ABSTRACT
Background
Childhood-onset allergic asthma is the best-known phenotype of asthma. Adult-onset asthma, also an important entity, is instead often shown to be more non-allergic. There is still a lack of studies concerning the association of allergies and age at asthma onset from childhood to late adulthood. The aim of the study was to assess the age at onset of asthma symptoms and age at asthma diagnosis among adults with allergic and non-allergic asthma.
Methods
Questionnaires were sent to 2000 randomly selected Finnish adults aged 18–80 years who were dispensed medication for obstructive airway diseases during the previous year. The corrected sample size was 1978 subjects after exclusion of non-analysable data. The response rate was 40.6%. Self-reported doctor-diagnosed asthma was considered allergic if a concomitant self-reported doctor-diagnosed pollen and/or animal allergy was reported with asthma symptoms upon allergen exposure.
Results
Of the 496 participants with asthma, 42.7% were considered to have allergic asthma. The median ages at asthma diagnosis and onset of asthma symptoms were 31 (IQR 17–46) and 20 (9.25–40) years in participants with allergic asthma and 49 (37.75–58) and 40.5 (30–50) years in participants with non-allergic asthma (p < 0.001), respectively. Of the participants with asthma diagnosed at ≥30 years of age, 18% of allergic and 7% of non-allergic participants reported having had asthma symptoms under 20 years of age.
Conclusions
Both the onset of symptoms and diagnosis occurred at a younger age among adults with allergic asthma than among those with non-allergic asthma. Only a minority of adults with non-allergic asthma had already had symptoms in younghood.
Introduction
Asthma is a chronic inflammatory airway disease that can begin at any age [Citation1]. The highest incidence is seen in young boys, and a second peak is seen in middle-aged women [Citation2]. Rather than a single uniform disease, asthma is a heterogeneous disorder expressing different phenotypes [Citation3]. Features and characteristics used to differentiate these phenotypes include disease severity and onset age, inflammatory pattern and atopic status [Citation3]. In particular, phenotyping asthma according to onset age has been of interest, with different characteristics and risk factors associated with different asthma onset ages [Citation4]. The most studied and well-known asthma phenotype is childhood-onset allergic asthma, but allergy seems to be less important in the aetiology of adult-onset asthma [Citation4,Citation5].
Defining allergic asthma, especially in epidemiological studies, can be challenging [Citation6]. Atopy is defined as a tendency to produce IgE antibodies against foreign proteins, whereas clinical allergy is allergic symptoms in a sensitized subject when exposed to the relevant allergen [Citation7]. However, asthma in an atopic person is not necessarily allergic in its mechanisms [Citation8]. In other words, the mere coexistence of asthma and allergic sensitization or another allergic disease, such as allergic rhinitis, does not by definition mean that the asthma is also driven by an allergy. Asthma can be diagnosed as allergic when, in a sensitized subject with asthma, exposure to allergen provokes asthma symptoms [Citation5,Citation7,Citation8].
It is common in studies to consider the asthma onset age equal to the age at diagnosis of the disease [Citation9], but variable symptoms may be present long before the diagnosis is formally established [Citation10]. Although it is not necessarily clear at what point in the natural history of asthma a subclinical disease becomes full-blown asthma, it is presumable that the first respiratory symptoms would indicate a clinically relevant manifestation of the disease [Citation10,Citation11]. Studies that address the duration from onset of asthma symptoms to asthma diagnosis are scarce, especially in adult asthma [Citation10,Citation12,Citation13]. It has also been speculated that a significant proportion of adult-diagnosed asthma originates in childhood, and that the disease is not truly adult-onset [Citation14,Citation15].
The aim of this study was to assess the age at onset of asthma symptoms and the age at diagnosis of asthma in adults with allergic and non-allergic asthma. We also analysed the proportion of participants with adult-diagnosed asthma reporting the onset of asthma symptoms in childhood and whether this differed between allergic and non-allergic adult-diagnosed asthma.
