ABSTRACT
Background
In patients with recurrent pleural effusion, therapeutic thoracentesis is one way of relief. Correct prediction of which patients will experience relief following drainage may support the management of these patients. This study aimed to assess the association between ultrasound (US) characteristics and a relevant improvement in dyspnoea immediately following drainage.
Methods
In a prospective, observational study, patients with recurrent unilateral pleural effusion underwent US evaluation of effusion characteristics and diaphragm movement measured by M-mode and the Area method before and right after drainage. The level of dyspnoea was assessed using the modified Borg scale (MBS). A minimal important improvement in dyspnoea was defined as delta MBS ≥ 1.
Results
In the 104 patients included, 53% had a minimal important improvement in dyspnoea following thoracentesis. We found no association between US-characteristics, including diaphragm shape or movement (M-mode or the Area method), and a decrease in dyspnoea following drainage. Baseline MBS score ≥ 4 and a fully drained effusion were significant correlated with a minimal important improvement in dyspnoea (OR 3.86 (1.42–10.50), p = 0.01 and 2.86 (1.03–7.93), p = 0.04, respectively).
Conclusions
In our study population, US-characteristics including assessment of diaphragm movement or shape was not associated with a minimal important improvement in dyspnoea immediately following thoracentesis.
Introduction
In patients with recurrent pleural effusion, dyspnoea is the main symptom [Citation1]. Therapeutic thoracentesis relieves dyspnoea in most, but not all patients [Citation2]. Patients with recurrent effusions who experience symptom relief following drainage may be considered for a definitive pleural treatment such as indwelling pleural catheter (IPC) or talc pleurodesis [Citation1,Citation3]. Thus, identifying which patients are likely to benefit from therapeutic thoracentesis may avoid superfluous drainages, including the patient risks and hospital costs, and may be suggestive of symptom relief following IPC and pleurodesis.
Previous studies have identified characteristics associated with a decrease in dyspnoea following thoracentesis including a larger volume drained [Citation2], less than five septations [Citation2], a higher level of dyspnoea at baseline [Citation4,Citation5], diaphragm shape or movement [Citation4,Citation6,Citation7], benign aetiology [Citation4] and high pleural pH [Citation4]. However, the findings are inconsistent [Citation2,Citation4–6], and some studies are limited by evaluating the effect of a combination of various pleural procedures [Citation2,Citation4]. Also, recurrent pleural effusions often have several causes but little is known about the impact of dyspnoea-associated comorbidities [Citation8,Citation9].
Impaired diaphragm movement is considered a key pathophysiological mechanism in effusion-related dyspnoea [Citation4,Citation6,Citation10–14]. Ultrasound (US) evaluation of diaphragm movement is often performed using the M-mode function or by simple ‘eyeballing’. The latter is easily performed, but imprecise and poorly validated concerning therapeutic thoracentesis [Citation4]. A novel alternative is the Area method, measuring the intrathoracic area above the diaphragm. The Area method has been shown to be feasible and accurate in assessing diaphragm movement in healthy individuals [Citation15] and to be associated with a relevant improvement in dyspnoea following therapeutic thoracentesis in a single-center study with 32 selected patients without pleural adhesions or evidence of trapped lung at imaging [Citation6]. However, pleural adhesions and trapped lung are common features of malignant pleural effusion [Citation16].
On this background, we set out to explore the association between US characteristics including diaphragm movement and change in dyspnoea immediately following thoracentesis in unselected patients with recurrent unilateral pleural effusion.
Materials and methods
We conducted a prospective, observational study of patients with unilateral pleural effusion and need of repeated therapeutic thoracentesis. Participants gave their informed consent and were recruited from the outpatient clinics of the Department of Respiratory Medicine, Zealand University Hospital, Naestved and Roskilde, Denmark.
The inclusion criteria were age ≥18 years, unilateral pleural effusion, need of therapeutic thoracentesis, minimum two previous thoracenteses and the ability to give informed consent. We collected data on basic demographics, smoking status, cause of pleural effusion, co-morbidities possibly contributing to dyspnoea and volume drained. All participants underwent a US evaluation of both hemi thoraces before and after drainage.
Ultrasound examinations
Two experienced and certified US operators (KF and JKP) performed all US examinations and thoracenteses after undergoing specific bedside training in the Area method.
