ABSTRACT
Endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNA) of the pancreas is performed routinely in many endoscopic centers as part of the diagnostic set-up for suspected pancreatic cancer. The use of transesophageal bronchoscopic ultrasound-guided fine needle aspiration (EUS-B-FNA) by pulmonologists has expanded significantly, since it enables effective diagnosis of lesions in the mediastinum and upper abdomen. The following case demonstrates the safety and feasibility of EUS-B-FNA in a patient with non-small cell lung cancer (NSCLC) cancer and a pancreatic mass of unknown origin. A patient who was previously diagnosed with NSCLC was referred to the Department of Respiratory Medicine, Odense University Hospital due to suspected recurrence of NSCLC. The patient underwent endobronchial ultrasound guided (EBUS)-FNA from several suspected mediastinal lymph nodes and combined EUS-B-FNA from a pancreatic mass during the same procedure. Pathology results from the pancreatic mass and from the mediastinal lymph nodes showed squamous-cell carcinoma, metastasis from the previous NSCLC. We here by demonstrated that EUS-B-FNA is a feasible and safe technique to obtain tissue samples from pancreatic lesions in patients under investigation for lung cancer.
Introduction
Interventional pulmonology for the assessment of patients with possible lung cancer has expanded in recent years with several additional techniques being implemented. Currently, pulmonologists can diagnose and stage lung cancer using minimally invasive techniques, several of which can also be used to assess extrathoracic disease [Citation1,Citation2].
Classically, a dedicated endoscopic ultrasound (EUS) scope is used to assess structures in the upper gastrointestinal system (e.g. esophagus and ventricle) and upper abdominal and retroperitoneal structures (e.g. lymph nodes, pancreas, and liver) [Citation3,Citation4]. Several studies have described pulmonologists using an endobronchial ultrasound (EBUS) scope as an alternative to dedicated EUS scope to assess both intrathoracic or subdiafragmatic lesions [Citation5–7].
The major clinical advantage of this EUS-B technique is that it can be combined with the conventional EBUS into a single procedure. A combined EBUS and EUS-B procedure enables pulmonologists to assess the mediastinum, pulmonary structures adjacent to the central airways or esophagus, and relevant structures in the upper abdomen in a single session. The combined technique expands the clinically relevant structures from which the pulmonologist can obtain fine-needle aspiration (FNA) during the same session and allows more accurate staging than either of the two procedures separately [Citation1,Citation2,Citation8,Citation9].
In the literature, most studies describe the diagnostic yield and safety of EUS-B-FNA in obtaining biopsies from classical sites of lung cancer metastasis (e.g. adrenal glands, liver). However, conventional EUS is used to assess several other structures, and as such, the clinical utility of EUS-B in patients with suspected lung cancer might be further exploited [Citation1,Citation2,Citation8]. In a recently published case report, EUS-B-FNA was performed to obtain a biopsy from a pancreatic lesion in a patient suspected of malignancy, and the pathology results showed primary pancreatic cancer [Citation10]. In our case, EUS-B-FNA was used in order to obtain biopsy from a pancreatic lesion in a patient with previous lung cancer suspected of having a metastatic relapse, confirming the safety and feasibility of the procedure not only in cases of primary pancreatic cancer but also for metastatic lung cancer.