Materials and methods
Study design and population
The study was a postal questionnaire survey conducted in Finland in 2017. The questionnaire was sent to a random sample of 2000 Finnish-speaking subjects aged 18–80 years who were dispensed medication for obstructive airway diseases (the Anatomical Therapeutic Chemical [ATC] Classification System code R03) [Citation16] during the previous 12 months. The Finnish Social Insurance Institution records all prescription medications bought in Finland. Since there are no over-the-counter R03 medications available in Finland, all dispensed R03 medications are recorded with the patient's identity.
The corrected sample size was 1978 subjects after exclusion of subjects with unsuccessful postal delivery of the questionnaire or non-analysable data. Written informed consent for the questionnaire was obtained from all responders. The study protocol was approved by the Ethics Committee of Tampere University Hospital (Approval number R15186).
A more detailed description of the study (e.g. study flow chart) has been previously published [Citation17].
Questionnaire and definitions
The English translation of the questionnaire has been previously published [Citation17]. The questions and definitions most relevant to the present study are outlined below (the total number of questions asked was 60).
Asthma was defined by a positive answer to the question ‘Do you have doctor-diagnosed asthma?’.
Age at asthma diagnosis was defined by an answer to the question ‘What age were you, when your asthma was diagnosed by a doctor?’.
Age at onset of asthma symptoms was defined by an answer to the question ‘Often asthma is diagnosed a long time after the first asthma symptoms have appeared. Now that you think about it, what age were you when your asthma symptoms appeared for the first time?’.
Allergy was defined by a positive response ‘pollen allergy’ and/or ‘animal allergy’ to the question ‘Do you have a doctor-diagnosed allergy?’.
The participant was considered to have allergic asthma if he or she had doctor-diagnosed asthma (see above) and a doctor-diagnosed allergy (see above) and responded positively to the question ‘If you have a doctor-diagnosed allergy, do the allergens also trigger asthma symptoms?’. As a sensitivity analysis, a different definition for allergic asthma was also used (asthma + pollen and/or animal allergy).
Statistical analysis
Statistical analyses were performed using IBM SPSS software version 27 (IBM Corp., Armonk, NY, USA). Pearson’s chi-square test was used for categorical variables, and the Mann‒Whitney U test was used for continuous variables. The Mantel‒Haenszel test of linear relationship was used for trend analysis. A p-value <0.05 was considered significant. The results are presented as percentages (%) or medians (interquartile range [IQR]).
Results
Characteristics of participants with asthma
A total of 803 subjects responded (response rate 40.6%), and 541 (67.4%) of them reported doctor-diagnosed asthma. The responders were, on average, older (median age 62 vs. 54 years, p < 0.001), more often women (61.3 vs. 54.6%, p = 0.003) and more often had a special reimbursement for asthma medication (54.9 vs. 35.5%, p < 0.001) than the non-responders [Citation17].
Forty-five participants were excluded from further analyses due to incomplete answers on the questionnaire on allergy or related asthma symptoms (corrected flow chart in Supplementary Figure S1). Of the remaining 496 participants with asthma, the majority were women and never-smokers (). The ever-smokers had smoked a median of 10.0 pack-years (IQR 3.8–21.5). Less than one-tenth of the participants with asthma also reported having a doctor-diagnosed chronic obstructive pulmonary disease (COPD), and approximately half of them reported allergies to pollen, animals or both.
Table 1. Characteristics of participants with self-reported doctor-diagnosed asthma. Data are presented as n (%) or median (IQR).
Characteristics of participants with allergic and non-allergic asthma
Of the 496 participants with asthma, 212 (42.7%) reported to have an allergy to pollen and/or animals and asthma symptoms on allergen exposure and were considered to have allergic asthma (). The rest (n = 284, 57.3%) were considered to have non-allergic asthma. The adults with non-allergic asthma were significantly older and more frequently ever-smokers than the adults with allergic asthma ().
