ABSTRACT
Purpose
When first-line chronic rhinosinusitis (CRS) treatment fails, patients can either be treated with oral or injected systemic corticosteroids. Although the EPOS and international guidelines for CRS do not mention injected corticosteroids, it is commonly used by ear, nose, and throat specialists. While the risks of systemic corticosteroids, in general, are known, the pros and cons of injected and oral corticosteroids (OCS) in CRS treatment are unclear.
Methods
A systematic review of studies that report the effects and/or side effects of injected and oral corticosteroids in the treatment of CRS was made according to the PRISMA guidelines.
Results
Altogether, 48 studies were included, only five studies reported on injected corticosteroids, and five attended with side effects. Three studies found beneficial effects of OCS perioperatively on sinus surgery, while four articles found no effect. Nineteen articles reported that OCS resulted in an improvement in symptoms. Two articles presented a longer-lasting effect of injected corticosteroids than OCS. Three studies reported adverse side effects of systemic corticosteroids, while two studies showed no adverse side effects. One study showed less adrenal suppression after injected corticosteroids compared to OCS. The evidence is not strong but shows a positive effect of systemic corticosteroids that lasts longer with injections.
Conclusion
Although systemic corticosteroids are widely used to treat CRS, there is a lack of studies comparing the OCS and injected corticosteroids. The evidence is sparse, however, injected steroids show longer effects with fewer side effects. An RCT study is needed to compare OCS and injected corticosteroids.
Introduction
Chronic rhinosinusitis (CRS) is a highly prevalent inflammatory disease of the upper airways.
CRS can be challenging to treat, and appropriate use of medical treatments is required to optimize patients’ daily functions and their quality of life (QoL). When patients have tried and failed standard local treatment or still have severe symptoms (VAS ≥5), treatment with a short course of systemic steroids may be used [Citation3–5].
Treatment with systemic corticosteroids almost always results in an improvement of symptoms, by reducing the inflammatory response, reducing polyp size, re-establishing normal mucosal function, and thereby improving sinus drainage and improving nasal airflow and reducing obstruction of the olfactory area; however, often the effect is transient [Citation3]. When corticosteroid injections are chosen as the treatment plan for CRS 14 mg of Betamethasone (Diprofos ®) is used as a single injection in Denmark, which compares to 88 mg equivalent dose of prednisolone (). When OCS is used, a 14-day treatment plan with 50 mg prednisolone daily (700 mg in total) could be chosen [Citation7]. New treatment options with monoclonal antibodies (biologicals) may in some countries be offered to CRS with nasal polyps (CRSwNP) to avoid the use of systemic corticosteroids or revision sinus surgery.
Table 1. Characteristics of various corticosteroids [Citation8].
The risks of adverse side effects heighten with increasing cumulative exposure and increasing mean daily exposure to systemic corticosteroids [Citation9]. The short- and long-term risks are summarized in . Even though systemic corticosteroids are recommended as standard treatment for CRS, there seems to be a lack of recommendations concerning the choice of drug, choice of treatment method (injection or oral corticosteroids (OCS)), dose, and duration of treatment. OCS is only sparsely mentioned in the 2020 edition of the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) and corticosteroid injections are not mentioned at all [Citation3]. Therefore, this review was made in an attempt to enhance the EPOS guidelines with a focus on the current evidence of effects and side effects of treating CRS with systemic corticosteroids and especially injected corticosteroids.
Table 2. Side effects to systemic corticosteroids [Citation15,Citation17,Citation20].
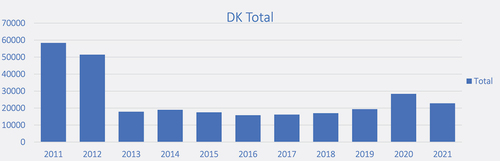
In Denmark, you need a prescription for buying systemic steroids. According to Amgros, who secure supplies of medicines for public hospitals, a total amount of 29.073 doses of 2 mL Diprofos was bought for Danish hospitals and pharmacies in 2020 (Amgros).
We believe that the 1 ml packages are used by rheumatologists, orthopedists, and in sports medicine, whereas the 2 mL packages primarily are used by ENT physicians. Hence, we assume that corticosteroid injections have regularly been used as a treatment for allergic rhinitis, non-allergic rhinitis, and CRS.
shows that injected corticosteroids are still used in Denmark but also show a decrease after 2012. The drop in injected corticosteroids in 2013 corresponds to an increase in nonsystemic corticosteroid symptomatic treatment [Citation21]. Our general experience (jet unpublished data) is that patients also report a longer lasting and better effectof injections than oral corticosteroids.
