ABSTRACT
Introduction
Spontaneous pneumothorax (SP) affects both young, otherwise healthy individuals and older persons with known underlying pulmonary disease. Initial management possibilities are evolving and range from observation to chest tube insertion. SP guidelines suggest an individualized approach based on multiple factors such as symptoms, size of pneumothorax, comorbidity and patient preference.
Aim
With this Danish national survey we aimed to map organization of care including involved specialties, treatment choice, training, and follow-up plans to identify aspects, and optimization of spontaneous pneumothorax management.
Method
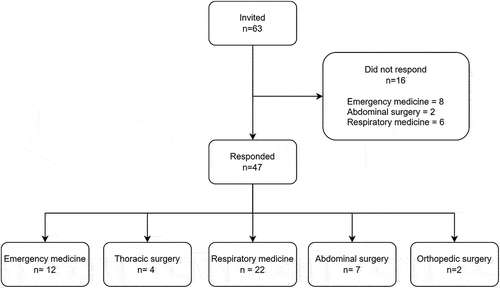
A survey developed by the national interest group for pleural medicine was sent to all departments of emergency medicine, thoracic surgery, respiratory medicine, and to relevant departments of abdominal or orthopaedic surgery.
Results
The response rate was 75 % (47 of 65). Overall, 21% of responding departments had no guideline for SP management, which was provided by multiple specialties with marked heterogeneity in choice of treatment including tube size, management during admission, and referral procedure to follow-up. Few departments required procedure training, and nearly all of the responders called for improvements in management of pneumothorax.
Conclusion
This survey suggests that SP management and care is delivered heterogeneously across Danish hospitals with marked difference between respiratory physicians, emergency physicians, general surgeons and thoracic surgeons. It is therefore likely that management is sub-optimal. There is a need for a common Danish SP guideline to ensure optimal treatment across involved specialties.
Introduction
Spontaneous pneumothorax (SP) is a common condition with an annual incidence of 24 cases in men and 9.8 in women per 100.000 population. Epidemiologically patients fall into two age categories, age < 40 and >40 years, with higher mortality in the latter [Citation1]. The terms primary and secondary SP (PSP and SSP) are used, respectively, for cases without and with underlying lung disease, most often COPD with emphysema [Citation2]. However, SP does not occur in healthy lungs [Citation3]. PSP may be the first presentation of clinically important lung diseases such as cystic lung disease or emphysema, or be caused by less pronounced local inflammation and pleural porosity [Citation3]. Guidelines suggest a structured work-up of patients with recurrent pneumothorax, and in some cases (e.g. women, family history of pneumothorax) after the first SP episode [Citation3,Citation4].
SP management ranges from conservative observation, aspiration, chest tube insertion to surgery. International guidelines suggest that conservative management or aspiration is reserved for younger, otherwise healthy individuals with PSP and none or minimal symptoms, while chest tubes are reserved for patients with unacceptable symptoms, especially in cases of SSP [Citation5–8]. Surgery after first episode, with pleurodesis and bullectomy, may be considered in selected cases. Recently ambulatory management i.e patients managed as outpatients with a small-bore device with Heimlich valve in place, has been shown to be effective for PSP [Citation9–11]. Thus, the palette of treatment options has evolved and allows for an individualised approach to SP treatment. Whilst these new treatment pathways may be of benefit for the patient, they provide significant challenges for the health care system: the simple ‘one-size-fits-all’ management of chest tube insertion with admission to hospital, and when resolved send home with no follow-up is no longer a valid way to manage SP [Citation4].
Patients with SP are most often admitted to the nearest emergency department as the typical presenting symptoms of chest pain, dyspnoea, or respiratory failure require immediate action [Citation7]. In most countries, thoracic surgery is centralized into tertiary university centres; in Denmark, with a total population of 5.5 million inhabitants, thoracic surgery is located in only four centres. Therefore, emergency departments in regional hospitals, without access to a 24-h thoracic surgery or a dedicated pulmonary pleural clinic, are likely to provide the first-line medical service to patients with SP. To handle this, regional hospitals must organize a service based on the locally available staff. Consequently, many patients are not attended by respiratory physicians or thoracic surgeons, but to what extent this practice leads to inferior SP treatment or follow-up is unknown.