Case report
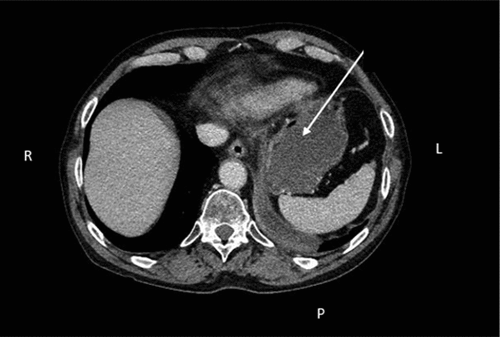
A 72-years-old man diagnosed Chronic Obstructive Pulmonary Disease (COPD) and previous non-small-cell lung carcinoma (NSCLC) of the subtype squamous-cell carcinoma was experiencing productive cough and mild fever. The patient’s general practitioner suspected pneumonia and COPD exacerbation and initiated treatment with antibiotics along with prednisolone. Due to lack of improvement, the patient was hospitalized at the Department of Oncology, and the recurrence of lung cancer was suspected due to the above disease course together with symptoms like tiredness, loss of weight and hemoptysis. Therefore, the Department of Oncology referred the patient to Computed Tomography (CT) of thorax and abdomen, which revealed irregularities in the right lobe of the liver along with edema and cystic changes in the tail of the pancreas (). Afterward, the patient was referred to the Department of Respiratory Medicine for further investigation, where a Positron emission tomography (PET) CT scanning was ordered.
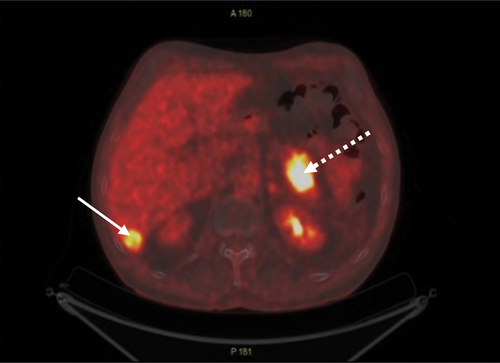
The PET CT scan showed increased flour-deoxy-glucose (FDG) uptake around the left main bronchus, in lymph nodes at station 11 R, in an 18 mm lesion in the liver, and in a 40 mm lesion in the tail of the pancreas ().
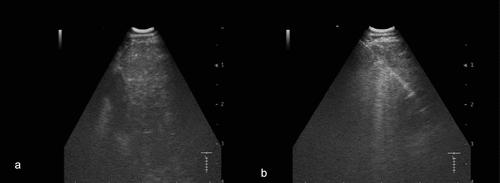
The patient underwent an invasive procedure with EBUS followed by EUS-B, where the suspected pancreatic lesion was visualized as a hyperechoic mass with irregular borders and biopsied using EUS-B-FNA (). EUS-B was chosen over the conventional EUS because sampling from the suspected pancreatic lesion could be combined with sampling from the suspected mediastinal lymph nodes into a single procedure, sparing the patient time, referral to another specialist, and an additional intervention.
Figure 3. Suspect lesion in the pancreas visualized by EUS-B (a, arrow). EUS-B-FNA from the suspected lesion in pancreas (b, dotted arrow).
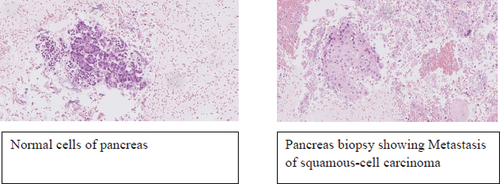
Pathological examination of the mediastinal lymph nodes and the suspected pancreatic lesion showed squamous cell carcinoma and metastasis from a previous NSCLC ().
Discussion
To our knowledge, this is one of the two papers reporting the utility of EUS-B in the diagnosis of malignant pancreatic lesions and the first to describe the use of EUS-B-FNA for obtaining biopsy from a pancreatic lesion, which showed to be metastatic NSCLC [Citation10].
Accurate staging of lung cancer is crucial for planning treatment via surgery, chemotherapy, radiotherapy, and palliation. Previous studies have shown that EUS-B-FNA is a feasible and safe technique for diagnosing and staging lung cancer, as metastases can be detected in the mediastinal lymph nodes, left adrenal gland, retroperitoneal lymph nodes and left lobe of liver [Citation2,Citation9]. Nonetheless, EUS-B-FNA is not routinely used in the diagnostic setup of pancreatic lesions.