At Zealand University Hospital, Naestved, Thoracic US (TUS) assessments were performed using LOGIQ S8 (GE Healthcare Wauwatosa, USA) and a C1–5 curved abdominal transducer (2–5 MHz), abdominal preset. At Zealand University Hospital, Roskilde, TUS was performed using an ALOKA ARIETTA V60 (Hitachi, Tokyo, Japan) and a C42 micro-convex transducer (4–8 MHz), liver preset. All US film clips were recorded for further analysis. The patient was placed in an erect position. TUS included an assessment of diaphragm shape (domed, flat, inverse), diaphragm movement (assessed by M-mode [Citation17] and Area method [Citation15]), effusion size (small: < 1/3 of hemi thorax, moderate: 2/3<>1/3 of hemi thorax and large > 2/3 of hemi thorax), echogenicity (simple, complex non-septated, complex septated, homogenously echogenic [Citation18]), septa score (number of septations visible in a single US field at the area of maximum septations: no septations, 1–2, 3–4, >5) [Citation2], swirling [Citation19] (present, non-present) and suspected trapped lung based on simple visual estimation of obtained images (e.g. impaired movement of the underlying lung). After ended drainage, the diaphragm was relocated and diaphragm shape and movement were reassessed, and it was evaluated whether the effusion was fully drained (if less than 0.1 liter pleural fluid remaining assessed by visual estimation).
Diaphragm movement assessment
The patients were instructed to breathe as calmly and unforced as possible during a US assessment of the diaphragm movement.
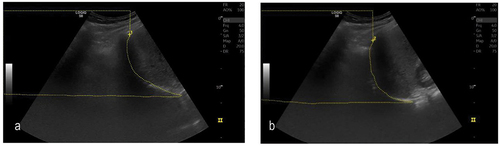
Area method was performed as previously described by Skaarup et al. [Citation15]. In a lateral view in the mid-axillary line, the diaphragm dome was visualised, and a film clip of a respiration was saved. The image frame of maximal inspiration and expiration was identified. The area above the diaphragm dome was measured by tracing the entire visual part of the diaphragm and using the area-calculation function. The change in intrathoracic area during respiration was measured by subtracting the area at maximal diaphragm contraction from the area at minimal diaphragm contraction, see
Figure 1. Diaphragm movement during respiration measured by the area method: (a) Full inspiration, maximal area over the diaphragm; (b) Full expiration, minimal area over the diaphragm.
M-Mode was performed using a subcostal view in the mid-clavicular line as previously described [Citation15,Citation17,Citation20,Citation21]. The M-mode line was placed on the part of the diaphragm with the largest movement. The amplitude during breathing was measured in millimetres.
Dichotomised M-mode was constructed as a post hoc variable (‘normal’ = M-mode ≥1.0 cm and ‘abnormal’ = M-mode <1.0 cm) with cut-off based on a previous study [Citation20] since simple visual estimation was not a pre-specified parameter in our protocol.
Thoracentesis
KF and JP performed all thoracentesis. US guided, using sterile technique and under local anaesthesia, a 7 French (or up to 16 French, to the choice of the clinician) pigtail catheter was inserted and connected to a sealed bag. To prevent symptoms caused by fast lung expansion, drainage was paused for 10 minutes after drainage of every liter or whenever symptoms started to appear. There was no standard minimum or maximum volume drained as drainage was guided by patient symptoms. Reasons for leaving residual fluid were symptoms of trapped lung (cough and chest pain) and no flow despite flushing.
Patient reported dyspnoea
On the day of inclusion, before thoracentesis all participants completed a questionnaire including the modified Borg 0–10 Scale [Citation22] (MBS) and the Medical Research Council (MRC) breathlessness scale [Citation23] of dyspnoea. Ten minutes after ended drainage, before the patients were mobilised, MBS was repeated. An improvement of minimal importance was defined as a decrease of ≥ 1 on MBS [Citation24], identifying the patient as a responder. Patients were not blinded to the amount of fluid removed.
Outcomes
Primary outcome: correlation between diaphragm movement measured by the Area method and an improvement in dyspnoea of minimal importance immediately following therapeutic thoracentesis.
Secondary outcomes: a) as above, but with M-mode and visual estimation as US method, b) correlation between earlier reported clinical and baseline US factors and relief in dyspnoea after thoracentesis, c) change in diaphragm shape and movement following thoracentesis.
