ABSTRACT
Background
Acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in the intensive care unit (ICU). Previous studies have suggested that blood group A increases the risk of developing ARDS following sepsis and major trauma. This study investigated the association between ABO and Rh blood groups and ARDS development and mortality in ARDS.
Methods
Patients admitted to the ICUs at Skåne University Hospital in Lund and Malmö, Sweden, in 2016 were retrospectively screened for ARDS according to the Berlin definition. Clinical data, patient characteristics, lab results, and survival data were collected from medical records and registry data. In addition, chest radiographs were reviewed by radiologists. ARDS development and 30-day mortality were analysed using multivariable logistic regression.
Results
A total of 1439 ICU patients were included. Of these, 10% had ARDS. Blood group O was associated with an increased risk of having or developing ARDS compared to blood group A (odds ratio [OR] 1.79, 95% confidence interval [CI] 1.13–2.84, p = 0.014). Among ARDS patients, blood group O had decreased 30-day mortality compared to blood group A (OR 0.25, 95% CI 0.09–0.67, p = 0.007). The Rh blood group was not associated with ARDS development or mortality.
Conclusion
In this study of ICU patients, blood group O was associated with an increased risk of having or developing ARDS but a decreased mortality in ARDS compared to blood group A. Further studies are needed to clarify the relationship and underlying mechanisms of the ABO blood group and ARDS.
Introduction
Acute respiratory distress syndrome (ARDS) is an acute hypoxemic respiratory failure with various etiologies [Citation1]. The current definition of ARDS is the Berlin Definition, where ARDS is defined by timing, radiology, aetiology of pulmonary oedema, and oxygenation [Citation2]. ARDS is a common cause of respiratory failure in the intensive care unit (ICU). A large multicentre study from 2016 showed that 10.4% of patients admitted to an ICU and 23.4% of patients in mechanical ventilation met the criteria for ARDS. Mortality is high, ranging from 35–46% [Citation3]. Attempts have been made to subdivide ARDS into subgroups based on physiological, clinical, biological, and genetic variables associated with different outcomes and treatment responses [Citation4].
Several systems classify human blood type based on antigens on the red blood cell surface and corresponding antibodies in plasma. The ABO and Rh blood group systems are the most important and clinically applicable. The ABO blood group is determined by the ABO gene, which encodes for glycosyltransferases that catalyse the final step in synthesising the A and B antigens [Citation5]. The Rh blood group system classifies the blood group as Rh positive or negative and is de-termined by the presence of the RhD protein on the red blood cell surface [Citation6].
It is well established that blood group O has lower von Willebrand factor (vWF) and factor VIII levels, which could explain why blood group O has a lower risk of vascular disease [Citation7–9]. In addition, the ABO blood groups are also thought to affect susceptibility to infections, for example, by regulating host-pathogen interactions and microbe attachment to the red blood cell surface [Citation10].
During the COVID-19 pandemic, ARDS incidence was markedly increased since COVID-19 may cause ARDS in severe cases. Many studies have recently been performed on potential prognostic factors in COVID-19, including blood groups. Several studies have shown that blood group A is associated with increased susceptibility and severity of COVID-19. In contrast, blood group O has been suggested to have a lower risk [Citation11–15]. A cohort study on 1.7 million blood donors has indicated that blood group A has a decreased risk of influenza and blood group B has a decreased risk of pneumonia compared to blood group O [Citation16].
Two studies have identified an association between blood group A and ARDS following major trauma and severe sepsis, especially from a non-pulmonary source. The most significant effect was shown in subtype A1, which has the highest density of A antigens [Citation17,Citation18]. Although there are few studies on the association between ABO blood groups and ARDS, several studies have implicated that blood groups may affect susceptibility and outcomes in ARDS risk factors, including sepsis and trauma. The results are however not consistent [Citation19–23]. On the other hand, one study failed to show any correlation between the ABO blood group and mortality or ICU length of stay in acute hypoxaemic respiratory failure [Citation24]. Furthermore, a recent multicenter study also concluded no association between blood group and mortality in ARDS patients or critically ill patients, in general [Citation25].