Materials and methods
Data sources and search strategy
We performed a systematic search on the PUBMED and EMBASE databases.
A search strategy was designed and used for each database to detect all articles concerning CRS and systemic corticosteroid treatment (Appendix 1 and 2). We conducted a broad search including allergic rhinitis to obtain all articles dealing with systemic corticosteroid treatment and/or side effects.
A systematic review was performed to identify all studies that reported the use of systematic corticosteroids and either the effects or side effects of the used medical therapy. This review was done accordingly to the elements in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) by using the 27-item checklist for items to include in a systematic review and a four-phase diagram illustrating the flow of information [Citation23].
Study selection
The studies were reviewed by two independent authors (STA and KA) and were selected by the inclusion and exclusion criteria. Titles and abstracts were screened using the web-based Covidence program for systematic review management. All our search results were uploaded to the program and assessed individually by the two authors. Conflicts were solved by full-text screening with STA. A full-text review was performed in the next phase, and the studies were analyzed and included if meeting the selection criteria. Finally, missing studies were searched manually and accessed from the library paper edition if possible.
Eligibility criteria
The selected inclusion criteria were:
Only papers from 1987 and forward were included.
Patients with diffuse primary CRS (eosinophilic chronic rhinosinusitis (eCRS)/non-eosinophilic chronic rhinosinusitis (Non-eCRS)/chronic rhinosinusitis with nasal polyps (CRSwNP)/chronic rhinosinusitis without nasal polyps (CRSsNP)/Central compartment atopic disease (CCAD)) and/or allergic rhinitis () according to the EPOS [Citation3] AND, OR
Papers reporting of effect, efficacy, and/or side effects of systemic corticosteroids AND,
Papers published from 1987 and forward AND, EITHER,
The exclusion criteria were:
Non-English
Case reports < 5 patients
Secondary CRS
Localized CRS
Allergic fungal and fungal sinusitis
Patients <18 years of age
Only local treatment with corticosteroids
Only local injection with corticosteroids in nasal polyps
Papers where both drug name and dosage/treatment length are not given
Letters, reviews, and animal studies
Furthermore, studies by the same authors, from the same area or possibly overlapping in time were closely reviewed to avoid any patient group overlap.
Data extraction
A data sheet was made to extract relevant data, and the following was entered into tables: author name, publication year, study design, studied drug/treatment plan, side effects, conclusions and quality of evidence.
For the data to be considered complete, it required a profound description of the treatment plan, its effects, or side effects. When the initial selection was done, the references cited in the authors’ articles were assessed to examine each article. A final data set was made once the articles were assessed for eligibility.
Details of the data and characteristics of the studies are summarized in tables.
A flow chart of our study retrieval and selection process is provided in .
Figure 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. PRISMA flowchart depicting the selection process in this study [Citation1,Citation2,Citation24].
![Figure 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. PRISMA flowchart depicting the selection process in this study [Citation1,Citation2,Citation24].](/cms/asset/710578ee-3c10-4905-b735-a30f7a0923e5/zecr_a_2240511_f0002_oc.jpg)
Results
Of the 1,177 identified articles 48 were selected for further review. The included studies have been divided and summarized into five tables:1) systemic corticosteroids perioperatively (n = 7; ), 2) the effect of oral corticosteroids (n = 20; ), 3) the effect of injected corticosteroids (n = 5; ), 4) side effects to systemic corticosteroids (n = 5; ), 5) other effects of corticosteroids (n = 11; ).
Table 3. Summary of results: systemic corticosteroids perioperatively.
Table 4. Summary of results: effect of oral systemic corticosteroids.
Table 5. Summary of results: effect of systemic injected corticosteroids.
Table 6. Summary of results: side effects of systemic corticosteroids.
Table 7. Summary of results: other effects of systemic corticosteroids.
Discussion
Although the use of systemic corticosteroids is a recommended treatment for CRS, there is a lack of a standardized prescribing regimen for oral and injected corticosteroids. In our study, we found 20 studies reporting the effects of OCS, but we only found five studies concerning injected corticosteroids although injected corticosteroids are frequently used [Citation21] ().