Table 2. Characteristics of participants with allergic (self-report of allergy to pollen and/or animals and asthma symptoms on allergen exposure) and non-allergic (self-report of no allergy to pollen or animals or asthma symptoms on allergen exposure) asthma. Data are presented as n (%) or median (IQR).
Age at asthma diagnosis, age at onset of asthma symptoms and duration from onset of asthma symptoms to asthma diagnosis
The 74 participants with missing answers on for either questions about age at asthma diagnosis or age at onset of asthma symptoms were excluded from this analysis. The median ages at onset of asthma symptoms and asthma diagnosis were 35 and 42 years, respectively, and the median duration from onset of symptoms to asthma diagnosis was 3 years ().
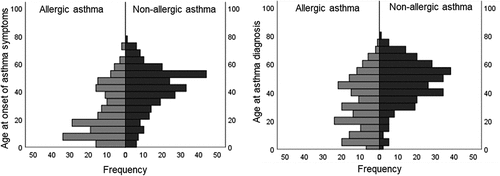
The median ages at both onset of asthma symptoms and asthma diagnosis were significantly lower in the adults with allergic asthma than in those with non-allergic asthma, but there was no difference in the median time from onset of symptoms to diagnosis between the groups (). The distributions of age at onset of asthma symptoms and age at diagnosis of asthma in the adults with allergic and non-allergic asthma are shown in . According to trend analysis, the proportion of participants with allergic asthma also decreased steadily with increasing age at onset of asthma symptoms and diagnosis of asthma (p < 0.001) (Supplementary Figure S2). The results did not considerably change when restricting the analysis only to never-smokers with asthma (Supplementary Table S1 and Supplementary Figure S3).
Proportion of participants with adult-diagnosed asthma reporting asthma symptom onset under 20 years of age
Of the participants with asthma diagnosed at ≥30, ≥40 or ≥50 years of age, 10.4%, 9.2% and 5.9%, respectively, reported having had asthma symptoms under 20 years of age. The proportions were clearly higher for adult-diagnosed allergic asthma than for adult-diagnosed non-allergic asthma ().
Table 3. Proportions of participants with adult-diagnosed asthma reporting the onset of asthma symptoms under 20 years of age. Allergic asthma was defined as self-report of asthma and allergy to pollen and/or animals and asthma symptoms on allergen exposure. Data are presented as n (%).
Sensitivity analysis
As a sensitivity analysis, we used a different definition of allergy and defined allergic asthma as asthma with pollen and/or animal allergy (asthma symptoms on allergen exposure were not required). Consequently, the proportion of participants labelled as having allergic asthma was higher with this definition (54.8%) than with the original definition (42.7%). However, the main results remained the same (Supplementary Tables S2 and 3, and Supplementary Figure S4). The adults with non-allergic asthma were older and more often ever-smokers than the adults with allergic asthma. Age at onset of asthma symptoms and age at diagnosis of asthma were also higher in non-allergic than in allergic asthma. Additionally, the proportion of participants with adult-diagnosed asthma having onset of asthma before the age of 20 years was higher in participants with allergic than non-allergic asthma.
Discussion
We found that the age at onset of asthma symptoms and age at diagnosis were significantly younger among adults with allergic asthma than among those with non-allergic asthma, but there was no difference in the median time from onset of asthma symptoms to diagnosis between allergic and non-allergic asthma. Approximately one-tenth of participants with adult-diagnosed asthma reported having an onset of asthma symptoms before the age of 20 years. This was much more infrequent among adults with non-allergic than allergic asthma, and it decreased with an increase in age at diagnosis.
Allergy has mostly been related to childhood-onset asthma and has been suggested to have a minor role in the aetiology of adult-onset asthma [Citation18]. In contrast, some studies have found a strong association between allergic diseases and adult-onset asthma, but these studies also included asthma onset in young adulthood [Citation19,Citation20]. In our previous study of a different sample of adult asthmatics, the median ages at diagnosis of allergic and non-allergic asthma were 19 and 35 years, respectively [Citation5]. Similar results have been reported in asthma cohorts from other countries as well; the mean age of onset varies from 8 to 18 years among allergic asthmatics and 21–42 years among non-allergic patients, with somewhat lower mean ages reported among severe asthma patients (4.5 and 19 years, respectively) [Citation21–26].