The Rh blood group, on the other hand, has not been linked to diseases to the same extent as the ABO blood group. The most well-known relationship between the Rh blood group and disease is allo-immunisation during pregnancy [Citation26]. In one study, Rh negativity was found protective against infection and severe outcomes in COVID-19 [Citation15].
This study investigated the association between ABO and Rh blood groups and ARDS development and mortality in ARDS.
Materials and methods
Study design and study population
All patients admitted to ICUs at Skåne University Hospital in Lund and Malmö during 2016 were included for data collection and ARDS screening. Patients without ABO or Rh blood group information were excluded when analyses were performed.
Data collection
Clinical data, including comorbidities, ARDS risk factors, and treatment variables, were retrospectively collected. The variables were predefined, and data collection was carried out systematically by trained data collectors. In cases of uncertainty, the study group collectively discussed and decided.
Chest radiographs were examined according to the radiographic criteria of the Berlin definition by a specialist cardiothoracic radiologist blinded to any clinical or laboratory data. Computer tomography and x-ray images were categorised as ’consistent with ARDS’, ’inconsistent with ARDS’, or ’equivocal’. Only the ’consistent with ARDS’ cases were considered as having ARDS. The first 100 assessed images were reviewed in the study group with a second cardiothoracic radiologist and compared to examples in the Berlin definition supplementary material.
Lab results were automatically extracted from electronic medical records. The ABO and Rh blood groups were also retrieved from medical records. Data on survival and the Simplified Acute Physiology Score 3 (SAPS-3) were retrieved from the Patient Administrative System for Intensive Care Units (PASIVA). In addition, survival data were extracted from the national population registry.
Independent variables and outcomes
Independent variables included age, sex, body mass index, smoking, comorbidities, ARDS risk factors, and SAPS-3. Outcomes included ARDS and 30-day mortality. ARDS risk factors included those in the Berlin definition [Citation2]. The updated Charlson Comorbidity Index (CCI) was used to adjust for chronic morbidity. CCI includes variables representing a range of different comorbidities; each is assigned a score based on the risk of mortality they represent. These include age, cardiovascular disease, lung disease, diabetes, kidney disease, and various others, and the total score is used to predict 10-year mortality [Citation27]. SAPS-3 was used to correct for acute morbidity. SAPS-3 evaluates various variables to assess the severity of illness in ICU patients. These include age, patient demographics, preexisting comorbidities, physiological parameters, organ function, and laboratory results, all collected within the first hour of ICU admission. The total SAPS-3 score predicts ICU mortality [Citation28]. When a patient had a documented ARDS risk factor at ICU admission, and all other criteria were fulfilled within the same 24-hour period, ARDS was defined as present. The mortality measure used was 30-day mortality after ICU admission.
Statistics
All analyses were performed using R, version 4.1.2 [Citation29].
Continuous variables were tested for normal distribution by inspecting histograms and Q-Q plots. The characteristics of the population were reported with frequencies for binary and categorical data. Continuous data were reported with the median and interquartile range. Differences in characteristics between the ABO blood groups were tested with Pearson’s Chi-squared test, Kruskal-Wallis rank-sum test, and Fisher’s exact test. Multiple testing correction was not undertaken, as the primary purpose of these analyses was to provide a descriptive overview, not to test specific hypotheses. The presence and development of ARDS were analysed using multivariable logistic regression adjusted for ABO blood group, Rh status, age, sex, smoking, and ARDS risk factors (pneumonia, aspiration, pulmonary contusion, lung vasculitis, non-pulmonary sepsis, non-cardiogenic shock, drug overdose, multiple blood transfusions, and major trauma). 30-day mortality was analysed in a multivariable logistic regression model adjusted for ABO blood group, Rh status, age, sex, smoking, CCI, and SAPS-3 score. A p-value <0.05 was considered significant.
