ABSTRACT
Introduction
Pulmonary lymphangitis carcinomatosa is a rare and severe manifestation of metastatic disease that causes pulmonary symptoms and radiologic patterns similar to interstitial lung diseases.
Case presentation
We report a case of a 78-year-old woman who presented to our department with insidiously developed symptoms of fatigue, dry cough, and severe dyspnea for 3 months. Chest radiography showed bilateral interstitial changes. On suspicion of interstitial lung disease, bronchoscopy and transbronchial cryobiopsy were carried out. Surprisingly, histopathological investigation revealed pulmonary lymphangitis carcinomatosa originating from primary breast adenocarcinoma.
Conclusion
To achieve an accurate diagnosis and prevent delay of initiation of proper treatment a thorough diagnostic approach is necessary. In case of doubt, biopsy should be performed to secure clarification. In this case report we discuss the diagnostic value of transbroncial cryobiopsy for this purpose.
Introduction
Diffuse interstitial lung diseases (ILD) are a large heterogeneous group of diseases all affecting the interstitial structures. The aetiology of the diseases is different, and, furthermore, a broad range of differential diagnoses should be considered including heart failure, infections, pulmonary metastasis, and lymphangitis carcinomatosa. All of these can cause diagnostic challenges and necessitate a thorough investigation [Citation1].
Pulmonary lymphangitis carcinomatosa (PLC) represents end-stage manifestation of metastatic malignancy with infiltration of the lymphatic vessels. In the late stage of PLC, classical radiological changes are nodular thickening of interlobular septa. PLC is present in 6–8% of patients with lung metastases [Citation2]. A recent review investigating case reports of PLC from 1970 to 2018 reveals that 80% of primary adenocarcinomas causing PLC are located in breast, lung, stomach, or pancreas [Citation3]. This case report elucidates the diagnostic challenges associated with this rare and severe manifestation of metastatic breast adenocarcinoma.
Case presentation
A 78-year-old woman was referred on the suspicion of an ILD due to a 3-month history of insidious symptoms of fatigue, dry cough, and severe dyspnoea corresponding to Medical Research Council dyspnoea score of 4. There were no extrapulmonary symptoms suggestive of connecting tissue disease or sarcoidosis. Until the onset of symptoms, her performance status was 1. She was a never smoker and only received medical treatment for hypertension and hypercholesterolaemia. Her family history was unremarkable, and there was no domestic or environmental exposure relevant for ILD. Physical examination only revealed bibasilar crackles.
Investigations
At the first outpatient appointment at Centre for Rare Lung Diseases, Department of Respiratory Diseases and Allergy, Aarhus University Hospital, blood results showed normal leukocytes with slight eosinophilia (0.62). IgM rheumatoid factor, anti-cyclic citrullinated peptide, antinuclear antibodies, and antineutrophil cytoplasmic antibodies were negative.
Lung function indicated a slightly obstructive pattern, and plethysmography showed normal lung volumes. Diffusing capacity for carbon monoxide was 60%.
A 6-minute walk test was performed. She had to stop after 2 min due to coughing and dyspnoea. Walking distance was 133 m. There was only a marginal rise in heart rate, and no desaturation was observed.
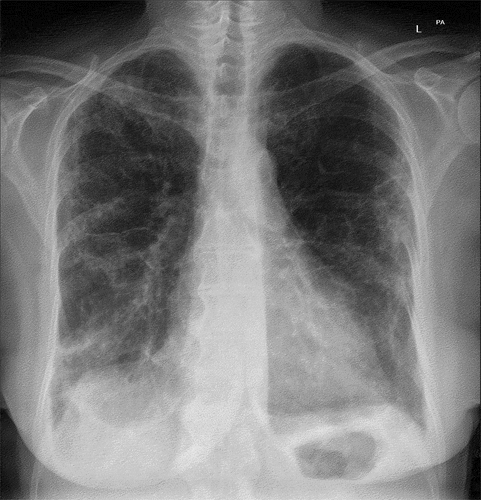
Chest X-ray showed bilateral scattered interstitial consolidations ().