To address this gap in literature, this study aimed to map the organization of pneumothorax care in Denmark by identifying the relevant specialties involved in pneumothorax care, availability of treatment choices including chest tube size and ambulatory management, training facilities for interventional procedures, follow-up pathways, and perceived need for optimization of spontaneous pneumothorax management in a national survey.
Methods
Design and approvals
The study was designed as a questionnaire survey according to the Cherries guidelines on reporting internet e-surveys [Citation12]. The STROBE guidelines on cross-sectional studies were followed [Citation13]. According to Danish law, approval from an ethical committee is not required in survey studies. Approval was obtained from hospital administration (Aarhus University Hospital) and data were handled in accordance with European Union law.
Development of questionnaire
The questionnaire was developed by the Danish Respiratory Society’s interest group for pleural diseases. This group consists of specialists in respiratory medicine from all five Danish health-care regions to ensure national representation. Firstly, the research aim was defined and themes of research questions were constructed to include: Organization of initial management, diagnostic approach, decisions behind chest tube insertion or observation, choice of tube type, management of chest tube during admission, follow-up plan, training aspects, and how management of pneumothorax could be optimized. Question types were dichotomous, multiple choice, or open-ended questions wherever relevant. Branching logic and stop questions were inserted to minimize irrelevant questions. The first questionnaire draft was developed by SHS and was revised by the group members in an iterative process until agreement on content and question type was reached. A thoracic surgeon and an emergency physician with special interest in pneumothorax reviewed the questionnaire, and inputs were integrated in the final questionnaire. The technical functionality of the questionnaire, understanding of questions, and time consumption were piloted in a group of two volunteers. The final survey consisted of total 83 questions divided into eight topics (participant information, PSP, SSP, management of tubes, training and needs for optimization of management. Due to the branching structure of the survey not all questions were available to be answered by each participant. A translated version of the questionnaire is available in supplementary material (supplemental table S1).
Recruitment
All Danish hospital departments of emergency medicine, respiratory medicine, and thoracic surgery were a priori selected as eligible participants for the survey. The questionnaire was sent to the head of department, or – if available – to the physician responsible to deliver pneumothorax care in each department. In the survey, the first stop-question was if the responding physician could recognize the role as a local expert, or if another physician in the department was better suited to answer the questionnaire. If positively answered, the participant was then asked to identify if representatives from other departments in their hospital were also engaged in SP care and should be included in the study. If other relevant participants were suggested, the questionnaire was sent to them. This expanding recruitment process was chosen to include, identify and respect different set-ups for pneumothorax care at the hospitals.
Survey administration
Participation was completely voluntary and no incentives were offered to the participants. Participants were informed that the survey was initiated by the Danish Respiratory Society’s interest group for pleural diseases and the purpose of survey was clearly stated. Consent was sought from all participants to store data.
The survey was constructed in the REDCap hosted by Aarhus University. In the REDCap survey distribution tool, each participant’s email-address was used as an identifier to send the questionnaire directly. No login or password protected access was needed as only the recipient of the email invitation could fill the questionnaire. Participants were allowed to save the questionnaire for later completion. Each participant and department contributed with maximally one response. A reminder of survey invitation was automatically sent up to four times with one-week interval until completion. If survey was not completed after four reminders, no further invitations were sent and the participant was considered as a non-responder.
Analysis and statistics
All available data from the questionnaires were used. Partly completed questionnaires were not excluded from the final analysis.
Data analyses were performed using statistical software (STATA version 14.2; StataCorp, Texas, USA) with no participant identification information. Descriptive data were presented as percent of responses and absolute numeric distribution. Fischer’s Exact test was used to test inter-group differences, and a p-value <0.05 was considered statistically significant.
Results
Total 63 eligible centres (and hence participants) were identified, and the questionnaire was completed by 47 (75%). shows participation among the involved specialties.