Statistical considerations
This study was a part of a larger study (clinical.trial.gov, ID: 19–000067); hence, the original power-calculation was not concerning the research questions of this paper. Categorical data was described as number (n) and percentage (%) and continuous variables as median and range/IQR or mean (SD) if normally distributed. Differences between paired observations were analysed using paired t-test and McNemar’s test for continuous variables and Wilcoxen signed rank test for categorical observations. Differences between unpaired observations were analysed using t-test for continuous variables and Chi-squared or Fisher’s exact test for categorical variables. Correlations concerning characteristics associated with being a responder were analysed using uni- and multivariate logistic regression analysis. Variables included in the univariate analyses were chosen a priory. The multivariate regression analysis included variables with p < 0.05 in the univariate analysis. No stepwise forward or backward selection was performed. MBS and MRC as predicting variables were dichotomised, since some categories contained very few patients. A cut-off of ≥ 4 was chosen to differentiate between moderate and severe dyspnoea, see Appendix A. Post hoc analysis on patients with non-domed diaphragm shape and not fully drained effusions, respectively, was performed to explore and explain unexpected results. Missing data was considered missing at random and p-values <0.05 considered the level of significance. All statistics were performed using STATA/BE 17 (Texas, US).
Results
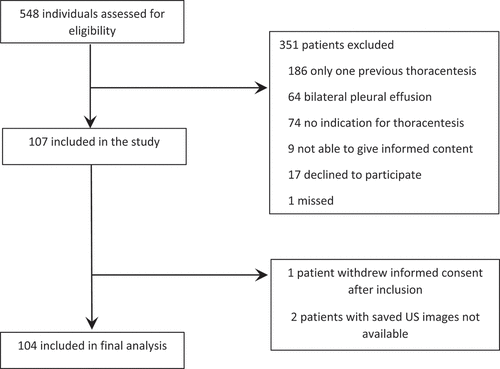
Between January 2020 and December 2021, 104 individual patients were included by screening 548 individual patients, see Patient characteristics are shown in . The mean age was 73.3, and the majority were male (60/104 (58%)). Most patients had malignant pleural effusion (75/104 (72%)), and 50/104 (48%) had at least one comorbidity known to cause dyspnoea (specifications are shown in Appendix B. Median MRC was 4 (IQR 2–5). The mean volume drained was 1313 ml (SD 668), and 46% (48/104) of the effusions were fully drained. The median MBS was 3 range (0–10) before drainage and 2(0–10) after ended drainage. Overall, patients experienced a relevant reduction in dyspnoea measured by MBS (median reduction 1.0 (IQR 0.0–3.0, p < 0.001), see ), and 55/104 (53%) met the criteria of being responders.
Figure 3. Box plot showing patient perceived dyspnoea before and after thoracentesis measured by modified Borg scale (MBS). Wilcoxen signed rank test for p-values.
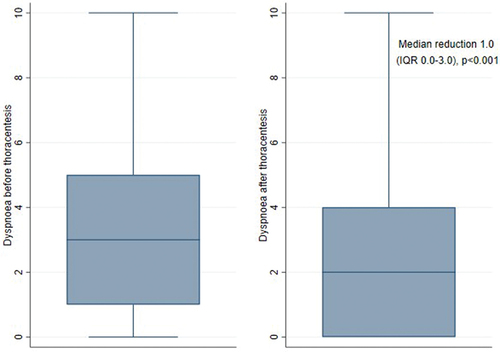
Table 1. Baseline characteristics of included patients.
Characteristics associated with a being a responder
As presented in , factors associated with being a responder in univariate analysis included baseline MRC ≥ 4 (OR 2.47 (1.05–5.78), p = 0.04, baseline MBS score ≥ 4 (OR 4.15 (1.81–9.55), p < 0.001) and a fully drained effusion (OR 2.43 (1.10–5.38), p = 0.03). Non-domed diaphragm shape at baseline and normalisation of the diaphragm shape following drainage was associated with not being a responder immediately after drainage (OR 0.36 (0.14–0.90), p = 0.03 and 0.36 (0.13–1.00), p = 0.05 respectively). In multivariate analysis, baseline MBS score ≥ 4 and a fully drained effusion were associated with being a responder (OR 3.86 (1.42–10.50), p = 0.01 and 2.86 (1.03–7.93), p = 0.04, respectively). We found no association between baseline diaphragm movement, measured by the Area method, M-mode, or dichotomised M-mode and being a responder. Likewise, no other US findings including septations, swirling or signs of trapped lung were correlated with being a responder, see .
Table 2. Correlation between different characteristics and having a clinical important improvement in dyspnoea measured by modified Borg scale (MBS), defined as a drop ≥ 1.