Results
A total of 1591 patients were screened for ARDS. Of these, 9.5% lacked information on the ABO blood group and were excluded. Therefore, the study population consisted of 1440 patients. The characteristics of the study population are presented in . In the logistic regression models, 1439 patients were included, as one had missing data on the Rh blood group.
Table 1. Characteristics of the studied patient population and outcomes. Baseline characteristics include patient demographics, comorbidities, acute morbidity as SAPS-3 score, ARDS risk factors, and outcomes for the study population in total and subdivided by ABO blood groups. Values are presented as medians with interquartile ranges unless otherwise specified.
In the 151 patients with missing data on blood group and thus excluded from the analysis, 7.3% fulfilled the ARDS criteria. The median age was 62 years, and 32% were female. The median SAPS-3 score was 56, and 30- day mortality was 25%.
ABO blood group and ARDS development
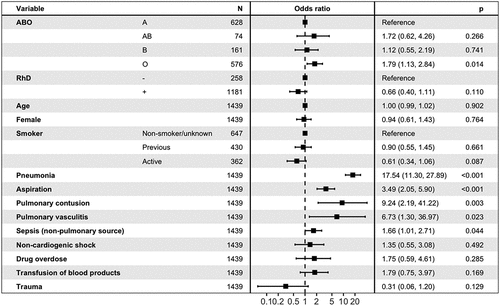
In multivariable logistic regression with blood group A as the reference and age, sex, smoking, and ARDS risk factors as independent variables, blood group O was associated with an increased risk of the presence or development of ARDS with an odds ratio (OR) of 1.79 (95% confidence interval [CI] 1.13–2.84, p = 0.014). Rh status was not associated with ARDS development. Due to very few observations, the ARDS risk factors inhalation injury, drowning, pancreatitis, and burn injury were not included as independent variables. See . Body mass index (BMI) was not included as 30% of the population had a missing BMI. BMI was not associated with ARDS development in bivariable logistic regression adjusted for age (p = 0.91).
ABO blood group and ARDS mortality
The outcomes for the subgroup with ARDS are presented in . For blood group and mortality analysis in ARDS, 140 patients were included. After adjusting for age, sex, smoking, CCI, and the SAPS-3 score, we found that blood group O was associated with lower mortality compared to blood group A with an OR of 0.25 (95% CI 0.09–0.67, p = 0.007). Rh status was not associated with ARDS mortality. See .
Figure 2. The association between blood groups, age, sex, smoking status, morbidity, and mortality in patients with acute respiratory distress syndrome (ARDS). Odds ratios and confidence intervals were calculated using multivariable logistic regression.
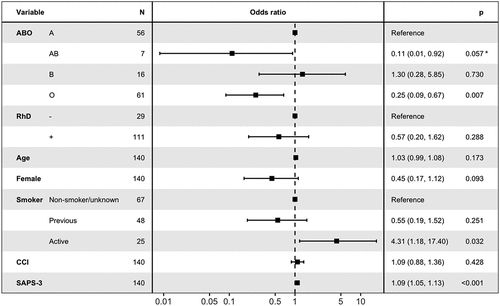
Table 2. Outcomes for the subgroup with ARDS. Values are presented as medians with interquartile ranges unless otherwise specified.
BMI was omitted as 33% of the ARDS subgroup had a missing BMI. BMI was not associated with mortality in ARDS in bivariable logistic regression adjusted for age (p = 1.0).
Discussion
In this retrospective study of 1439 ICU patients, we found that patients with blood group O had an increased risk of having or developing ARDS on ICU admission but a decreased mortality in ARDS compared to patients with blood group A. To our knowledge, this is the first study to report this specific association between ARDS and blood group O. In addition, we did not find any association between Rh status and ARDS development or mortality. ARDS mortality in this study (36.4%) was similar to previously reported mortality [Citation3].
Previous studies have shown that blood group A might be associated with an increased risk of ARDS compared to blood group O in Caucasian sepsis and trauma patients [Citation17,Citation18]. However, several factors could explain why this study found different results. Reilly et al. only studied ARDS following trauma and sepsis, whereas this study included a general ICU population and ARDS with other etiologies. We did not categorise patients based on race or study any race-related associations. Furthermore, the models were not adjusted for the same variables. Although a different patient population, a study from 2017 found that blood group O was associated with an increased risk of ARDS and respiratory failure in pregnant patients [Citation30].