Organization of spontaneous pneumothorax management within the departments
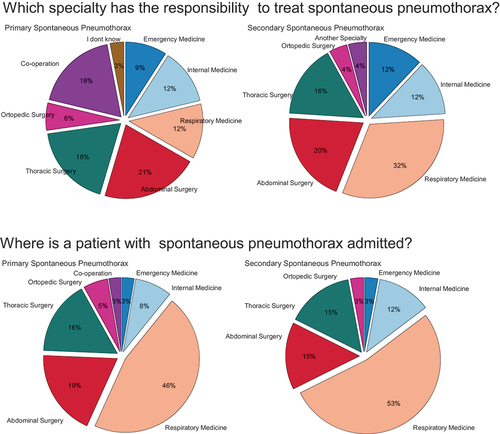
Only a minority of departments had formal guidelines for SP management: 11% (4/37) for PSP, and 21% (7/34) for SSP. Where guidelines were available, multiple specialties were responsible for the management of pneumothorax as shown in . Approximately half of the units responded when patients were admitted to a department of respiratory medicine (PSP 46% and SSP 52%), see .
Figure 2. Questionnaire results on how management of primary (PSP) and secondary spontaneous pneumothorax (SSP) is organized in Danish hospitals. Results show that there is a difference in which specialty has the responsibility to treat patients with SP and which department the patient is admitted to.
Diagnosis of pneumothorax
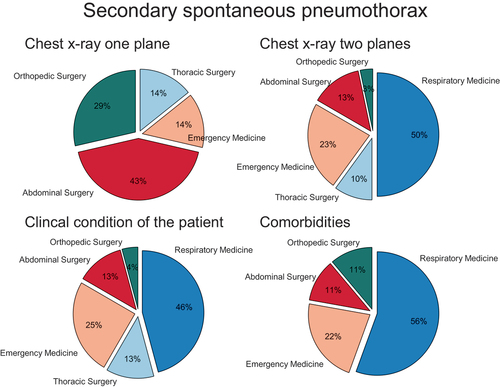
In most departments, the diagnosis was made on a chest x-ray in either erects in two planes (Posterior-anterior plane and lateral plane) (75%; 35/47), erect (Posterior-anterior plane only) or supine (Anterior-posterior plane only) (15%; 7/47). Thoracic ultrasound was used by 34% (16/47) but significantly more in the departments of emergency medicine (75%; 9/12) than in respiratory medicine (23%; 5/22) or thoracic surgery (25%, 1/4), p = 0.012. Computed tomography was reported to be used by 32% (15/47) of the departments, see Supplementary Figure S1.
Choice of intervention and management of chest tubes
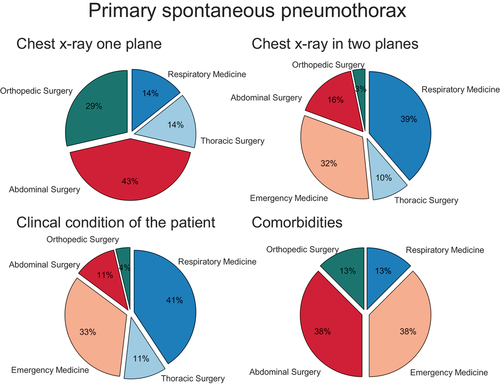
The decision to treat SP invasively by chest tube insertion was reported to be based on a combination of factors including size of pneumothorax on chest X-ray (81%, 38/47), clinical condition (57%, 27/47) and number of comorbidities (17%, 8/47). However, clinical decision-making varied significantly between specialties (p = 0.001), see .
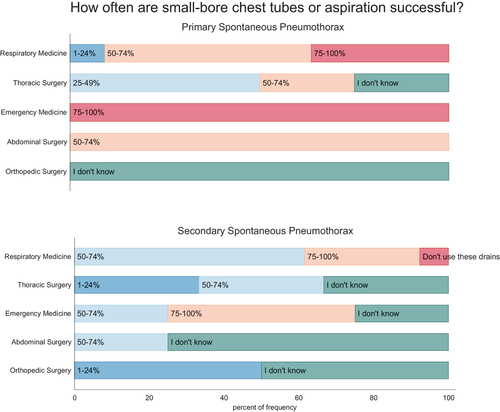
Figure 3. Use and perceived efficacy of small-bore chest tubes in primary (PSP) and secondary spontaneous pneumothorax (SSP) among specialties. Participants were asked how often they use small-bore chest tubes and how often they estimate these tubes are successful. Questionnaire results are shown in intervals ranging from never, red; 1–24% of the cases, blue; 25–49% of the cases, dark blue; 50–74% of the cases – light blue; 75–100% of the cases, yellow; or if not known; green.