It is noteworthy that the choice of OCS dosage and length of treatment seems to reflect personal experience, as we found that very different dosages have been given with a dosing interval from 0.25 mg to 50 mg daily and a treatment length interval from 5 days to 4 weeks for the OCS (). One injection of 14 mg betamethasone corresponds to only 88 mg prednisolone (), which is a much lower dose than the usual given oral treatment course (for example 37.5 mg/d for 10 days). Given that OCS is widely used with different doses and treatment lengths and injected corticosteroids are also frequently used, it would be intuitive to assume that systemic corticosteroids are efficacious and relatively safe to use. However, as our results show, there is a lack of high-level evidence to support the use of systemic corticosteroids. No study has studied the efficacy and side effects of OCS compared to injected corticosteroid use in CRS patients or any of the subgroups from our inclusions.
Of the 20 studies regarding the effect of OCS, 19 reported beneficial effects and/or symptom improvements and one concluded that the effect of OCS was similar to topical corticosteroids (). However, in the study by Karaki et. al, the dose of OCS for seasonal allergic rhinitis was very low (0.25 mg twice daily), and this could explain their results pointing to no significant effect of OCS [Citation34]. Several studies have presented evidence that corticosteroids are beneficial for CRS patients concerning improving symptoms and quality of life (). There seems to be support for the positive effect on olfactory function after systemic corticosteroids. Three studies have found an improvement in olfactory function with systemic corticosteroids [Citation22,Citation42,Citation49]. Shriver et al. reported that 26.6% of 425 patients showed an improvement in smell function after treatment with oral methylprednisolone, but the effect was transient [Citation49]. Yet, the efficacy of injected corticosteroids is implied to be bigger and longer lasting [Citation22]. Overall, there is a lot of data reporting on the improvement of symptoms with systemic corticosteroids, but the period for this effect is also addressed in some studies. Two studies have reported symptom relief from OCS use [Citation36,Citation52]. However, Jankowski et al. addressed a longer-lasting effect from administrating patients with depot injection of triamcinolone 80 mg than oral prednisolone as the VAS score decreased from 6.4 to 2.0 (mean values) after the injection and stayed stable between 2.0 and 2.6 (mean values) in the next 12 months compared to the re-increase of VAS from 3.2 to 6.4 (mean values) 2 months after the oral treatment [Citation52].
In a study by Aasbjerg et al., it is reported that on average 1.6 corticosteroid injections are prescribed each year for patients with allergic rhinitis [Citation21]. This speaks for injected corticosteroids being used frequently and being effective. Essentially, the Norwegian criteria for treatment with monoclonal antibodies for nasal polyps include having had treatment with systemic corticosteroids within the past year [Citation69]. Similarly, it is also a criterion in Denmark. Having had treatment with systemic corticosteroids in the past year gives points for treatment with monoclonal antibodies [Citation70].
It has been reported that the injected and oral treatments with corticosteroids affect adrenal function differently. In a study by Laursen et al., they concluded that the adrenal gland function decreased after oral prednisolone treatment in contrast to the injected Betamethasone [Citation53]. However, it is not mentioned whether the adrenal gland function continues to be low or returns to normal.
The results of our study imply a positive effect on symptom relief with systemic corticosteroids and a better and longer-lasting effect with injection. Yet, it is noteworthy that none of the studies have studied the efficacy of single-modality oral and injected corticosteroid use and there has been no RCT that has examined the efficacy, duration of effect, or side effects of systemic corticosteroids in CRS or allergic rhinitis patients. In addition, some further limitations were detected in the body of identified evidence stemming from a focus on surrogate outcomes rather than patient-important ones’ and trials’ size. Limited studies were also discovered in our review, mostly with low-quality evidence, making it difficult to draw scientific conclusions with high levels of evidence. Thus, there is a lack of strong evidence to support recommendations for systemic corticosteroids, but the existing literature points to symptom relief with systemic corticosteroid use and a longer-lasting effect and lower accumulated doses with injected corticosteroids compared to OCS.
We found seven studies concerning the administration of systemic corticosteroids perioperatively (). All seven articles concerned OCS. Three of these concluded that there were beneficial effects after OCS treatment mostly regarding the facilitation of surgery, while four articles concluded that there was no effect of the treatment. Keeping in mind the limited found studies with varying levels of evidence, there seems to be no definitive recommendations in the literature regarding OCS or injected corticosteroids perioperatively.