Kander et al. found no differences in ARDS mortality among blood groups; however, blood group A was associated with increased ventilator time in ARDS, in accordance with increased ARDS mortality in blood group A compared to O in this study. Their study included a much larger cohort but with a less robust identification of ARDS compared to this study [Citation25].
In COVID-19, several studies have shown that blood group O has a decreased risk of severe illness but also a lower risk of infection overall [Citation11–15]. The contrasting results of this study may underscore that ABO blood groups have different effects in COVID-19-associated ARDS and non-COVID-19 ARDS.
The results of this study could have several explanations. Reilly et al. found that increased levels of vWF and thrombomodulin in plasma were associated with ARDS risk and that blood group A was associated with higher levels of vWF and thrombomodulin. They suggested that high levels of vWF and thrombomodulin, resulting in microvascular and coagulation dysfunction, might explain increased ARDS mortality in patients with blood group A [Citation18]. Increased plasma concentrations of thrombomodulin and vWF have been associated with ARDS mortality in other studies [Citation31,Citation32]. This might explain the higher ARDS mortality in blood group A compared to O in this study but does not offer a clear explanation of the increased ARDS prevalence in blood group O.
Similar to blood group O, blood group AB also had lower mortality in our results, although not significant. This finding is surprising since blood group AB has both A and B antigens, unlike blood group O, which has neither A nor B antigens. Furthermore, blood group B did not show any significant association or trend regarding ARDS development or mortality. Therefore, our results might implicate that the A and B antigens do not play a significant role in ARDS pathogenesis. In contrast with this study, Reilly et al. suggested a decreased ARDS risk for blood group AB [Citation18].
If the ABO effect on ARDS depends on the A antigen levels, it is logical that blood groups O and B should be associated with similar outcomes. However, we did not see such results in our study.
The increased presence and development of ARDS and decreased mortality seem contradictory. Our results indicate that blood group O has a higher rate of mild ARDS and a lower rate of severe ARDS compared to other ABO blood groups. Although not statistically significant, this may be part of the explanation.
A possible hypothesis could be host-pathogen interaction, considering the ABO blood group system’s connection to pathogen susceptibility [Citation33,Citation34]. Blood groups A, B, and AB may enhance pathogen or toxin adhesion, potentially increasing the severity of ARDS. Conversely, individuals with blood group O who lack these antigens may have decreased severity in ARDS due to reduced pathogen binding. However, they might produce anti-A or anti-B antibodies, contributing to pathogen clearance and enhancing the inflammatory response. Thus, blood group O individuals might face a higher risk of ARDS development due to increased inflammation but have improved outcomes due to better pathogen elimination, aligning with the study’s findings.
The lack of association between the Rh blood group and ARDS development and mortality is in line with previous studies, where Rh status did not seem to affect morbidity or mortality to the same extent as the ABO blood group [Citation25,Citation35].
An overrepresentation of peripheral vascular disease and diabetes mellitus with complications was shown in blood group AB. It is well established that non-O blood groups have a higher risk of vascular disease, including peripheral vascular disease [Citation7]. However, only a few studies have shown that blood group AB, particularly, would be associated with a higher risk of vascular disease than other non-O blood groups [Citation9,Citation36]. Diabetes mellitus has previously been linked to blood group B and AB [Citation37,Citation38].
In our study, active smoking was associated with increased mortality in ARDS but did not appear to influence the risk of developing ARDS. This contrasts with previous research, which have suggested that smoking is a risk factor for ARDS but is linked to lower ARDS mortality [Citation39,Citation40]. The absence of an association between active smoking and ARDS development in our study might be attributed to nicotine’s anti-inflammatory properties [Citation41]. Adjustment for confounding factors could account for the differing results in our study regarding the relationship between smoking and ARDS mortality compared to prior studies.
