards higher number of deaths in patients with comorbid diabetes, but the difference disappeared with adjustment for confounders. Diabetes may have contributed to cardiovascular disease. As for depression and anxiety in patients with severe COPD, these conditions are often treated with benzodiazipines and opioids [Citation37,Citation38]. Even if we failed to show an association between depression and mortality in our study, there is a theoretical possibility that increased plasma concentrations of benzodiazipines and opioids due to impaired kidney function may have contributed to a higher mortality risk. However, we have no data on these medications in this study.
Interestingly, a statistically significant difference by sex was found in the impact of musculoskeletal problems in COPD. Musculoskeletal dysfunction is known to predict mortality, but our analyses rather showed that it was associated with lower mortality in men. As musculoskeletal dysfunction in this study was defined as a condition needed to treat and that treatment includes physiotherapy, we speculate that the therapy in itself may have a positive impact on the mortality risk. Physical activity is known to be a very important predictor of mortality [Citation39], and muscular treatment against musculoskeletal problems may have increased physical function in the male part of the study population.
Strengths of our study are that it is a multicenter study including a large number of secondary care respiratory units in Sweden with data on several established mortality predictors. Due to compulsory report to the national cause of death register, all-cause mortality data are complete in the present population.
Limitations are that the definitions of comorbid conditions are based on the assessment of the responsible physician of existing pharmacologically or non-pharmacologically treated comorbidities. As the diagnoses were not objectively confirmed by laboratory or physiological examinations, under- as well as overdiagnostics may have occurred. Another limitation is that mortality data were not available in all patients from the original study. However, an attrition analysis showed no difference in factors that may have influenced mortality rates, such as age, lung function, exacerbation rate, BMI or comorbid conditions, between units with and without available mortality data (data not shown).
A limitation for the analyses of cause-specific death is that death certificates are not very accurate in terms of the claimed cause of death that is rarely based on autopsy. Previous studies have confirmed poor congruence for registered and actual cause of death and it has also been suggested that COPD is underreported on death certificates [Citation40–42]. However, in our study, respiratory death was the most common cause of death.
Even if the number of patients with impaired kidney function was low, the clear association with mortality indicates that renal impairment should be considered in the medical care of severe COPD. Future research should focus on the association of more objective findings of mild as well as advanced renal failure with mortality.
Conclusion
In addition to the well-known predictors high age, low BMI and poor lung function; we demonstrate that impaired kidney function is an important predictor of long-term mortality in patients with severe COPD, which should be taken into account in the medical care of these patients.
Availability of data and materials
Data cannot be made freely available as they are subject to secrecy in accordance with the Swedish Public Access to Information and Secrecy Act, but can be made available to researchers upon request, after approval from the Swedish Ethical Review Authority has been obtained.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Global Initiative for Chronic Obstructive Lung Disease (webpage on the internet) Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023. cited 2023 Jan 31. http://www.goldcopd.org
- Ekberg-Aronsson M, Pehrsson K, Nilsson JA, et al. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res. 2005 Aug 25;6(1):98. DOI:10.1186/1465-9921-6-98.
- Gudmundsson G, Gislason T, Lindberg E, et al. Mortality in COPD patients discharged from hospital: the role of treatment and co-morbidity. Respir Res. 2006 Aug 16;7(1):109. DOI:10.1186/1465-9921-7-109.
- Henoch I, Ekberg-Jansson A, Löfdahl CG, et al. Early predictors of mortality in patients with COPD, in relation to respiratory and non-respiratory causes of death - a national register study. Int J Chron Obstruct Pulmon Dis. 2020;15:1495–8.
- Sundh J, Janson C, Lisspers K, et al. Clinical COPD questionnaire score (CCQ) and mortality. Int J Chron Obstruct Pulmon Dis. 2012;7:833–842.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005 Nov;60(11):925–931. DOI:10.1136/thx.2005.040527.
- Antonelli Incalzi R, Fuso L, De Rosa M, et al. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J. 1997 Dec;10(12):2794–2800. DOI:10.1183/09031936.97.10122794.
- Almagro P, Cabrera FJ, Diez J, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en servicios de medicina interna (ESMI) study. Chest. 2012 Nov;142(5):1126–1133.
- Wijnhoven HA, Kriegsman DM, Hesselink AE, et al. The influence of co-morbidity on health-related quality of life in asthma and COPD patients. Respir med. 2003 May;97(5):468–475. DOI:10.1053/rmed.2002.1463.
- Sundh J, Johansson G, Larsson K, et al. Comorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:173–183.
- Cazzola M, Bettoncelli G, Sessa E, et al. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2010;80(2):112–119.
- Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med. Jul 9 2012;12:26. DOI:10.1186/1471-2466-12-26.
- Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir med. 2013 Sep;107(9):1376–1384. DOI:10.1016/j.rmed.2013.05.001.
- Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007 Sep 1;370(9589):797–799. DOI:10.1016/s0140-6736(07)61383-x.
- Corsonello A, Antonelli Incalzi R, Pistelli R, et al. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011 Dec;17(Suppl 1):S21–8. DOI:10.1097/01.mcp.0000410744.75216.d0.
- Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–888.
- Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006 Jan;16(1):63–70. DOI:10.1016/j.annepidem.2005.04.008.
- Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008 Oct;32(4):962–969. DOI:10.1183/09031936.00012408.
- Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J. 2006 Dec;28(6):1245–1257. DOI:10.1183/09031936.00133805.
- Sundh J, Janson C, Johansson G, et al. Characterization of secondary care for COPD in Sweden. Eur Clin Respir J. 2017;4(1):1270079.
- Sundh J, Johansson G, Larsson K, et al. The phenotype of concurrent chronic bronchitis and frequent exacerbations in patients with severe COPD attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:2327–2334.
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007 Sep 1;370(9589):786–796. DOI:10.1016/s0140-6736(07)61382-8.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009 Sep;34(3):648–654. DOI:10.1183/09031936.00102509.
- Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City heart study. Am J Respir Crit Care Med. 2006 Jan 1;173(1):79–83. DOI:10.1164/rccm.200506-969OC.
- de Torres Jp, Cote CG, López MV, et al. Sex differences in mortality in patients with COPD. Eur Respir J. 2009 Mar;33(3):528–535.
- Gjerde B, Bakke PS, Ueland T, et al. The prevalence of undiagnosed renal failure in a cohort of COPD patients in western Norway. Respir med. 2012 Mar;106(3):361–366. DOI:10.1016/j.rmed.2011.10.004.
- AbdelHalim HA, AboElnaga HH. Is renal impairment an anticipated COPD comorbidity? Respir Care. 2016 Sep;61(9):1201–1206. DOI:10.4187/respcare.04516.
- Domenech P, Perez T, Saldarini A, et al. Kidney-lung pathophysiological crosstalk: its characteristics and importance. Int Urol Nephrol. 2017 Jul;49(7):1211–1215. DOI:10.1007/s11255-017-1585-z.
- Sorino C, Scichilone N, Pedone C, et al. When kidneys and lungs suffer together. J Nephrol. 2019 Oct;32(5):699–707. DOI:10.1007/s40620-018-00563-1.
- Ganguly K, Levänen B, Palmberg L, et al. Cadmium in tobacco smokers: a neglected link to lung disease? Eur Respir Rev. 2018 Mar 31;27(147):170122.
- Sundblad BM, Ji J, Levänen B, et al. Extracellular cadmium in the bronchoalveolar space of long-term tobacco smokers with and without COPD and its association with inflammation. Int J Chron Obstruct Pulmon Dis. 2016;11:1005–1013.
- Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013 Mar;1(1):73–83. DOI:10.1016/s2213-2600(12)70060-7.
- Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005 Oct;128(4):2099–2107.
- Gershon A, Hwee J, Victor JC, et al. Mortality trends in women and men with COPD in Ontario, Canada, 1996-2012. Thorax. 2015 Feb;70(2):121–126. DOI:10.1136/thoraxjnl-2014-205956.
- Hambraeus K, Tydén P, Lindahl B. Time trends and gender differences in prevention guideline adherence and outcome after myocardial infarction: data from the SWEDEHEART registry. Eur J Prev Cardiol. 2016 Mar;23(4):340–348. DOI:10.1177/2047487315585293.
- Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A Randomized, Double-Blind, Multicenter, Parallel-Group Study. Am J Respir Crit Care Med. 2021 Mar 1;203(5):553–564. DOI:10.1164/rccm.202006-2618OC.
- Ahmadi Z, Bernelid E, Currow DC, et al. Prescription of opioids for breathlessness in end-stage COPD: a national population-based study. Int J Chron Obstruct Pulmon Dis. 2016;11:2651–2657.
- Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine use among older adults with chronic obstructive pulmonary disease: a population-based cohort study. Drugs Aging. 2013 Mar;30(3):183–192. DOI:10.1007/s40266-013-0056-1.
- Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011 Aug;140(2):331–342.
- Jensen HH, Godtfredsen NS, Lange P, et al. Potential misclassification of causes of death from COPD. Eur Respir J. 2006 Oct;28(4):781–785. DOI:10.1183/09031936.06.00152205.
- Drummond MB, Wise RA, John M, et al. Accuracy of death certificates in COPD: analysis from the TORCH trial. COPD. 2010 Jun;7(3):179–185. DOI:10.3109/15412555.2010.481695.
- Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003 Nov;22(5):809–814. DOI:10.1183/09031936.03.00031403.