Our results showed that 11 studies focused on other effects of systemic corticosteroids. Different inflammatory markers have been reported to be regulated by systemic corticosteroids [Citation32,Citation35,Citation43,Citation44,Citation48,Citation51,Citation68], and different factors have been addressed to have an impact on the responsiveness to systemic corticosteroids [Citation59,Citation60,Citation62,Citation63,Citation66]. This suggests there is a possibility to determine which patients are fitted for systemic corticosteroids to ensure the beneficial effects of the treatment and avoid the risks of side effects for those unresponsive to systemic corticosteroids [Citation61]. However, studies have shown low or very low-quality evidence, making it unmanageable to make recommendations based on the scientific conclusions.
With prolonged or short-term use of systemic corticosteroids, there are well-known side effects (). Corticosteroid use can cause metabolic effects, osteoporosis, and infections, among other things. It has been reported that 15% of the patients presented with truncal adiposity, facial adiposity (moon face), and dorso-cervical adiposity (buffalo hump) after prednisolone treatment (10—30 mg/day) after short-term therapy (<3 months) [Citation19]. Also, the risk of hospital admission for sepsis has been reported to be 0.05% for systemic corticosteroid users even at relatively low doses compared with 0.02% for non-corticosteroid users [Citation71]. The risks of adverse side effects heighten with increasing cumulative exposure and increasing mean daily exposure to systemic corticosteroids [Citation9]. Hence, it is relevant to consider the equivalent dose of OCS which is higher compared to injected corticosteroids ().
While the risks of long-term systemic corticosteroids are well known, from other diseases, there is a lack of studies concerning this in rhinitis and CRS treatment. We found five studies focusing on the side effects of systemic corticosteroids and three of these articles presented adverse side effects to OCS, while two studies concluded on no adverse side effects (). Among the severe side effects, it has been shown that there is an increased risk of bone loss and adrenal insufficiency. Bonfils et al. reported that only 39% had normal lumbar bone mineral density (BMD) and only 56% had normal BMD of the femoral neck out of 46 patients treated with OCS [Citation18]; their included patients had an oral corticosteroid consumption during the past year that was greater than three short courses of systemic corticosteroid treatments. This should be compared with a prevalence of osteoporosis of 18.3% in the general population [Citation72]. It is highly recommended to perform DXA evaluation if a cumulated prednisolone dosage of 350 mg is reached within 1 year i.e. 35 mg of prednisolone for 10 days [Citation73]. Also, guidelines in Denmark recommend performing a DXA evaluation if patients receive 5 mg of prednisolone daily for 3 months or treatment with corticosteroids equivalent to at least 5 mg daily with an accumulated dose equivalent to 450 mg in 1 year [Citation74]. The American College of Rheumatology’s (ACR) Glucocorticoid-Induced Osteoporosis Guideline (GIOP) recommends that all patients continuing corticosteroid therapy ≥2.5 mg/day for >3 months or having a high dose of OCS defined as >30 mg/d or an accumulated dose of >5 g/d should have a DXA-evaluation [Citation75].
Another severe side effect has been reported to be adrenal insufficiency. In the study by Bonfils et al., 48.8% of 41 patients were found to have adrenal insufficiency after OCS intake [Citation18], while the study by Laursen et al. [Citation53] showed adrenal suppression with OCS but not with injected steroids. Among the side effects of injected corticosteroids, subcutaneous atrophy at the insertion site has been reported [Citation15]. Other side effects regarding the injected corticosteroids have also been addressed, namely avascular bone necrosis. Nasser et al. reported that a man with severe hay fever was given at least one depot corticosteroid injection each year for 11 years, leading to avascular necrosis of both femoral heads [Citation76]. These studies outline the risks of corticosteroid injections. On the other hand, fewer injections may cause fewer complications with lower risks of side effects. It was reported by Ostergaard and colleagues in a study that included 1,362 patients that a single injection of corticosteroids have no complications and supported no concern for adrenal suppression, osteoporosis, or serious tissue atrophy [Citation77]. Other authors have foreseen the actual safety of systemic corticosteroids without the reported side effects [Citation43,Citation54,Citation55].
In summary, there is a lack of studies that report on the prevalence of side effects of systemic corticosteroids. Only osteoporosis, adrenal insufficiency, risk of allergy, and tissue atrophy after injections are mentioned in the reviewed studies. There is especially a deficiency in the literature concerning dosage and treatment length to avoid any adverse side effects and there are no studies that report on how long it takes for side effects to arise after ending treatment with systemic corticosteroids. It can be critically discussed whether many of the studies were not citing side effects after systemic treatment with corticosteroids simply because rhinologists do not see them. Patients go to their ophthalmologists to have their glaucoma treatment, others go to the emergency room if they have (pathological) fractures, GP for diabetes, etc.