Allergic asthma is mostly defined in previous studies as asthma with sensitization to an aeroallergen (≥1 positivity either in skin prick test and/or allergen-specific IgE) [Citation21–26]. However, according to the definition of allergic disease, mere sensitization to an allergen is not the same as a clinically relevant allergy, and the coexistence of asthma and another allergic disease does not necessarily mean that asthma is also allergic (e.g. a patient with allergic rhinitis due to pollen in childhood with only mild symptoms in adulthood and typical non-allergic eosinophilic asthma at the age of 55 years with no relation between asthma symptoms and pollen season) [Citation5,Citation7]. It is therefore recommended to consider and combine both the sensitization pattern and symptoms of asthma on allergen exposure to diagnose asthma as either allergic or non-allergic [Citation1,Citation27]. Consequently, a significant proportion of asthma among atopics is considered not to be attributable to atopy but to represent incidental coexistence of two fairly common features, non-allergic asthma and atopy [Citation8].
Approximately one-third of non-sensitized asthmatics have been reported to have allergy-like symptoms [Citation22,Citation28]. Romanet-Manent et al. reported seasonal exacerbation of asthma and rhinitis symptoms in almost 40% of asthmatics without sensitization, but these occurred mainly during winter months and were probably more related to chronic rhinosinusitis than allergies [Citation23]. Overall, there seems to be a weaker association between allergic sensitization and allergy-like symptoms in adult-onset asthma than in childhood-onset asthma [Citation4,Citation29]. Consequently, especially in adult-onset asthma, it is important to consider the relation between allergen exposure and asthma symptoms in addition to allergic sensitization when defining asthma as either allergic or non-allergic.
We found that the proportion of allergic asthma declined quite steadily with increasing age at onset of asthma symptoms as well as age at asthma diagnosis. Two previously published studies from Sweden and Brazil have reported sensitization to an aeroallergen among asthmatics to decrease with advancing age at asthma onset (86%, 56% and 26% in onset age groups of ≤6 years, 7–19 and ≥20 years in Sweden and 72%, 69%, 64%, 58% and 38% in age groups of symptom onset ≤12, 13–20, 21–39, 40–54 and ≥55 years in Brazil) [Citation29,Citation30]. The proportion of concurrent allergic rhinitis has also been shown to similarly decrease with increasing age at asthma diagnosis in a previous Finnish study, as well as in a large European study of multiple asthma cohorts [Citation5,Citation31]. To our knowledge, the present study is the first to show the proportions of allergic and non-allergic asthma in consequent age groups of asthma onset stratified both by age at asthma symptom onset and age at diagnosis.
Studies published in recent years have shown that a significant proportion of asthma cases are diagnosed in adulthood [Citation32,Citation33]. However, some previous studies have suggested that asthma onset in adulthood is mostly a relapse of childhood asthma, but again, these studies focus on asthma onset in young adulthood before the age of 26 years [Citation14,Citation15]. In our study, only a small minority of participants with adult-diagnosed asthma reported having symptoms already in younghood. This was much more frequent among adult-diagnosed allergic asthma, and the proportion of participants having onset of symptoms in younghood was lower in non-allergic asthma and at a later age at diagnosis. This suggests that adult-diagnosed non-allergic asthma is a separate disease from allergic asthma that often originates in childhood.