High BMI has previously been associated with an increased risk of ARDS but not with increased mortality in ARDS [Citation42]. BMI was not adjusted for in the multivariable models of this study, mainly due to missing values. This is a limitation. However, there was no difference in BMI between different ABO blood groups in the study population. Bivariable analysis, adjusted for age, did not show an association between BMI and ARDS development or mortality in ARDS.
As this study is retrospective, it comes with limitations such as insufficient documentation and the risk of different information interpretations by different data collectors. Some patients have more than one ICU admission and, therefore, occur as more than one case in the database. This circumstance could affect the outcomes. However, whether ARDS risk factors and the other ARDS criteria are present can change fast, and it is logical to see multiple admissions as different cases. The study population consists only of ICU patients. Thus, a selection of patients has already been made. If the ABO blood group affects morbidity and mortality in general, the ICU population might be disproportionate regarding blood group distribution, comorbidities, and risk factors. However, this is difficult to avoid in a retrospective study design. Furthermore, treatments that may influence ARDS risk factors, such as antibiotics and steroids, were not documented but could affect results.
The small number of ARDS patients in this study represents a significant limitation, leading to even smaller subgroups when divided according to blood group.
Moreover, it is important to acknowledge that variations in characteristics between the entire study cohort and the ARDS subgroup may impact the study results. Nonetheless, we have tried to mitigate this influence by meticulously adjusting for crucial demographic and clinical variables in the multivariable logistic regression analysis.
Our study has some specific strengths. The multicentre approach, with two ICUs in large university hospitals, applies the results to ICU populations in regions with demographics similar to Sweden´s. The collected data covers a broad range of parameters and patient characteristics, giving good knowledge of the studied patient population and the opportunity to correct potential confounders or mediators in the analysis. Moreover, the ARDS diagnostics in the study are robust and do not depend on a correct and documented diagnosis by the treating physician. Finally, data collection was systematically performed, following a well-defined protocol, resulting in a limited amount of missing data on important variables. In the analysis of ARDS development, mediators regarding ARDS risk factors were adjusted to isolate the blood group effect on ARDS development. Mortality analysis was adjusted for both chronic (CCI) and acute morbidity (SAPS-3). Even though age is included in both CCI and SAPS-3, we included it as a separate variable to sufficiently adjust for age as it has been shown that age is not sufficiently accounted for [Citation43].
Although blood groups are non-modifiable risk factors, studies like this are essential to investigate the role of blood groups in the pathogenesis of different diseases. In addition, the results of such studies can be applied in the search for potential treatment targets related to the blood group system. Furthermore, identifying subgroups with an increased risk of ARDS development or mortality can guide clinical surveillance and help predict ARDS prognosis. A better understanding of ARDS risk factors, including blood groups, helps us identify high-risk patients early and may potentially lead to more timely ICU admission and interventions. Future studies investigating the association of ARDS and blood groups in a prospective study setting would be of great value. For example, a larger study population would make it possible to study the ABO blood group effect in specific subgroups based on ARDS severity and different ARDS risk factors. In addition, studies on biomarkers associated with ABO blood group and ARDS are necessary to find the specific pathways through which ABO blood group affects ARDS development and outcome.
Conclusion
This study of ICU patients indicates a potential correlation between the ABO blood group and both development of ARDS and mortality in ARDS. Blood group O was associated with an increased risk of having or developing ARDS in the ICU but a decreased 30-day mortality in ARDS compared to blood group A. However, further studies are needed to clarify the relationship and underlying mechanisms of the ABO blood group and ARDS.
Abbreviations
ARDS: Acute respiratory distress syndrome; ICU: Intensive care unit; vWF: von Willebrand factor; SAPS-3: Simplified Acute Physiology Score 3; PASIVA: Patient Administrative System for Intensive Care Units; CCI: Charlson Comorbidity Index; OR: Odds ratio; CI: Confidence interval; BMI: Body mass index.