Limited studies have been discovered in our paper implicating the lack of high level of evidence of the efficacy, duration of effect, and side effects of OCS and injected corticosteroids. Also, the limited number of studies indicates no strong evidence for the scientific conclusions emphasizing the need for a well-conducted RCT regarding this topic.
Conclusions
Oral and injected glucocorticosteroids are often effectively used in CRS treatment. Nevertheless, evidence of drug of choice, dosage, treatment length, number of treatment courses pr. year, side effects, and treatment methods are surprisingly poor. The only side effects reported were osteoporosis and adrenal insufficiency. A careful estimate shows that the effect of OCS lasts 2 months and a little longer for injected corticosteroids. Additionally, the corticosteroid injection has a much lower dose compared to OCS with less adrenal suppression, but the effect cannot be stopped if side effects occur. Altogether, the presented data indicate that an RCT study is needed to compare OCS and injected corticosteroids, focusing on their efficacy, duration of effect and side effects.
Acknowledgments
The authors would like to thank Jeppe Skou Petersen, seniorspecialist, from Amgros for providing data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Thank References
- Ho J, Li W, Grayson JW, et al. Systemic medication requirement in post-surgical patients with eosinophilic chronic rhinosinusitis. Rhinology. 2021;59(1):59–18. doi: 10.4193/Rhin20.073
- Giordano J, Darras J, Chevalier D, et al. Preoperative corticosteroid treatment and nasal polyposis. Ann Otolaryngol Chir Cervicofac. 2009;126(3):120–124. doi: 10.1016/j.aorl.2009.03.005
- Fokkens WJ, Lund VJ, Hopkins C, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology. 2020;58(2):82–111. doi: 10.4193/Rhin20.601
- Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: position paper of the european academy of allergy and clinical immunology. Allergy. 2017;72(11):1657–1665. doi: 10.1111/all.13200
- Hellings PW, Scadding G, Bachert C, et al. EUFOREA treatment algorithm for allergic rhinitis. Rhinology. 2020;58(6):618–622. doi: 10.4193/Rhin20.376
- Parente L. Deflazacort: therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol Toxicol. 2017;18(1):1. doi: 10.1186/s40360-016-0111-8
- Kirtsreesakul V, Wongsritrang K, Ruttanaphol S. Clinical efficacy of a short course of systemic steroids in nasal polyposis. Rhinology. 2011;49(5):525–532. doi: 10.4193/Rhino11.140
- ClinCals. (2015, October 24). Corticosteroid Conversion Calculator. Retrieved from ClinCals: https://clincalc.com/corticosteroids/
- Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi: 10.2147/JAA.S176026
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361–1367. doi: 10.4065/81.10.1361
- Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27(7):1059–1066. doi: 10.1007/s00467-011-1928-4
- Hirsch IB, Paauw DS. Diabetes management in special situations. Endocrinol Metab Clin North Am. 1997;26(3):631–645. doi: 10.1016/S0889-8529(05)70271-1
- Segal BH, Sneller MC. Infectious complications of immunosuppressive therapy in patients with rheumatic diseases. Rheum Dis Clin North Am. 1997;23(2):219–237. doi: 10.1016/S0889-857X(05)70327-6
- Da Silva JA, Jacobs JW, Kirwan JR, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis. 2006;65(3):285–293. doi: 10.1136/ard.2005.038638
- Ameratunga R. Gluteal subcutaneous atrophy after depot steroid injection for allergic rhinitis. World Allergy Organ J. 2012;5(11):168–169. doi: 10.1097/WOX.0b013e3182758d80
