?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Bronchiectasis is a disease with predominantly neutrophilic inflammation. As a readily available biomarker, there is little evidence to support the use of blood neutrophil-to-lymphocyte ratio (NLR) to predict bronchiectasis exacerbation severe enough to warrant hospitalization.
Methods
A registry-based retrospective cohort study was conducted at a in Hong Kong. Chinese patients with non-cystic fibrosis (CF) bronchiectasis were retrospectively reviewed and subsequently followed up to investigate the association of NLR and the need for hospitalization for bronchiectasis exacerbation. Data on the NLR for patients in a clinically stable state in 2018 were collected and patients followed up from 1 January 2019 to 31 December 2022. The primary outcome was the need for hospitalization due to bronchiectasis exacerbation over the next 4 years.
Results
We reviewed 473 Chinese patients with non-CF bronchiectasis, of whom 94 required hospitalization for bronchiectasis exacerbation during the 4-year follow-up period. Multi-variable logistic regression adjusted for E-FACED score (Exacerbation, Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea score), gender, age, smoking status, and presence of co-existing chronic obstructive pulmonary disease (COPD) was conducted to compare patients with highest and lowest quartile NLR. Results revealed that those with NLR at the highest quartile were at increased risk of hospitalization for bronchiectasis exacerbation with an adjusted odds ratio (aOR) of 2.02 (95% confidence interval = 1.00–4.12, p = 0.05).
Conclusion
Blood NLR may serve as a marker to predict the need for hospitalization due to bronchiectasis exacerbation.
Background
Bronchiectasis is a chronic airway disease as a result of airway insults. Recurrent or persistent airway infections result in subsequent progressive airway damage [Citation1,Citation2]. According to the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) Bronchiectasis Registry, approximately 50% of patients with bronchiectasis experience two or more exacerbations annually [Citation3] with a consequent negative impact on morbidity, quality of life and mortality, and increased healthcare costs [Citation4–12].
As a disease with predominantly neutrophilic inflammation [Citation13–16] the neutrophil:lymphocyte ratio (NLR) has been evaluated as a potential biomarker in bronchiectasis. In a Spanish study, higher NLR correlated with more severe bronchiectasis according to the commonly used scores (FACED: Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea score, E-FACED: Exacerbation, Forced expiratory volume in 1 s (FEV1), Age, Chronic colonization, Extension, and Dyspnea score and Bronchiectasis severity index (BSI)), as well as poorer quality of life (as measured by St George’s Respiratory Questionnaire [SGRQ]). There was also a higher number of comorbidities (Charlson comorbidity index) and infections with Pseudomonas aeruginosa and other micro-organisms. NLR correlated more strongly with severity scores than other systemic inflammatory biomarkers such as blood neutrophil count, C-reactive protein (CRP) and fibrinogen [Citation17]. In the same study, NLR was a predictor of the incident number and severity of exacerbations [Citation17]. Nonetheless, the association of NLR with BSI and FACED scores was not confirmed in another study [Citation18]. In a smaller scale study conducted in Greece, NLR was higher in patients with positive sputum cultures, but the study did not compare the risk of bronchiectasis exacerbation [Citation19]. In another small-scale pediatric study conducted in Turkey, NLR was also shown to be a biomarker to predict acute exacerbations [Citation20].
Although NLR is a readily available biomarker, its use in bronchiectasis has been limited. The current literature is focused mainly on Caucasian cohorts with limited data for Asian populations that have a higher prevalence of bronchiectasis (174.45 per 100,000 population in China [Citation21] and 94.8 per 100 000 population in Germany in 2017 [Citation22]. A larger scale study conducted in an Asian population with predominantly post-infective (in particular, post-tuberculosis) bronchiectasis and a long follow-up is warranted to determine the role of NLR in predicting bronchiectasis exacerbationin particular, the need for hospitalization. In this study, we investigated the role of NLR at a stable state in predicting the risk of bronchiectasis exacerbation requiring hospitalization.