Adult-onset asthma is usually found to be less often allergic, more symptomatic, having a greater decline in lung function and a higher prevalence of obesity and female predominance compared to childhood-onset asthma [Citation2,Citation4,Citation23,Citation27,Citation31,Citation34]. Sensitization to allergens in general tends to decrease after early adulthood, which is suggested to be due to low incidence and high remission [Citation35] or a possible cohort effect [Citation36]. One of the non-allergic adult-onset phenotypes is eosinophilic asthma, which is commonly associated with chronic rhinosinusitis with or without polyposis and mostly onsets at middle age [Citation3]. Longstanding exposure to inhaled respiratory irritants such as tobacco smoke or occupational exposure has also been associated with non-allergic mechanisms of inflammation (e.g. neutrophilic), especially in adult asthma [Citation27,Citation34]. Accordingly, in the present study, most of the self-reported COPD and predominance of ever-smokers were among the participants with non-allergic asthma.
The strength of the present study was that it represented an unselected random sample of Finnish adults who had bought any medication for obstructive lung diseases in the preceding year. We analysed allergic and non-allergic asthma according to the age at onset of asthma symptoms in addition to age at asthma diagnosis for a better indication of the asthma onset age. Due to the selection method, asthmatics who had not bought any medication for their asthma were not included. This would exclude childhood asthmatics in remission or mild, less symptomatic asthmatics who had not even bought reliever medication. It is possible that exclusion of the mildest asthma may have shortened the reported time from first symptoms of asthma to asthma diagnosis, since severe, more symptomatic asthmatics might seek help from a health-care provider more promptly than less symptomatic, mild asthmatics [Citation1]. Additionally, the response rate was quite low (41%), but there was no substantial difference in the basic characteristics of responders vs. non-responders according to the non-responder analysis. Recall bias is a possible confounder, as it is possible for a responder in a questionnaire study to incorrectly remember the time of diagnosis or symptom onset. In the study, the median age of the responders was older than of the non-responders (62 vs. 54 years), which could increase the risk of recall bias among the responders. The self-reported age at asthma onset has previously been published to be both mostly accurate [Citation37] and inaccurate [Citation38].
In the present study, there were more women among the responders than among the non-responders. Since the phenotype of childhood asthma is more prominent in boys, it could be underrepresented among the women-dominated responders whose asthma incidence usually peaks in middle age [Citation2]. The median age of the participants with asthma was also quite high, and nearly half were ever-smokers. Nevertheless, only approximately 8% reported that they had doctor-diagnosed COPD, and the main results did not considerably change after excluding ever-smokers from the analyses.
We did not have the results of lung function tests, but a majority of the participants with asthma (76%) had a special reimbursement right for their asthma medication, which in Finland requires an asthma diagnosis confirmed by lung function tests. Since our study was an epidemiological questionnaire study, allergic asthma was defined by self-report of doctor-diagnosed asthma and an aeroallergen allergy without objective measures of the sensitization patterns of the participants. However, we included the requirement of asthma symptoms on allergen exposure in the definition of allergic asthma for a better indication of clinically relevant allergic disease. We also performed a sensitivity analysis with a different definition of allergic asthma (asthma + doctor-diagnosed pollen and/or animal allergy), and the results were similar. We inquired about a doctor-diagnosed allergy to pollens and/or animals because these are the most common aeroallergen sensitizations in Finland [Citation36,Citation39]. Sensitization to house dust mites and moulds, even though they are important perennial aeroallergies globally, is found in only a small minority (2–6%) of the Finnish population [Citation39].
In conclusion, we found that the symptom onset and diagnosis of asthma occurred at a significantly younger age in adults with allergic asthma than in those with non-allergic asthma who had bought medication for obstructive airway diseases during the preceding year. To the best of our knowledge, the proportion of allergic asthma was shown to decrease steadily with increasing age at onset of asthma stratified by both onset of symptoms and diagnosis of asthma for the first time. Only a minority of participants with asthma diagnosed after 30 years of age reported asthma symptoms from childhood, and this was most evident in adult-diagnosed non-allergic asthma. Consequently, the older the age at which adult asthma is diagnosed, the more likely it is non-allergic, and triggers other than exposure to allergens should be considered, especially in uncontrolled disease.
Author contribution
JP, JK and LL designed the study, created the questionnaire and collected the data. JP, PS and PJ conducted the statistical analyses with help from LL and JK. JP, PS, PJ, JK and LL interpreted the data, critically reviewed the manuscript, read and approved the manuscript before submission.