- Stanbury RM, Graham EM. Systemic corticosteroid therapy–side effects and their management. Br J Ophthalmol. 1998;82(6):704–708. doi: 10.1136/bjo.82.6.704
- Keenan GF. Management of complications of glucocorticoid therapy. Clin Chest Med. 1997;18(3):507–520. doi: 10.1016/S0272-5231(05)70398-1
- Bonfils P, Halimi P, Malinvaud D. Adrenal suppression and osteoporosis after treatment of nasal polyposis. Acta Otolaryngol. 2006;126(11):1195–1200. doi: 10.1080/00016480600672667
- Fardet L, Kassar A, Cabane J, et al. Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf. 2007;30(10):861–881. doi: 10.2165/00002018-200730100-00005
- Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33(4):289–294. doi: 10.1097/00004836-200110000-00006
- Aasbjerg K, Torp-Pedersen C, Backer V. Specific immunotherapy can greatly reduce the need for systemic steroids in allergic rhinitis. Allergy. 2012;67(11):1423–1429. doi: 10.1111/all.12023
- Jankowski R, Bodino C. Olfaction in patients with nasal polyposis: effects of systemic steroids and radical ethmoidectomy with middle turbinate resection (nasalization). Rhinology. 2003;41(4):220–230.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160
- Akiyama K, Makihara S, Uraguchi K, et al. Impact of preoperative systemic corticosteroids on the histology and diagnosis of eosinophilic chronic rhinosinusitis. Int Arch Allergy Immunol. 2019;179(2):81–88. doi: 10.1159/000496437
- Rives P, Espitalier F, Michel G, et al. Prospective evaluation of oral corticosteroid as a predictor of postoperative olfactory recovery after functional endoscopic surgery for nasal polyposis. Eur Arch Otorhinolaryngol. 2019;276(12):3359–3366. doi: 10.1007/s00405-019-05537-y
- Shen KH, Wang YH, Hsu TW, et al. Differential effects of postoperative oral corticosteroid on eosinophilic vs. non-eosinophilic CRSwNP subtypes. Am J Otolaryngol. 2019;40(1):22–29. doi: 10.1016/j.amjoto.2018.09.005
- Ecevit MC, Erdag TK, Dogan E, et al. Effect of steroids for nasal polyposis surgery: A placebo-controlled, randomized, double-blind study. Laryngoscope. 2015;125(9):2041–2045. doi: 10.1002/lary.25352
- Wright ED, Agrawal S. Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: evaluation with the novel Perioperative Sinus Endoscopy (POSE) scoring system. Laryngoscope. 2007;117(11 Pt 2 Suppl 115):1–28. doi: 10.1097/MLG.0b013e31814842f8
- Chang MT, Noel J, Ayoub NF, et al. Oral corticosteroids following endoscopic sinus surgery for chronic rhinosinusitis without nasal polyposis: a randomized clinical trial. JAMA Otolaryngology–Head & Neck Surg. 2021;147(5):434–441. doi: 10.1001/jamaoto.2021.0011
- Arancibia C, Langdon C, Mullol J, et al. Lack of additive benefit of oral steroids on short-term postoperative outcomes in nasal polyposis. Laryngoscope. 2020;130(12):2742–2747. doi: 10.1002/lary.28347
- Workman AD, Miyake MM, Nocera AL, et al. Unexpected effects of systemic steroids on the CRSwNP proteome: is protein upregulation more important than inhibition? Int Forum Allergy Rhinol. 2020;10(3):334–342. doi: 10.1002/alr.22497
- Watanabe S, Pinto JM, Bashir ME, et al. Effect of prednisone on nasal symptoms and peripheral blood T-cell function in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(8):609–616. doi: 10.1002/alr.21336
- Karaki M, Akiyama K, Mori N. Efficacy of intranasal steroid spray (mometasone furoate) on treatment of patients with seasonal allergic rhinitis: comparison with oral corticosteroids. Auris Nasus Larynx. 2013;40(3):277–281. doi: 10.1016/j.anl.2012.09.004