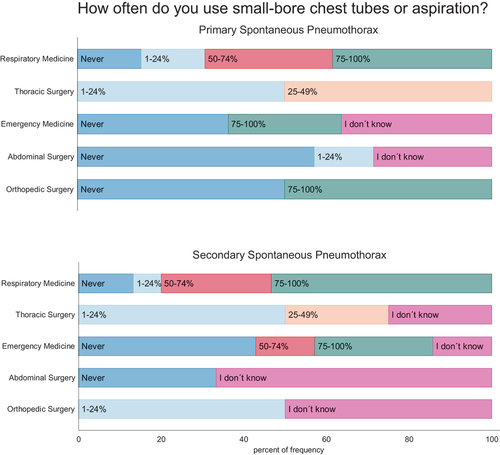
Needle aspiration and small-bore chest tubes were used more commonly (p = 0.001) in the department of respiratory medicine compared to other specialties, see . Likewise, the perceived efficacy of small-bore chest tubes to successfully treat PSP and SSP in > 75% of the cases was significantly higher in respondents from departments of emergency medicine or respiratory medicine versus the surgical specialties (PSP: p = 0.011; SSP: p = 0.014), see and supplementary figure S2 and supplementary figure S4. Difference between specialties that have the lungs as their primary organ (respiratory medicine and thoracic surgery) versus other involved specialties (emergency medicine, abdominal surgery and orthopaedic surgery), presented in supplementary figure s3 and supplementary figure s5 show difference between these groups (PSP: p = 0.001; SSP: p = 0.026)
Figure 4. Decision to treat primary (PSP) and secondary spontaneous pneumothorax (SSP) invasively. Participants were asked which of the variables they primarily based the decision to treat the patients with or without chest tube insertion on.
On the contrary, large-bore surgical chest tubes were reported as the preferred treatment by 85% (6/7) of abdominal-, 50% (1/2) of orthopaedic, and 50% (2/2) of thoracic surgical departments. This was significantly more common than the usage in respiratory 14% (3/22) and emergency medicine departments 33% (4/12) (p = 0.004). Interestingly, 73% (11/15) of respiratory medicine and 29% (2/7) of emergency medicine departments reported that large-bore chest tubes were inserted in case of ongoing air-leak and/or incomplete lung expansion after 24-h treatment with a small-bore chest tube, see Supplementary Figure S1. The large-bore chest tube insertion was mostly performed by surgeons, and the patients were subsequently admitted to the departments of respiratory medicine or abdominal surgery (see Supplementary Figure S6).
In-hospital chest tube management
Thoracic suction was only used in the department of respiratory medicine 63% (7/11) and thoracic surgery, 75% (3/4) with no usage at all in the departments of abdominal or orthopaedic surgery. For analgesia, paracetamol was used by 68% (32/47) of departments, non-steroid anti-inflammatory drugs by 55% (26/47), and morphine by 61% (29/47), but the usage was statistically different among departments (p = 0.001). A chest X-ray following chest tube insertion was performed by all specialties except the emergency medicine (p = 0.13). A trial of clamping of the chest tube, once air leak had visibly stopped, to assess early recurrence of pneumothorax before chest tube removal was conducted in the departments of respiratory medicine, 45% (9/20) and abdominal surgery, 29% (2/7) only (p = 0.003). Chest X-ray prior to the chest tube removal was done in respiratory medicine, 75% (15/20), abdominal surgery, 57% (4/7) and in all orthopaedic surgery departments, but not in thoracic surgery or emergency medicine (p = 0.003).
Patients were routinely encouraged to walk around independently in 55% (26/47) departments but were mobilized by a physiotherapist in 17% (8/47) of the departments.