Author’s contributions
PJ and AF conceived and designed the study. AD collected data. HK, AD, and PJ performed the calculations and prepared the tables and figures. HK and AD wrote the draft manuscript. HK, PJ, and AF finalised the manuscript.
Ethics approval and consent to participate
This study was approved by the Regional Ethical Committee in Lund (2020–02395) and KVB, the local group handling the disclosure of personal information such as medical records.
Acknowledgments
We want to thank all staff at the ICUs of Skåne University Hospital in Lund and Malmö for contributing to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting this study’s findings are available on request from the corresponding author. However, the data are not publicly available due to privacy.
Additional information
Funding
References
- Thompson BT, Chambers RC, Liu KD, et al. Acute respiratory distress syndrome. N Engl J Med. 2017 Aug;377(6):562–9.
- Ranieri VM, Rubenfeld F, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012 Jun;307(23):2526–2533.
- Bellani F, Laffey J, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016 Feb;315(8):788–800.
- Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019, 02;25(1):12–20. doi: 10.1097/MCC.0000000000000571
- Storry JR, Olsson ML. The ABO blood group system revisited: a review and update. Immunohematology. 2009;25(2):48–59. doi: 10.21307/immunohematology-2019-231
- Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000 Jan;95(2):375–387. doi: 10.1182/blood.V95.2.375
- Wu O, Bayoumi N, Vickers MA, et al. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008 Jan;6(1):62–69.
- Wiggins KL, Smith NL, Fllazer NL, et al. ABO genotype and risk of thrombotic events and hemorrhagic stroke. J Thromb Haemost. 2009 Feb;7(2):263–269.
- He M, Wolpin B, Rexrode K, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2314–2320.
- Abegaz SB. Human ABO Blood groups and their associations with different diseases. Biomed Res Int. 2021;2021:6629060. doi: 10.1155/2021/6629060
- Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe covid-19 with respiratory failure. N Engl J Med. 2020 10;383(16):1522–1534. doi: 10.1056/NEJMoa2020283
- Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the coronavirus disease 2019 (COVID-19) susceptibility. Clin Infect Dis. 2021 07;73(2):328–331. doi: 10.1093/cid/ciaa1150
- Hultström M, Persson B, Eriksson O, et al. Blood type a associates with critical COVID-19 and death in a Swedish cohort. Crit Care. 2020 08;24(1):496. doi: 10.1186/s13054-020-03223-8
- Li J, Wang X, Chen J, et al. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br J Haematol. 2020 07;190(1):24–27. doi: 10.1111/bjh.16797
- Ray JG, Schull MJ, Vermeulen MJ, et al. Association Between ABO and Rh Blood groups and SARS-CoV-2 infection or severe COVID-19 illness: a population-based cohort study. Ann Intern Med. 2021 Mar;174(3):308–315. doi: 10.7326/M20-4511
- Su S, Guo, Fluo L T, et al. Association of ABO blood group with respiratory diseases hospitalisation and severe outcomes: a retrospective cohort study in blood donors. Int J Infect Dis. 2022 May;122:21–29. doi: 10.1016/j.ijid.2022.05.019
- Reilly JP, Meyer NJ, MflS S, et al. ABO blood type a is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest. 2014 Apr;145(4):753–761.
- Reilly JP, Meyer NJ, Mfl S, et al. The ABO histo-blood group, endothelial activation, and acute respiratory distress syndrome risk in critical illness. J Clin Invest. 2021 01;131(1). doi: 10.1172/JCI139700
- Itenov TS, Sessler DI, Khanna AK, et al. ABO blood types and sepsis mortality. Ann Intensive Care. 2021 Apr;11(1):61.
- Hasegawa D, Nishida K, Kawaji T, et al. Impact of blood type O on mortality of sepsis patients: a multicenter retrospective observational study. Diagnostics (Basel). 2020 Oct;10(10):826.