- Ekinci A. The effects of systemic steroid therapy on macrophage migration inhibitory factor concentrations in patients with nasal polyps. J Laryngol Otol. 2018;132(10):891–895. doi: 10.1017/S0022215118001652
- Grammer LC. Doxycycline or oral corticosteroids for nasal polyps. J Allergy Clin Immunol Pract. 2013;1(5):541–542. doi: 10.1016/j.jaip.2013.04.010
- De Silva AP, Schembri MA, Sarah AH, et al. Short-term Oral Steroids Significantly Improves Chronic Rhinosinusitis without Nasal Polyps. Laryngoscope. 2021;131(10):E2618–e26. doi: 10.1002/lary.29495
- Yigit O, Acioğlu E, Gelişgen R, et al. The effect of corticosteroid on metalloproteinase levels of nasal polyposis. Laryngoscope. 2011;121(3):667–673. doi: 10.1002/lary.21462
- Vaidyanathan S, Williamson P, Anderson K, et al. Effect of systemic steroids on humming nasal nitric oxide in chronic rhinosinusitis with nasal polyposis. Ann Allergy Asthma Immunol. 2010;105(6):412–417. doi: 10.1016/j.anai.2010.08.017
- Won TB, Jang E, Min SK, et al. Treatment outcomes and predictors for systemic steroids in nasal polyposis. Acta Otolaryngol. 2012;132 Suppl 1:S82–7. doi: 10.3109/00016489.2012.659753
- Yazici D, Ü T, Uğuz A. The effect of corticosteroid therapy on cyclooxygenase 2, vascular endothelial growth factor, and inducible nitric oxide synthase expression levels in nasal polyposis. Eur Arch Otorhinolaryngol. 2014;271(6):1541–1547. doi: 10.1007/s00405-013-2718-3
- Goektas O, Lau L, Olze H. Treatment of chronic rhinosinusitis with pressure-pulsed corticosteroid inhalation. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 2):402–405. doi: 10.1007/s12070-013-0625-y
- Lennard CM, Mann EA, Sun LL, et al. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. 2000;14(6):367–373. doi: 10.2500/105065800779954329
- Bolger WE, Joshi AS, Spear S, et al. Gene expression analysis in sinonasal polyposis before and after oral corticosteroids: a preliminary investigation. Otolaryngol Head Neck Surg. 2007;137(1):27–33. doi: 10.1016/j.otohns.2007.01.023
- Tuncer U, Soylu L, Aydogan B, et al. The effectiveness of steroid treatment in nasal polyposis. Auris Nasus Larynx. 2003;30(3):263–268. doi: 10.1016/S0385-8146(03)00051-8
- Hissaria P, Smith W, Wormald PJ, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measures. J Allergy Clin Immunol. 2006;118(1):128–133. doi: 10.1016/j.jaci.2006.03.012
- Berkiten G, Salturk Z, Topaloğlu İ. Efficacy of systemic steroid treatment in sinonasal polyposis. J Craniofac Surg. 2013;24(3):e305–8. doi: 10.1097/SCS.0b013e31828f261f
- Woodworth BA, Joseph K, Kaplan AP, et al. Alterations in eotaxin, monocyte chemoattractant protein-4, interleukin-5, and interleukin-13 after systemic steroid treatment for nasal polyps. Otolaryngol Head Neck Surg. 2004;131(5):585–589. doi: 10.1016/j.otohns.2004.05.028
- Schriever VA, Merkonidis C, Gupta N, et al. Treatment of smell loss with systemic methylprednisolone. Rhinology. 2012;50(3):284–289. doi: 10.4193/Rhino.11.207
- Benítez P, Alobid I, de Haro J, et al. A short course of oral prednisone followed by intranasal budesonide is an effective treatment of severe nasal polyps. Laryngoscope. 2006;116(5):770–775. doi: 10.1097/01.mlg.0000205218.37514.0f
- Fernandes AM, Babeto E, Rahal P, et al. Expression of genes that encode the annexin-1 and galectin-1 proteins in nasal polyposis and their modulation by glucocorticoid. Braz J Otorhinolaryngol. 2010;76(2):213–218. doi: 10.1590/S1808-86942010000200011
- Jankowski R, Bodino C. Evolution of symptoms associated to nasal polyposis following oral steroid treatment and nasalization of the ethmoid–radical ethmoidectomy is functional surgery for NPS. Rhinology. 2003;41(4):211–219.