Post hoc analysis showed that patients with non-domed diaphragm shape at baseline had a significantly higher proportion of large effusions and of suspected trapped lung, a larger volume drained and a smaller proportion of fully drained effusions, see Appendix D.
Patients with not fully drained effusions had a higher proportion of suspected trapped lung and a lower proportion of initial responders see Appendix E.
Besides a higher proportion of non-domed diaphragm shape at baseline and a smaller proportion of fully drain effusions, patients with signs of suspected trapped lung also had larger effusions and a higher proportion of non-simple effusions and septated effusions, see Appendix F. Diaphragm movement before drainage was significantly more often impaired, when measured by dichotomised M-mode, not by the Area-method, see Appendix F.
Changes in diaphragm shape following thoracentesis
At initial US assessment, 26/104 (26%) had flat or inversed diaphragm shape, see . Of these, 21/26 (81%) became domed following drainage. The diaphragm was not visible in one patient due to thorax deformity and pneumothorax.
Table 3. Diaphragm shape before and after thoracentesis. N = 104.
Changes in diaphragm movement following thoracentesis
At the side of effusion, diaphragm movement increased significantly following thoracentesis, both measured by M-mode (mean (SD) 1.3 cm (0.8) vs 1.6 cm (1.0), mean delta = 0.3 cm (SD 1.0), p-value = 0.004) and the Area method (mean (SD) 8.6 cm2 (6.2) vs 14.6 cm2 (9.7), mean delta = 6.5 cm2 (SD 9.4), p < 0.001), see . At the opposing side, there was no significant change in diaphragm movement neither by M-mode (mean delta = −0.02 cm (SD 1.3), p = 0.91) nor the Area method (mean delta = −0.3 cm2 (SD 8.7), p = 0.79).
Table 4. Diaphragm movement pre and post thoracentesis assessed by M-mode and area method. N = 104.
Discussion
In this study, we found baseline level of dyspnoea to be associated with a minimal relevant reduction in dyspnoea. This is in line with previous studies [Citation4,Citation5]. In addition, a fully drained effusion was associated with being a responder. We found no correlation between US assessed diaphragm shape or movement or any other US findings and improvement in dyspnoea immediately following therapeutic thoracentesis. Hence, we could not identify any US characteristics which prior to thoracentesis enabled prediction of relief in dyspnoea following drainage.
Our findings concerning no predictive effect of diaphragm shape were unexpected since previous studies have shown this correlation [Citation4,Citation7]. Perhaps, patients with non-domed shape, having the largest volumes of fluid to start with, still had a significant volume remaining after thoracentesis, thus needing further drainage to fully respond, though their diaphragm shape was normalised.
Our quite high proportion of not fully drained effusions (56/104 (54%)) may be explained by a higher proportion of trapped lung in this group.
Skaarup et al. has previously found a clear correlation between improvement in dyspnoea immediately following thoracentesis and initial diaphragm movement, measured by the Area method (n = 32). Compared to Skaarup et al., we found a smaller increase in diaphragm movement on the side of effusion following thoracentesis (mean 6.5 cm2 (SD 9.4) vs. mean 18.6 cm2). Since our measurements by the Area method on the unaffected side are comparable (before thoracentesis 27.8 cm2 (SD 10.3) vs 26.3 cm2 (95% CI 21–31.63), after thoracentesis 24.6 cm2 (SD 10.7) vs 27.9 cm2 (95% CI 20.35–35.47)) [Citation6], there is no indication of differences in US techniques. Compared to our study, Skaarup et al. found a higher decrease in dyspnoea measured by MBS (median 2.5 vs. 1.0) and a lower proportion of non-responders measured by MBS (15.6% vs 47%). A possible explanation is the higher level of baseline dyspnoea (MBS mean (SD) 5.6 ± 2.5 vs. 3.3 ± 2.5). Also, these differences may be caused by distinct differences in the study populations, as Skaarup et al. excluded patients with pleural adhesions and trapped lung. Thus, our study population is likely to have more pronounced modifications in their pleural effusion, e.g. pleural thickening, trapped lung and septations, possible influencing the diaphragm movement in addition to the pleural fluid. The dyspnoea in our study population may be explained by other factors than restored diaphragm movement, e.g. re-expansion of the lung tissue, the underlying cause of the pleural effusion or comorbidity.