Follow-up after an episode of spontaneous pneumothorax
Patients with a first episode of PSP were reported to be referred for follow-up with respiratory medicine by 32% (8/25) and for SSP by 65% (17/26) of the departments. The follow-up was reported to include high resolution computed tomography (HRCT), spirometry and reversibility tests, body plethysmography, alfa-1-antitrypsin level, and support for smoking cessation, if relevant. None of the departments had a guideline or standard program for follow up and all examinations were chosen on an individual basis. None reported referral to follow-up in thoracic surgery and there were no data to suggest collaboration between respiratory medicine and thoracic surgery.
Training of physicians in chest tubes insertion
The survey respondents answered that completion of a training program was required prior to insertion of small-bore chest tubes in only 28% (13/47) of the departments, mostly in respiratory medicine, 41% (9/22) and in thoracic surgery, 75% (3/4) versus 14% (1/7) in abdominal surgery and 0% in emergency medicine and orthopaedic surgery, p = 0.008. The training program included theoretical education, 23% (11/47), simulation on phantom, 15% (7/47), supervision, 28% (13/47) and an assessment test, 2% (1/47). Large-bore chest tube insertion training was demanded by 100% (4/4) in thoracic and orthopaedic surgery but only by 43% (3/7) in abdominal surgery. The training program included theoretical education, 19% (9/47), phantom simulation, 10% (5/47), supervision, 21% (10/47) but no departments used a test to assess competency.
Optimal treatment of pneumothorax
Most of the responders considered that the initial SP treatment should be provided by the departments of respiratory medicine, thoracic surgery, or emergency medicine. Respiratory physicians and thoracic surgeons reported that they were better suited to manage pneumothorax than other specialties, ().
Table 1. Heatmap showing the distribution of which specialty should be responsible for the initial management of primary (PSP) and secondary spontaneous pneumothorax (SSP) and the distribution differences between responding specialties. Color coded based on percentage; green > 80%, light green 60–80%, dark blue 40–60%, light blue 20–40%, orange < 20%.
Most survey participants responded that SP management needs improvement (major: 32% (13/41); minor: 44% (18/41)). Only 2% (1/41) found current SP management optimal. All but one (97%; 40/41) considered that a national trans-speciality guideline for SP management was the most pivotal initiative to improve future SP treatment and care.
Discussion
This nationwide Danish survey study mapped the organization, diagnostic modalities, choice of treatment, management of chest tubes, follow-up standards, and level of procedural training in SP management. The main finding is that SP management involves multiple specialties that do not share pivotal aspects of patient care such as clinical decision making, in-hospital management or follow-up.
Traumatic pneumothorax is a surgical diagnosis, often managed with large-bore drains. The responses on SP management by the participating surgical departments probably reflect that SP is managed with the standard approach to traumatic pneumothorax with large-bore drains, suction, and no follow-up. This is supported by the reported absence of local SP guidelines in the majority of surgical departments, and the lack of routine referral to respiratory medicine for evaluation of possible prophylactic interventions to reduce SP recurrence risk [Citation14]. In Denmark, departments of thoracic surgery are centralized to only four hospitals that therefore are mostly involved in secondary prophylactic SP surgery rather than in resolving ongoing air leak. Our survey therefore highlights a profound need for shared SP care pathways across specialties to ensure contemporary guideline-based SP treatment, follow-up, and training of caregivers [Citation15]. Almost all participants responded in favour of a national multidisciplinary guideline for SP, and considered that an improvement in SP care is needed. British Thoracic Society guidelines on pleural diseases are due to be published in 2023 and a taskforce publication from the European Respiratory Society is awaited [Citation4,Citation5,Citation16]. We expect that these important documents may facilitate the development of shared Danish guidelines and care pathways aiming to disseminate best practice to the clinicians and surgeons across the Denmark. A recent UK survey on initial management of pneumothorax shows that this is achievable, as the choice of treatment depends on symptom severity and pneumothorax size – and less on department – to individualize treatment to conservative management, aspiration, use of an ambulatory device, or in-hospital chest tube drainage [Citation17].