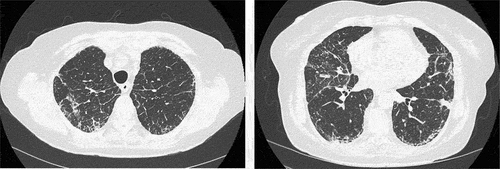
High-resolution computed tomography (HRCT) clarified interstitial changes with irregular reticulation and basal traction bronchiectasis as well as subpleural consolidation (). There was no lymphadenopathy in the hilar, mediastinal, or axillar regions. Cryptogenic organizing pneumonia, chronic eosinophilic pneumonia, and non-specific interstitial pneumonia were considered at a multidisciplinary meeting consisting of a thoracic radiologist, pulmonologist, and pathologists. Bronchoscopy followed by bronchoalveolar lavage (BAL) and transbronchial cryobiopsy (TBCB) were recommended for further investigations.
Figure 2. High-resolution computed tomography revealed interstitial changes with irregular reticulation (left) and basal traction bronchiectasis and subpleural consolidation (right).
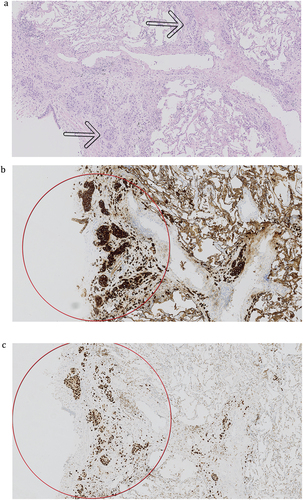
The BAL fluid was normal except for the finding of atypical cells with large, pleomorphic nuclei. TBCB revealed lymphangitis carcinomatosa. Immunohistochemically, the tumour cells were positive for cytokeratin 7 (CK7), GATA3, and mammaglobin A and sporadic for GCDFP, while they were negative for estrogen receptor, thyroid transcription factor 1 (TTF-1), napsin A, p40, and cytokeratin 5/6. The immune profile was consistent with metastatic primary breast adenocarcinoma (). As the next step, a mammography was performed where a 31 mm area was found in the left breast, and breast biopsy confirmed the diagnosis of lobular carcinoma. When re-investigating the HRCT scans, the tumour in the left breast was clearly visible.
Treatment
Due to her disseminated disease, no curable treatment was possible, and the patient was referred for palliative chemotherapy. She received several series of chemotherapy with Vinorelbine and Herceptin without clinical improvement and died 20 months after the onset of symptoms.
Discussion
This case of a 78-year-old woman with rapidly evolving fatigue, dry cough, and severe dyspnoea shows the diagnostic challenges and pitfalls when diagnosing ILD. Despite an HRCT highly suggestive of ILD, investigations revealed a metastatic breast cancer with PLC. Similar cases reporting PLC as a primary manifestation of metastatic disease have been published [Citation4–6]. As in our case, the radiological findings gave rise to the suspicion of ILD rather than cancer or PLC. In a case report by Irena Hammen, she presents diagnostic consideration of diffuse interstitial lung disease in a case regarding a 46-year-old woman with exertional dyspnoea, non-productive cough, and HRCT showing ground-glass opacities and interlobular septal thickening. Idiopathic interstitial pneumonia was suspected, but transbronchial lung biopsy revealed lepidic lung adenocarcinoma and PLC [Citation7].
Unfortunately, the primary tumour was not detected on scanning prior to clinical examination and therefore the clinical investigation was focused on pulmonary and extra-pulmonary findings related to ILD. As breast cancer was not suspected at the first visit in our clinic, palpation of mammae was not performed and is not routinely performed in an ILD clinic when there is no suspicion of disease in the breast. The question is then if cryobiopsies of the pulmonary changes were redundant and whether we exposed the patient to unnecessary risks of complication by performing the biopsies. Even if the tumour was detected on HRCT and a biopsy of the tumour had revealed breast cancer, further examinations of the pulmonary lesions would still be necessary to determine if the changes were due to PLC or simultaneously ILD. This would be important to find out if the changes were due to metastasis or simultaneously ILD due to other causes.