A large and comprehensive study by Muruganandan et al. [Citation4] including 145 patients found clinically relevant relief in dyspnoea measured 24–36 hours after thoracentesis to be associated with ‘abnormal, paralysed or paradoxical’ in contrast to ‘normal or reduced’ diaphragm movement prior to thoracentesis [Citation4]. We were not able to confirm this finding using the more sophisticated Area method for assessment of diaphragm movement or on dichotomised M-mode measures. A possible explanation is the difference in time of post drainage evaluation. In addition, there are certain differences in the study populations. Our population had a lower level of baseline dyspnoea (VAS mean (SD) 4.1 ± 3.0 vs. 5.6 ± 2.1, 10 being the worse) and a lower proportion of responders (55/104 (53%) vs. 106/145 (73%)). We included patients with effusions smaller than 500 ml (11/106 (10%)) and fewer patients with initial abnormal diaphragm shape (26/104 (25%) vs. 72/145 (50%)). As mentioned above, our study population may have other considerable causes of relief in dyspnoea following thoracentesis besides improved diaphragm movement.
Certain limitations specific to our study must be acknowledged. First, no dedicated calculation of sample size was performed regarding the primary outcome of this paper. Thus, there is a risk of type II errors. Also, we did not correct for multiple imputations despite a rather small sample size and large amount of analysis. This was chosen since it was an exploratory study, and we would risk overcorrection of type 1 errors. Second, we did not evaluate inter or intra-observer variability, as this have previously shown to be low using the Area method [Citation15]. Third, measurements of physiological parameters (e.g. respiratory rate or peripheral oxygenation), functional capacity, and lung volumes were not included, since previous studies have shown limited changes shortly after drainage [Citation6,Citation7,Citation10,Citation11]. Fourth, since some studies suggest the maximal effect of pleural fluid drainage occur one to two days after thoracentesis [Citation5,Citation13,Citation25], one could argue our measurement of dyspnoea after drainage were performed too early. In such case, we systematically underestimate the number of responders, and risk to neglect a true relationship between, e.g. dyspnea relief and diaphragm movement. However, we would expect to observe a trend towards such an association, which we did not (p-values >0.70). Fifth, our study exclusively included patients with recurrent need of therapeutic thoracentesis, who may have more pronounced pleural changes (e.g. pleural thickening, trapped lung and septations [Citation26]) than the average patient presenting with unilateral pleural effusion. Sixth, our definition of suspected trapped lung was based on subjective visual estimation by the examiner with no use of more objective measurements. Last, we chose to define a responder by a clinical important improvement in dyspnoea measured by MBS, even though MBS have not been validated in patients with pleural effusion.
Patients with recurrent pleural effusion and symptom relief following thoracentesis should be considered for definitive pleural treatment [Citation3,Citation16]. Early prediction of who will benefit clinically significant from thoracentesis would enable patients and clinicians to proceed directly to definitive pleural treatments and thus reduce number of pleural interventions and hospital visits [Citation27]. Our study found that US findings are not reliable to predict a clinical important relief in dyspnoea following therapeutic thoracentesis in unselected patients with recurrent pleural effusions and should as such be used with caution.
Conclusions
In patients with large pleural effusions, no ultrasound characteristics (e.g. diaphragm shape or movement) reliably identified patients with dyspnoea relief following therapeutic thoracentesis. Our findings address the complexity of dyspnea and do not question the crucial role of thoracic ultrasound in the diagnosis and management of pleural disease.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Appendix.docx
Download MS Word (19.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2024.2337446
Additional information
Funding
References
- Roberts ME, Rahman NM, Maskell NA, et al. British thoracic society guideline for pleural disease. Thorax. 2023 Jul;78(Suppl 3):s1–12.