The perceived efficacy of small-bore chest tubes varied between specialties; only the respiratory- and emergency medicine departments reported that they were effective. It could be speculated that this difference was due to the transfer of complicated cases with persistent air-leak refractory to the small tube to departments of thoracic or abdominal surgery where a large chest tube could be inserted. But the survey data do not support this since the surgical specialties reported that large-bore tube insertion is their first-line treatment choice, and most patients with large-bore chest tubes were then admitted to the departments of respiratory medicine. Moreover, facility of thoracic suction, which is often used in persistent air-leak despite poor evidence for efficacy, was only available in the departments of thoracic surgery, emergency-, and respiratory medicine indicating that the complicated SP patients should be treated in these departments [Citation4,Citation18]. Analgesic medications were used in all departments but the type of medication used differed. Only a few departments used physiotherapy to mobilize patients while mostly relied on patients to mobilise independently.
Recurrence of pneumothorax is not uncommon and prevention is a major concern for the patients [Citation14]. A minority of the departments referred patients for follow-up after pneumothorax. Majority were referred to the respiratory medicine with only a subset being referred to thoracic surgery. Follow-up was not standardized and the survey did not report any criteria used for patient selection for consideration of elective preventive surgery (bullectomy or pleurodesis).[5] [Citation4,Citation19] Hence, the current practice might miss the diagnosis of patients with underlying genetic diseases who present with SP and this can lead to significant clinical consequences [Citation20,Citation21].
Chest tube insertion is an invasive procedure with potentially life-threatening complications. It is worrying that only a small number of departments mandated the staff to complete a training program for chest tube insertion. Data suggest that procedural training programs including simulation and validated assessment tests are not only available but also have proven efficacy and shown to improve success rate and safety of invasive thoracic procedures [Citation22,Citation23].
The present study has several limitations. Firstly, the data originate from a questionnaire and not from the observation of actual clinical practice. Recollection bias is thus unavoidable. However, we tried to minimize this by approaching the most qualified person in each department; i.e. the physician/surgeon responsible for each department’s pneumothorax care. Thus, it is reasonable to conclude that the described discrepancies reflect real and significant differences in current SP care, though the true magnitude of differences is unknown as chart review was not used to validate participants’ responses. Secondly, despite the response rate of 75% which is relatively high for surveys, we still missed 25% of invited departments. Non-participation bias is plausible, and we predict that the non-participation reflects a lower priority of SP care [Citation24]. Thirdly, our questionnaire did not cover advanced treatments such as blood-patch pleurodesis or endobronchial valve placement or surgical intervention [Citation5,Citation25,Citation26].
In conclusion, this national survey clearly highlights that the treatment of spontaneous pneumothorax is poorly organized in the Danish healthcare. Patients receive different treatments based on the locality and the speciality leading pneumothorax care, rather than the clinical presentation. The procedural training for interventional management of SP is inadequate and a cross-speciality national guideline of spontaneous pneumothorax management is desired for delivery of standardised care.
Supplementary files.docx
Download MS Word (59.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2024.2307648
References
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax. 2000;55(8):666–11. doi: 10.1136/THORAX.55.8.666
- Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320(14):1471–1480. doi: 10.1001/JAMA.2018.14299
- Hallifax R. Aetiology of primary spontaneous pneumothorax. J Clin Med. 2022;11(3):11. doi: 10.3390/JCM11030490
- Maskell N, Najib Rahman MR. BTS Guideline for Pleural Disease. On behalf of the BTS Pleural Guideline Development Group. Draft Public Consult. 2022 June 20. https://www.brit-thoracic.org.uk/document-library/guidelines/pleural-disease/pleural-disease-2010/.