There are several ways to perform a pancreatic biopsy, such as EUS-FNA, endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous pancreas biopsy with the use of Computed Tomography (CT) or ultrasound (US) [Citation11–13]. Each of these techniques has advantages and disadvantages. Small pancreatic lesions are difficult to detect using US and CT. Furthermore, CT- or US-guided biopsy of pancreatic tumors is usually challenging to perform due to the retroperitoneal placement of the pancreas [Citation14,Citation15]. EUS-FNA should be performed when a mass lesion is detected by EUS, where lesions located at the tail of pancreas are typically accessed transgastric, while head and uncinate masses are aspirated through transduodenal approach [Citation16]. In cases of localized stenosis of the pancreatic duct, caliber change, and dilatation of the branch duct are found, ERCP followed by serial pancreatic juice aspiration cytological examination is recommended [Citation17]. ERCP is better for diagnosing pancreatic cancer at an early stage [Citation17], but post-procedural complications such as pancreatitis, bleeding, infections, and perforations have also been reported [Citation17,Citation18].
Compared to EUS-B, the main advantages of EUS are its larger ultrasonic window angle and improved ultrasonic imaging with higher resolution. However, the EUS-B scope is thinner and may therefore cause less patient discomfort. Furthermore, pulmonologists are routinely trained to perform EUS-B but not EUS; thus, EUS-B can be combined with EBUS in a single procedure [Citation19]. EUS-B-FNA is also minimally invasive and can therefore be used as a diagnostic technique for patients with poor lung function [Citation20]. Nonetheless, EUS-B has limitations, as it only allows assessment of lesions located at the tail of pancreas [Citation16]. For pancreatic lesions that require transduodenal approach, EUS is a more favorable technique because the EUS-B scope is too short to reach the duodenum. Complications of EUS-B are rare; however, perforation of the esophagus has been reported [Citation21].
Literature comparing the utility of EBUS combined with EUS-B versus EUS alone is scarce. One study showed that the utility of combined EBUS and EUS-B is similar to that of EBUS and EUS performed by a pulmonologist and a gastroenterologist [Citation22].
In conclusion, we recommend that interventional pulmonologists performing bronchoscopic procedures such as EBUS and EUS-B consider the possibility of performing EUS-B-FNA for suspected pancreatic lesions for patients that are under investigation for metastatic lung cancer.
title page.docx
Download MS Word (13.1 KB)Acknowledgments
The authors gratefully acknowledge the contributions of Karen Ege Olsen and the Department of Pathology at Odense University Hospital for providing the pathological slides and descriptions.
Staff of Radiology Department, Odense University Hospital, Odense, Denmark.
Staff of Nuclear medicine Department, Odense University Hospital, Odense, Denmark
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2023.2294545.
References
- Shafiq M, Lee H, Yarmus L, et al. Recent advances in interventional pulmonology. Ann Am Thorac Soc. 2019;16(7):786–5. doi: 10.1513/AnnalsATS.201901-044CME
- Christiansen IS, Ahmad K, Bodtger U, et al. EUS-B for suspected left adrenal metastasis in lung cancer. J Thorac Dis. 2020;12(3):258–263. doi: 10.21037/jtd.2020.01.43
- Tanaka K, Hayashi T, Utsunomiya R, et al. Endoscopic ultrasound-guided fine needle aspiration for diagnosing pancreatic mass in patients with surgically altered upper gastrointestinal anatomy. Dig Endosc. 2020;32(6):967–73. doi: 10.1111/den.13625
- Ding S, Lu A, Chen X, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration: a single-center analysis. Int J Med Sci. 2020;17(17):2861–2868. doi: 10.7150/ijms.48882
- Crombag LMMJ, Szlubowski A, Stigt JA, et al. EUS-B-FNA vs EUS-FNA for left adrenal gland analysis in lung cancer patients. Lunger Cancer. 2017;108:38–44. doi: 10.1016/j.lungcan.2017.02.011
- Crombag L, Bonta P, Annema J. Left adrenal gland analysis using the EBUS scope: a feasibility study in lung cancer patients.14 interventional pulmonology. European Respiratory Societypp. PA779. 2015.