Disclaimer
None of the funders were involved in the planning, execution, drafting or write-up of this study.
Supplemental Material
Download Zip (611.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available on request from the authors.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2023.2269653.
Additional information
Funding
References
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. [cited 2022 December 1]. Available from: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
- Honkamäki J, Hisinger-Mölkänen H, Ilmarinen P, et al. Age- and gender-specific incidence of new asthma diagnosis from childhood to late adulthood. Respir med. 2019;154:56–7. doi: 10.1016/j.rmed.2019.06.003
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678
- Miranda C, Busacker A, Balzar S, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–108. doi: 10.1016/j.jaci.2003.10.041
- Pakkasela J, Ilmarinen P, Honkamäki J, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020;20(1):9–2. doi: 10.1186/s12890-019-1040-2
- Pape K, Schlünssen V, Lodge CJ, et al. Is self-reported history of eczema and hay fever a valid measure of atopy in those who report current asthma? Allergy. 2020 Nov;75(11):2981–2984. doi: 10.1111/all.14440
- Johansson SGO, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy: an EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56(9):813–824. doi: 10.1111/j.1398-9995.2001.00002.x-i1
- Arbes SJ. Do all asthmatics with atopy have atopic asthma? J Allergy Clin Immunol. 2012;130(5):1202–1204. doi: 10.1016/j.jaci.2012.06.040
- Mirabelli MC, Beavers SF, Chatterjee AB, et al. Age at asthma onset and subsequent asthma outcomes among adults with active asthma. Respir med. 2013;107(12):1829–1836. doi: 10.1016/j.rmed.2013.09.022
- Martyn M, Weaver AL, Jacobson RM, et al. Characterization of the duration from onset of asthma symptoms to asthma disease. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2008;100(6):589–595. doi: 10.1016/S1081-1206(10)60059-2
- Pattaro C, Locatelli F, Sunyer J, et al. Using the age at onset may increase the reliability of longitudinal asthma assessment. J Clin Epidemiol. 2007;60(7):704–711. doi: 10.1016/j.jclinepi.2006.10.010
- Sauni R, Kauppi P, Helaskoski E, et al. Audit of quality of diagnostic procedures for occupational asthma. Occup Med. 2009;59(4):230–236. doi: 10.1093/occmed/kqn165
- Santos MS, Jung H, Peyrovi J, et al. Occupational asthma and Work-Exacerbated asthma: factors associated with time to Diagnostic Steps. Chest. 2007;131(6):1768–1775. doi: 10.1378/chest.06-2487
- Stern DA, Morgan WJ, Halonen M, et al. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372(9643):1058–1064. doi: 10.1016/S0140-6736(08)61447-6
- Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–1422. doi: 10.1056/NEJMoa022363
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment, 2023. Oslo, 2022. [cited 2023 January 15]. Available from: https://www.whocc.no/filearchive/publications/2023_guidelines_web.pdf
- Pakkasela J, Salmela P, Juntunen P, et al. Adherence to treatment guidelines and good asthma control in Finland. Eur Clin Respir J. 2023;10(1):2149918. doi: 10.1080/20018525.2022.2149918
- Rackemann FM. A working classification of asthma. Am J Med. 1947;3(5):601–606. doi: 10.1016/0002-9343(47)90204-0
- Torén K, Hermansson BA. Incidence rate of adult-onset asthma in relation to age, sex, atopy and smoking: a Swedish population-based study of 15813 adults. Int J Tuberc Lung Dis. 1999;3(3): 192–197. PMID: 10094318.