- Online Ap p en dix A 1 BTS Guideline for Pleural Disease. 2022. Available from: https://www.brit-thoracic.org.uk/media/455876/section-a-online-ap pen dic es-a1-a5-for-consultation-jun-2022.pdf
- Brown SGA, Ball EL, Perrin K, et al. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382(5):405–415. doi: 10.1056/NEJMOA1910775
- Brown SGA, Ball EL, Macdonald SPJ, et al. Spontaneous pneumothorax; a multicentre retrospective analysis of emergency treatment, complications and outcomes. Intern Med J. 2014;44(5):450–457. doi: 10.1111/IMJ.12398
- Liu WL, Lv K, Deng HS, et al. Comparison of efficiency and safety of conservative versus interventional management for primary spontaneous pneumothorax: a meta-analysis. Am J Emerg Med. 2021;45:352–357. doi: 10.1016/J.AJEM.2020.08.092
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: a systematic review and network meta-analysis of randomized clinical trials. Respir med. 2018;137:152–166. doi: 10.1016/J.RMED.2018.03.009
- Hallifax RJ, McKeown E, Sivakumar P, et al. Ambulatory management of primary spontaneous pneumothorax: an open-label, randomised controlled trial. Lancet (London, England). 2020;396:39–49. doi: 10.1016/S0140-6736(20)31043-6
- Walker SP, Keenan E, Bintcliffe O, et al. Ambulatory management of secondary spontaneous pneumothorax: a randomised controlled trial. Eur Respir J. 2021;57(6):57. doi: 10.1183/13993003.03375-2020
- Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34. Available from: https://www.jmir.org/2004/3/e34
- von EE, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD
- Walker SP, Bibby AC, Halford P, et al. Recurrence rates in primary spontaneous pneumothorax: a systematic review and meta-analysis. Eur Respir J. 2018;52(3):1800864. doi: 10.1183/13993003.00864-2018
- Shiu T, Hospital K, Kong H, et al. Management of patients admitted with pneumothorax: a multi-centre study of the practice and outcomes in Hong Kong. Hong Kong Med J. 2009;15(6):427–433.
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J. 2015;46(2):321–335. doi: 10.1183/09031936.00219214
- Hallifax RJ, Roberts M, Russell N, et al. Pneumothorax management: current state of practice in the UK. Respir Res. 2022;23(1):1–4. doi: 10.1186/S12931-022-01943-9/FIGURES/2
- Jablonski S, Brocki M, Wawrzycki M, et al. Efficacy assessment of the drainage with permanent airflow measurement in the treatment of pneumothorax with air leak. Thorac Cardiovasc Surg. 2014;62(6):509–515. doi: 10.1055/S-0033-1359714
- Olesen WH, Katballe N, Sindby JE, et al. Surgical treatment versus conventional chest tube drainage in primary spontaneous pneumothorax: a randomized controlled trial†. Eur J Cardiothorac Surg. 2018;54(1):113–121. doi: 10.1093/EJCTS/EZY003
- Grimes HL, Holden S, Babar J, et al. Combining clinical, radiological and genetic approaches to pneumothorax management. Thorax. 2022;77(2):196–198. doi: 10.1136/THORAXJNL-2021-217210
- Scott RM, Henske EP, Raby B, et al. Familial pneumothorax: towards precision medicine. Thorax. 2018;73(3):270–276. doi: 10.1136/THORAXJNL-2017-211169
- Berger M, Weber L, McNamara S, et al. Simulation-based mastery learning course for tube thoracostomy. MedEdPORTAL J Teach Learn Resour. 2022;18:11266. doi: 10.15766/MEP_2374-8265.11266
- Yee J, Miguel CS, Khandelwal S, et al. Procedural curriculum to verify Intern competence prior to patient care. West J Emerg Med. 2022;24(1):8–14. doi: 10.5811/WESTJEM.2022.11.58057
- Sedgwick P. Non-response bias versus response bias. BMJ. 2014;348(apr09 1):g2573–g2573. doi: 10.1136/BMJ.G2573
- Shakir S, Choo-Kang B, Ross C, et al. Autologous blood patch pleurodesis for secondary spontaneous pneumothorax: a narrative review, a Retrospective Case series and state of play in the UK. Pulm Ther. 2023;9(1):165–172. doi: 10.1007/S41030-022-00212-W
- Ibrahim IM, Elaziz MEA, El-Hag-Aly MA. Early autologous blood-patch pleurodesis versus conservative management for treatment of secondary spontaneous pneumothorax. Thorac Cardiovasc Surg. 2019;67(3):222–226. doi: 10.1055/S-0038-1642028