- Laursen LC, Faurschou P, Pals H, et al. Intramuscular betamethasone dipropionate vs. oral prednisolone in hay fever patients. Allergy. 1987;42(3):168–172. doi: 10.1111/j.1398-9995.1987.tb02194.x
- Gelardi M, Barbara F, Covelli I, et al. Long-Term Therapy with Corticosteroids in Nasal Polyposis: A Bone Metabolism Assessment. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 3):2050–2056. doi: 10.1007/s12070-018-1466-5
- Sahlstrand-Johnson P, Holmström M, Ehnhage A. Does the oral steroid treatment of patients with nasal polyposis cause osteopenia or osteoporosis? Clinical Otolaryngology: Official Journal Of ENT-UK; Official Journal Of Netherlands Society For Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2019;44(6):1011–1016. doi: 10.1111/coa.13431
- Hansel K, Marietti R, Bianchi L, et al. Cross-reactions to systemic corticosteroids in patients contact sensitized to budesonide. Contact Dermatitis. 2020;83(4):321–324. doi: 10.1111/cod.13597
- Rajasekaran K, Seth R, Abelson A, et al. Prevalence of metabolic bone disease among chronic rhinosinusitis patients treated with oral glucocorticoids. Am J Rhinol Allergy. 2010;24(3):215–219. doi: 10.2500/ajra.2010.24.3445
- Walford HH, Lund SJ, Baum RE, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155(1):126–135. doi: 10.1016/j.clim.2014.09.007
- Wen W, Liu W, Zhang L, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129(6):1522–8.e5. doi: 10.1016/j.jaci.2012.01.079
- Lu H, Lin XS, Yao DM, et al. Increased serum amyloid a in nasal polyps is associated with systemic corticosteroid insensitivity in patients with chronic rhinosinusitis with nasal polyps: a pilot study. Eur Arch Otorhinolaryngol. 2018;275(2):401–408. doi: 10.1007/s00405-017-4809-z
- Hong H, Chen F, Sun Y, et al. Nasal IL-25 predicts the response to oral corticosteroids in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018;141(5):1890–1892. doi: 10.1016/j.jaci.2017.10.050
- Milara J, Peiró T, Armengot M, et al. Mucin 1 downregulation associates with corticosteroid resistance in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2015;135(2):470–476. doi: 10.1016/j.jaci.2014.07.011
- Milara J, Morell A, Ballester B, et al. MUC4 impairs the anti-inflammatory effects of corticosteroids in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;139(3):855–62.e13. doi: 10.1016/j.jaci.2016.06.064
- Radajewski K, Kalińczak-Górna P, Zdrenka M, et al. Short Term Pre-Operative Oral Corticosteroids—Tissue Remodeling in Chronic Rhinosinusitis with Nasal Polyps. J Clin Med. 2021;10(15):3346. doi: 10.3390/jcm10153346
- Edward JA, Sanyal M, Ramakrishnan VR, et al. Systemic prednisone administration selectively alters granulocyte subsets in nasal polyps from aspirin-exacerbated respiratory disease and chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2013;3(11):866–876. doi: 10.1002/alr.21221
- Salman S, Akpinar ME, Yigit O, et al. Surfactant protein a and D in chronic rhinosinusitis with nasal polyposis and corticosteroid response. Am J Rhinol Allergy. 2012;26(2):e76–80. doi: 10.2500/ajra.2012.26.3739
- Kapucu B, Cekin E, Erkul BE, et al. The effects of systemic, topical, and intralesional steroid treatments on apoptosis level of nasal polyps. Otolaryngol Head Neck Surg. 2012;147(3):563–567. doi: 10.1177/0194599812446678
- Acıoğlu E, Yigit O, Alkan Z, et al. The effects of corticosteroid on tissue lactoferrin in patients with nasal polyposis. Am J Rhinol Allergy. 2012;26(1):e28–31. doi: 10.2500/ajra.2012.26.3735
- Legemiddelverk S (18 Oct 2022). Nye Metoder. Retrieved from nyemetoder.no: https://nyemetoder.no/metoder/dupilumab-dupixent-indikasjon-iv
- Medicinrådet. (26 Oct 2022). Medicinrådet. Retrieved from medicinraadet.dk: https://medicinraadet.dk/media/tx2aecsh/medicinrådets-samling-af-vurderinger-vedr-svær-crswnp-vers-1-1.pdf
- Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415.
- Salari N, Ghasemi H, Mohammadi L, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):609. doi: 10.1186/s13018-021-02772-0
- Walsh LJ, Lewis SA, Wong CA, et al. The impact of oral corticosteroid use on bone mineral density and vertebral fracture. Am J Respir Crit Care Med. 2002;166(5):691–695. doi: 10.1164/rccm.2110047
- Selskab DE (10 2020). Glukokortikoid-Induceret Osteoporose. Retrieved from Dansk Endokrinologisk Selskab: https://endocrinology.dk/nbv/calcium-og-knoglemetabolisme/4-glukokortikoid-induceret-osteoporose/
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis & Rheumat. 2017;69(8):1521–1537. doi: 10.1002/art.40137
- Nasser SM, Ewan PW. Lesson of the week: Depot corticosteroid treatment for hay fever causing avascular necrosis of both hips. BMJ. 2001;322(7302):1589–1591. doi: 10.1136/bmj.322.7302.1589
- Østergaard MS, Østrem A, Söderström M. Hay fever and a single intramuscular injection of corticosteroid: a systematic review. Prim Care Respir J. 2005;14(3):124–130. doi: 10.1016/j.pcrj.2004.08.001