- Psallidas I, Yousuf A, Talwar A, et al. Assessment of patient-reported outcome measures in pleural interventions. BMJ Open Respir Res [Internet]. 2017 Jul 1 [cited 2021 Feb 3];4(1):e000171. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28883922
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J [Internet]. 2018;52(1):1–23. doi: 10.1183/13993003.00349-2018
- Muruganandan S, Azzopardi M, Thomas R, et al. The pleural effusion and symptom evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion. Eur Respir J [Internet]. 2020;55(5):1900980. doi: 10.1183/13993003.00980-2019
- Boshuizen RC, Vincent AD, van den Heuvel MM. Comparison of modified Borg scale and visual analog scale dyspnea scores in predicting re-intervention after drainage of malignant pleural effusion. Support Care Cancer [Internet]. 2013;21(11):3109–3116. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23842597
- Skaarup SH, Lonni S, Quadri F, et al. Ultrasound evaluation of hemidiaphragm function following thoracentesis: a study on mechanisms of dyspnea related to pleural effusion. J Bronchol Interv Pulmonol [Internet]. 2019;27(3):172–178. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31651544
- Wang L, Cherng J, Wang J. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology. 2007;12(5):719–723. doi: 10.1111/j.1440-1843.2007.01124.x
- Walker S, Maskell N. Identification and management of pleural effusions of multiple aetiologies. Curr Opin Pulm Med. 2017 Jul;23(4):339–345. doi: 10.1097/MCP.0000000000000388
- Bintcliffe OJ, Hooper CE, Rider IJ, et al. Unilateral pleural effusions with more than one apparent etiology. A prospective observational study. Ann Am Thorac Soc. 2016 Jul;13(7):1050–1056
- Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following Thoracocentesis. Chest. 1978 Nov 1;74(5):540–542. doi: 10.1378/chest.74.5.540
- Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med. 1983 May 1;74(5):813–819. doi: 10.1016/0002-9343(83)91072-0
- Karetzky MS, Kothari GA, Fourre JA, Khan AU. Effect of thoracentesis on arterial oxygen tension. Respiration [Internet]. 1978 [cited 2021 Dec 23];36(2):96–103. Available from https://www.karger.com/Article/FullText/193932
- Perpina M, Benlloch E, Marco V, et al. Effect of thoracentesis on pulmonary gas exchange from the ciudad. Thorax [Internet]. 1983;38(10):747–750. Available from: http://thorax.bmj.com/
- Wang JS, Tseng CH. Changes in pulmonary mechanics and gas exchange after thoracentesis on patients with inversion of a hemidiaphragm secondary to large pleural effusion. Chest. 1995 Jun 1;107(6):1610–1614. doi: 10.1378/chest.107.6.1610
- Skaarup SH, Lokke A, Laursen CB. The area method: a new method for ultrasound assessment of diaphragmatic movement. Crit Ultrasound J [Internet]. 2018;10(1):15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29946769
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med [Internet]. 2018;198(7):839–849. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30272503
- Houston JG, Cowan MD, McMillan NC, et al. Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax. 1994;49(5):500–503. doi: 10.1136/thx.49.5.500
- Yang PC, Luh KT, Chang DB, et al. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. Am J Roentgenol. 1992;159(1):29–33. doi: 10.2214/ajr.159.1.1609716
- Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration [Internet]. 2014;87(4):270–278. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24480900
- Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by M-Mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009 Feb 1;135(2):391–400. doi: 10.1378/chest.08-1541
- Parreira F, Machado R, Tomás T, et al. A reliable M- mode ultrasound protocol for the assessment of diaphragm mo7on. Chest World conference in Madrid, Spain, 2014 Mar 1–24. 2012;37(1):5896606.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc [Internet]. 1982;14(5):377–381. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7154893
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581
- Oxberry SG, Bland JM, Clark AL, et al. Minimally clinically important difference in chronic breathlessness: every little helps. Am Heart J. 2012 Aug 1;164(2):229–235. doi: 10.1016/j.ahj.2012.05.003
- Brandstetter RD, Cohen RP. Hypoxemia after Thoracentesis: a predictable and treatable condition. JAMA [Internet]. 1979 Sep 7;242(10):1060–1061. doi: 10.1001/jama.1979.03300100038019. Available from
- Chung C-L, Chen Y-C, Chang S-C. Effect of repeated thoracenteses on fluid characteristics, cytokines, and fibrinolytic activity in malignant pleural effusion. Chest [Internet]. 2003 Apr;123(4):1188–1195. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12684310
- Evison M, Blyth KG, Bhatnagar R, et al. Providing safe and effective pleural medicine services in the UK: an aspirational statement from UK pleural physicians. BMJ Open Respir Res. 2018;5(1):e000307. doi: 10.1136/bmjresp-2018-000307
Appendix
A
Table A. Modified Borg Scale (MBS).
Appendix
B
Specification of comorbidities associated with dyspnoea, n = 50
Appendix
C
Specification of aetiology of the pleural effusion, n = 104
Appendix
D
Selected variables by completion of drainage (fully drained vs. not fully drained)
Appendix
E
Selected variables by completion of drainage (fully drained vs. not fully drained)
Appendix
F
Selected variables by signs of trapped lung (n = 104)