- Toppila-Salmi S, Chanoine S, Karjalainen J, et al. Risk of adult-onset asthma increases with the number of allergic multimorbidities and decreases with age. Allergy. 2019;74(12):2406–2416. doi: 10.1111/all.13971
- Kanani AS, Broder I, Greene JM, et al. Correlation between nasal symptoms and asthma severity in patients with atopic and nonatopic asthma. Ann Allergy Asthma Immunol. 2005;94(3):341–347. doi: 10.1016/S1081-1206(10)60985-4
- Nieves A, Magnan A, Boniface S, et al. Phenotypes of asthma revisited upon the presence of atopy. Respir med. 2005;99(3):347–354. doi: 10.1016/j.rmed.2004.08.004
- Romanet-Manent S, Charpin D, Magnan A, et al. Allergic vs nonallergic asthma: what makes the difference? Allergy. 2002;57(7):607–613. doi: 10.1034/j.1398-9995.2002.23504.x
- Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes Using Cluster analysis in the severe asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC
- Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi: 10.1164/rccm.200711-1754OC
- de Carvalho-Pinto RM, Cukier A, Angelini L, et al. Clinical characteristics and possible phenotypes of an adult severe asthma population. Respir med. 2012;106(1):47–56. doi: 10.1016/j.rmed.2011.08.013
- Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–813. doi: 10.1016/S0140-6736(06)69290-8
- Ray P, Vervloet D, Charpin D, et al. Evaluation of atopy through an expert system: description of the database. Clin Exp Allergy. 1995;25(11):1067–1073. doi: 10.1111/j.1365-2222.1995.tb03253.x
- Warm K, Hedman L, Lindberg A, et al. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J Allergy Clin Immunol. 2015;136(6):1559–1565.e2. doi: 10.1016/j.jaci.2015.06.015
- Agondi RC, Andrade MC, Takejima P, et al. Atopy is associated with age at asthma onset in elderly patients. J Allergy Clin Immunol Pract. 2018;6(3):865–871. doi: 10.1016/j.jaip.2017.10.028
- Baan EJ, de Roos EW, Engelkes M, et al. Characterization of asthma by age of onset: a multi-database cohort study. J Allergy Clin Immunol Pract. 2022;10(7):1825–1834.e8. doi: 10.1016/j.jaip.2022.03.019
- Kankaanranta H, Tuomisto LE, Ilmarinen P. Age-specific incidence of new asthma diagnoses in Finland. J Allergy Clin Immunol Pract. 2017;5(1):189–191.e3. doi: 10.1016/j.jaip.2016.08.015
- Sood A, Qualls C, Schuyler M, et al. Adult-onset asthma becomes the dominant phenotype among women by age 40 years. The longitudinal CARDIA study. Ann Am Thorac Soc. 2013;10(3):188–197. doi: 10.1513/AnnalsATS.201212-115OC
- Ilmarinen P, Tuomisto LE, Phenotypes KH. Risk factors, and mechanisms of adult-onset asthma. Mediators Inflamm. 2015;2015:514868. doi: 10.1155/2015/514868
- Warm K, Backman H, Lindberg A, et al. Low incidence and high remission of allergic sensitization among adults. J Allergy Clin Immunol. 2012;129(1):136–142. doi: 10.1016/j.jaci.2011.08.033
- Haarala AK, Sinikumpu SP, Vaaramo E, et al. Incidence and remission of aeroallergen sensitization in adults in Northern Finland: 15 years longitudinal study. Sci Rep. 2021;11(1):4249. doi: 10.1038/s41598-021-83326-6
- Torén K, Palmqvist M, Löwhagen O, et al. Self-reported asthma was biased in relation to disease severity while reported year of asthma onset was accurate. J Clin Epidemiol. 2006;59(1):90–93. doi: 10.1016/j.jclinepi.2005.03.019
- Burgess JA, Walters EH, Byrnes GB, et al. Who remembers whether they had asthma as children? J Asthma. 2006;43(10):727–730. doi: 10.1080/02770900601028587
- Pallasaho P, Rönmark E, Haahtela T, et al. Degree and clinical relevance of sensitization to common allergens among adults: a population study in Helsinki, Finland. Clin Exp Allergy. 2006;36(4):503–509. doi: 10.1111/j.1365-2222.2006.02460.x