A PET-CT is often performed to investigate possible metastasis but cannot distinguish between activity due to metastasis and ILD. Other invasive possibilities could be endobronchial ultrasound-guided aspiration of the lymph nodes, but there was no mediastinal lymph node enlargement and therefore a sample negative for cancer cells would not be sufficient. Forceps biopsies could be another option and does have a lower rate of complications compared to cryobiopsies and has a high diagnostic yield in diffuse lung disease like PLC. However, due to crush artefacts and the small sample size, repeated invasive procedures are sometimes necessary to achieve final diagnosis. Over the last decade, studies have shown that when comparing cryobiopsies to forceps biopsies in the diagnosis of peripheral lesions and endobronchial tumours, the diagnostic yield is higher and the quality is superior for both the histopathological and the molecular diagnosis when performing cryobiopsies [Citation8,Citation9]. Some centres have started using cryobiopsies when suspecting PLC and similar results are expected. BAL may show malignant cells, but a negative report cannot rule out malignancy.
Getting the right diagnosis is important as it would influence the choice of treatment both if the result was PLC, but also if it was an underlying ILD as the risk of drug-induced pneumonitis is increased if treated with, e.g., immunotherapy [Citation10].
Typical radiologic findings in PLC are diffuse smooth (early stage) or nodular (late stage) thickening of the peribronchovascular interstitium and interlobular septae [Citation11]. Subpleural nodularity may sometimes be seen [Citation12,Citation13]. The changes are usually bilateral and symmetric, but can be focal and unilateral, especially when the primary cancer originates from the lung. Pleural effusion and lymphadenopathy are also common findings. For differential diagnosis, other typical septal patterns with smooth reticulation are pulmonary oedema, sarcoidosis, Erdheim-Chester disease, and, in some cases, pulmonary fibrosis [Citation12]. A differentiating feature of PLC is the preservation of the lung architecture.
In the case presented, the radiological findings were not typical for PLC: basal traction bronchiectasis, irregular reticulation, and consolidation in a bilateral symmetrical distribution without enlargement of lymph nodes or pleural effusion.
The exact mechanism of the spread of cancer cells as is seen in PLC is still unknown. One theory suggests a hematogenous spread and subsequently lymphatic involvement. Another theory is a retrograde route from mediastinal or hilar lymph nodes when involved [Citation2]. Apart from being found in the lymphatic vessels, tumour cells can also be found in the adjacent interstitium. The result is thickening of the bronchovascular bundles and alveolar septae due to the infiltration of tumour cells corresponding to reticulation on imaging. Oedema, tumour secretions, and reactive and sometimes fibrotic changes, called desmoplasia, also contribute to the changes [Citation14]. The histopathology in the case presented showed alveolar lung with a small degree of interstitial fibrosis and no inflammation but several intralymphatic clusters of tumour cells with enlarged, hyperchromatic nuclei.
Similarities between cancer and fibrotic ILD have been described, mostly for idiopathic pulmonary fibrosis (IPF) [Citation15]. Both have high mortality, and some consider IPF a neoproliferative disorder of the lung sharing some of the pathogenesis, e.g. genetic alterations, response to growth and inhibitory signals, resistance to apoptosis, myofibroblasts origin and behaviour, altered cellular communications, and intracellular signalling pathways with cancer [Citation15].
The prognosis of metastatic cancer with PLC is poor, with a life expectancy of only 6 months, although new treatment options for various cancers may increase the mean survival [Citation3]. The 5-year survival rate of patients with invasive breast cancer in developed countries is estimated to be 80% [Citation16]. In comparison, the 3-year survival of progressive fibrotic ILD (PF-ILD) not treated with anti-fibrotic drugs is found to be 64% in a real-life study by Galiardi et al. [Citation17] and is similar to IPF and what other studies have found depending on which PF-ILD definition is used [Citation18].
PLC is a rare and severe presentation of cancer, and respiratory symptoms like dyspnoea and cough are non-specific, which potentially lead to delayed diagnosis [Citation19], especially when symptoms from the primary cancer or other typical cancer-related symptoms like weight loss, night sweat, and fatigue are absent. However, with the increased use of transbronchial cryobiopsies of ILD diagnostics, more timely diagnosis of PLC is anticipated.