- Vilmann P, Clementsen P, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European society of gastrointestinal endoscopy (ESGE) guideline, in cooperation with the European respiratory society (ERS) and the European society of thoracic surgeons (ESTS). Jun 1. Endoscopy. 2015;47(6):545–559. 06. doi: 10.1055/s-0034-1392040
- Christiansen IS, Bodtger U, Naur TMH, et al. EUS-B-FNA for diagnosing liver and celiac metastases in lung cancer patients. Respiration. 2019;98(5):428–433. doi: 10.1159/000501834
- Skovgaard Christiansen I, Kuijvenhoven JC, Bodtger U, et al. Endoscopic ultrasound with bronchoscope-guided fine needle aspiration for the diagnosis of paraesophageally located lung lesions. Respiration. 2019;97(4):277–283. doi: 10.1159/000492578
- Issa MA, Sidhu JS, Tehrani SG, et al. Endoscopic ultrasound-guided pancreas biopsy in the hands of a chest physician. Respir Med Case Rep. 2023;43:101833. doi: 10.1016/j.rmcr.2023.101833
- Mohammad Alizadeh AH, Shahrokh S, Hadizadeh M, et al. Diagnostic potency of EUS-guided FNA for the evaluation of pancreatic mass lesions. Endosc Ultrasound. 2016;5(1):30–4. doi: 10.4103/2303-9027.175879
- Gruber-Rouh T, Langenbach MC, Eichler K, et al. CT-guided percutaneous biopsy of suspect pancreatic lesions: radiological and clinical outcome. Clin Radiol. 2019;74(11):899.e7–.e12. doi: 10.1016/j.crad.2019.07.022
- Huang Y, Shi J, Chen YY, et al. Ultrasound-guided percutaneous core needle biopsy for the diagnosis of pancreatic disease. Ultrasound Med Biol. 2018;44(6):1145–1154. doi: 10.1016/j.ultrasmedbio.2018.02.016
- Rodriguez J, Kasberg C, Nipper M, et al. CT-guided needle biopsy of the pancreas: a retrospective analysis of diagnostic accuracy. Am J Gastroenterol. 1992;87(11):1610–1613.
- Pinto MM, Avila NA, Criscuolo EM. Fine needle aspiration of the pancreas. A five-year experience. Acta Cytol. 1988;32(1):39–42.
- Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 2010;71(1):91–98. doi: 10.1016/j.gie.2009.06.017
- Hanada K, Minami T, Shimizu A, et al. Roles of ERCP in the early diagnosis of pancreatic cancer. Diagnostics. 2019;9(1):30. doi: 10.3390/diagnostics9010030
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102(8):1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x
- Vilmann P, Clementsen PF. Combined EUS and EBUS are complementary methods in lung cancer staging: do not forget the esophagus. Endosc Int Open. 2015;3(4):E300–1. doi: 10.1055/s-0034-1392786
- Shen Y, Qin S, Jiang H. Endobronchial ultrasound-guided transbronchial needle aspiration combined with either endoscopic ultrasound-guided fine-needle aspiration or endoscopic ultrasound using the EBUS scope-guided fine-needle aspiration for diagnosing and staging mediastinal diseases: a systematic review and meta-analysis. Clinics (Sao Paulo). 2020;75:e1759.
- Tournoy KG, Burgers SA, Annema JT, et al. Transesophageal endoscopic ultrasound with fine needle aspiration in the preoperative staging of malignant pleural mesothelioma. Clin Cancer Res. 2008;14(19):6259–63. doi: 10.1158/1078-0432.CCR-07-5283
- Hernández JAC, Pérez RC, Heras MI, et al. Usefulness of endoscopic ultrasound performed by pulmonologists in lung cancer. Eur Respir J. 2019;54(suppl 63):A4771.