- Griffin RL, Jansen JO, Bosarge PL, et al. The association between ABO blood type and mortality among severely injured trauma patients. Shock. 2020 Aug;54(2):205–208. doi: 10.1097/SHK.0000000000001497
- Takayama W, Endo A, Koguchi H, et al. The impact of blood type O on mortality of severe trauma patients: a retrospective observational study. Crit Care. 2018 05;22(1):100. doi: 10.1186/s13054-018-2022-0
- Takayama W, Endo A, Murata K, et al. The impact of blood type on the mortality of patients with severe abdominal trauma: a multicenter observational study. Sci Rep. 2021 08;11(1):16147. doi: 10.1038/s41598-021-95443-3
- Rezoagli E, Flatti S, Villa S, et al. ABO blood types and major outcomes in patients with acute hypoxaemic respiratory failure: a multicenter retrospective cohort study. PloS One. 2018;13(10):e0206403. doi: 10.1371/journal.pone.0206403
- Kander T, Bjurström MF, Frigyesi A, et al. ABO and RhD blood group are not associated with mortality and morbidity in critically ill patients; a multicentre observational study of 29 512 patients. BMC Anesthesiol. 2022 Apr;22(1):91. doi: 10.1186/s12871-022-01626-4
- Urbaniak SJ, Flreiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000 Mar;14(1):44–61. doi: 10.1054/blre.1999.0123
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011 Mar;173(6):676–682.
- Moreno RP, Pfl M, Almeida E, et al. SAPS 3–from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive care Med. 2005 Oct;31(10):1345–1355.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria; 2022. https://www.R-project.org/
- Blazer A, Mitsakakis N, Lapinsky S. ABO blood type association with respiratory failure in pregnancy. Chest. 2017 Oct;152(4):A374. doi: 10.1016/j.chest.2017.08.400
- Ware LB, Eisner MD, Thompson BT, et al. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004 Oct;170(7):766–772.
- Sapru A, Calfee CS, Liu KD, et al. Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive care Med. 2015 Mar;41(3):470–478.
- Cooling L. Blood groups in infection and Host susceptibility. Clin Microbiol Rev. 2015 Jul;28(3):801–870. doi: 10.1128/CMR.00109-14
- Zhong X, Wang DL, Xiao LH, et al. ABO blood groups and nosocomial infection. Epidemiol Infect. 2023 Apr;151:e64. doi: 10.1017/S0950268823000432
- Dahlén T, Clements M, Zhao J, et al. An agnostic study of associations between ABO and RhD blood group and phenome-wide disease risk. Elife. 2021 04;10.doi: 10.7554/eLife.65658
- Blais C, Flermain M, Delage F, et al. The association between blood group and the risk of vascular disease in Quebec blood donors. Blood Transfus. 2016, 09;14(5):455–459. doi: 10.2450/2016.0303-15
- Meo SA, Rouq FA, Suraya F, et al. Association of ABO and Rh blood groups with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2016;20(2):237–242.
- Kamil M, Al-Jamal HA, Yusoff NM. Association of ABO blood groups with diabetes mellitus. Libyan J Med. 2010 Feb 5;5(1):4847.
- Moazed F, Hendrickson C, Jauregui A, et al. Cigarette Smoke Exposure and Acute Respiratory Distress Syndrome in Sepsis: Epidemiology, Clinical Features, and Biologic Markers. Am J Respir Crit Care Med. 2022 Apr;205(8):927–935.
- Calfee CS, Matthay MA, Kangelaris KN, et al. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med. 2015 Sep;43(9):1790–1797.
- Zhang W, Lin H, Zou M, et al. Nicotine in inflammatory diseases: anti-inflammatory and pro-inflammatory effects. Front Immunol. 2022;13:826889. doi: 10.3389/fimmu.2022.826889
- De Jong A, Verzilli D, Jaber S. ARDS in obese patients: specificities and management. Crit Care. 2019 Mar;23(1):74. doi: 10.1186/s13054-019-2374-0
- Holmgren F, Andersson P, Jakobsson A, et al. Artificial neural networks improve and simplify intensive care mortality prognostication: a national cohort study of 217,289 first-time intensive care unit admissions. J Intensive Care. 2019;7(1):44. doi: 10.1186/s40560-019-0393-1