Conclusion
Similarities between ILD and malignancy like PLC can delay and complicate the correct diagnosis. Common pathogenic pathways and overlap in radiological patterns require wide diagnostic approach. In case of doubt, biopsy should be performed to secure the exact diagnosis and to prevent delay of the initiation of proper treatment, and one could consider using cryobiopsies for this purpose.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–5. doi: 10.1164/rccm.202202-0399ST
- Kumar AA, Mantri SN. Lymphangitis carcinomatosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- Klimek M. Pulmonary lymphangitis carcinomatosis: systematic review and meta-analysis of case reports, 1970-2018. Postgrad Med. 2019;131(5):309–318. doi: 10.1080/00325481.2019.1595982
- Hecimovic A, Jakopovic M, Vukic Dugac A, et al. Metastatic cancer mimics interstitial lung disease. Cases when we need fast diagnosis and treatment. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace. Monaldi Archives For Chest Disease. 2019;89(2). doi: 10.4081/monaldi.2019.1041
- Gilchrist FJ, Alton H, Brundler MA, et al. Pulmonary lymphangitic carcinomatosis presenting as severe interstitial lung disease in a 15-year-old female. Eur Respir Rev. 2011;20(121):208–210. doi: 10.1183/09059180.00000911
- Ağca M, Akyıl FT, Hörmet M, et al. A rare case of progressive dyspnea and bilateral lung infiltration in a young male. Turk Thorac J. 2017;18(3):96–99. doi: 10.5152/TurkThoracJ.2017.16052
- Hammen I. Interstitial lung disease pattern turned out to be a predominantly lepidic lung adenocarcinoma. Respir Med Case Rep. 2017;21:56–58. doi: 10.1016/j.rmcr.2017.02.016
- Ganganah O, Guo SL, Chiniah M, et al. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: A systematic review and meta-analysis. Respirology. 2016;21(5):834–841. doi: 10.1111/resp.12770
- Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J. 2012;39(3):685–690. doi: 10.1183/09031936.00033011
- Conte P, Ascierto PA, Patelli G, et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. 2022;7(2):100404. doi: 10.1016/j.esmoop.2022.100404
- Munk PL, Müller NL, Miller RR, et al. Pulmonary lymphangitic carcinomatosis: CT and pathologic findings. Radiology. 1988;166(3):705–709. doi: 10.1148/radiology.166.3.3340765
- Oikonomou A, Prassopoulos P. Mimics in chest disease: interstitial opacities. Insights Imaging. 2013;4(1):9–27. doi: 10.1007/s13244-012-0207-7
- Pasławski M, Krzyzanowski K, Złomaniec J. Lymphangitis carcinomatosa in thin section computed tomography. Annales Universitatis Mariae Curie-Sklodowska Sectio D: Medicina. 2004;59(1):1–5.
- Johkoh T, Ikezoe J, Tomiyama N, et al. CT findings in lymphangitic carcinomatosis of the lung: correlation with histologic findings and pulmonary function tests. AJR. 1992;158(6):1217–1222. doi: 10.2214/ajr.158.6.1590110
- Vancheri C. Common pathways in idiopathic pulmonary fibrosis and cancer. Eur Respir Rev. 2013;22(129):265–272. doi: 10.1183/09059180.00003613
- Akram M, Iqbal M, Daniyal M, et al. Awareness and current knowledge of breast cancer. Biological Res. 2017;50(1):33. doi: 10.1186/s40659-017-0140-9
- Gagliardi M, Berg DV, Heylen CE, et al. Real-life prevalence of progressive fibrosing interstitial lung diseases. Sci Rep. 2021;11(1):23988. doi: 10.1038/s41598-021-03481-8
- Khor YH, Farooqi M, Hambly N, et al. Patient characteristics and survival for progressive pulmonary fibrosis using different definitions. Am J Respir Crit Care Med. 2023;207(1):102–105. doi: 10.1164/rccm.202205-0910LE
- Pandey S, Ojha S. Delays in diagnosis of pulmonary lymphangitic carcinomatosis due to benign presentation. Case Rep Oncol Med. 2020;2020:4150924. doi: 10.1155/2020/4150924