Methods
This was a retrospective single-center registry-based cohort study. All Chinese patients who were followed up in 2018 in the respiratory clinic for non-CF bronchiectasis at the Department of Medicine, Queen Mary Hospital (QMH), were identified through the bronchiectasis database managed by the Division of Respiratory Medicine, Department of Medicine, The University of Hong Kong (HKU). QMH is a tertiary public hospital in Hong Kong West Cluster managed by the Hospital Authority (HA) and affiliated to HKU. The study commenced on 1 January 2019 and patients were followed up until 31 December 2022. Patient clinical records for the subsequent 4 years were reviewed. Patients with bronchiectasis were diagnosed according to Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations [Citation23]. Radiological criteria were as follows: inner airway – artery diameter ratio ≥1.5, an outer airway – artery diameter ratio ≥1.5 or a lack of tapering of the airways, and visibility of airways in the periphery [Citation23]. Patients were excluded if they had traction bronchiectasis resulting from interstitial lung disease (ILD), bronchiectasis due to allergic bronchopulmonary aspergillosis (ABPA), were non-Chinese in ethnicity or lost to follow-up. Exclusions comprised five patients with traction bronchiectasis due to interstitial lung disease, two with bronchiectasis due to allergic bronchopulmonary aspergillosis, five with non-Chinese ethnicity and 10 who were lost to follow-up. Demographic data (age, sex, smoking history), clinical information (exacerbation history), and investigation results (lung function test results, extent of bronchiectasis, sputum microbiology results, blood test results) were retrieved from the database. The primary outcome was bronchiectasis exacerbation, which mandated hospitalization. Patients who required hospitalization and from 1 January 2019 to 31 December 2022 were identified and their records retrieved from the electronic patient records (ePR) of the HA that contains all inpatient and outpatient records of patients who attend clinics and hospitals managed by the HA) Bronchiectasis exacerbation was defined as [1] a deterioration of ≥key symptoms (cough, sputum volume and/or consistency, sputum purulence, dyspnea and/or exercise tolerance, fatigue and/or malaise, hemoptysis) for ≥48 hours AND [2] a clinician’s assessment that a change in bronchiectasis treatment with systemic antibiotic(s) prescription was required [Citation24]. Pseudomonas aeruginosa colonization, one of the prognostic markers of bronchiectasis exacerbation and a component of the FACED and E-FACED score, was defined as the persistence of Pseudomonas aeruginosa in repeated sputum specimens/bronchoalveolar lavage fluid taken at a clinically stable state with no clinical evidence of active infection or tissue damage in the prior 2 years prior [Citation25]. Lung function testing with spirometry was performed using the Vmax® Encore 22 System (CareFusion, San Diego, CA, USA). Spirometry data were interpreted using the updated spirometric reference values for Hong Kong Chinese adults [Citation26]. NLR was calculated by the following formula:
The blood sample was obtained in 2018 while the patient was clinically stable and at least 3 months after the last bronchiectasis exacerbation. For patients with multiple blood samples available, the highest NLR was recorded.
The study was approved by the Institutional Review Board with approval number UW 20–435. Patient informed consent was waived in this study by the IRB of the University of Hong Kong and Hospital Authority Hong Kong West Cluster as the study was retrospective without active patient recruitment.
Sample size calculation
According to Martinez-García et al., the mean ± standard deviation number of exacerbations requiring hospitalization was 1.1 ± 1.7 times in the high NLR group and 0.4 ± 1.3 in the low NLR group. A sample size of 248 subjects could provide 90% power to detect a difference in means between the matched pairs using a 2-sided type I error of 0.05.
Statistical analysis
The demographic (age, gender, smoking history) and clinical data (exacerbation history, lung function parameters, extent of bronchiectasis, history of Pseudomonas aeruginosa colorization, blood test results) are described as frequency or mean ± standard deviation (SD). Demographic and clinical data were compared between the groups with or without bronchiectasis exacerbation during follow-up by independent t-test. Since NLR was not normally distributed, it was compared in the group with or without hospital-requiring bronchiectasis exacerbation by Wilcoxon signed rank test. NLR was classified in four quartiles (first quartile [Q1]: 0–25%, second quartile [Q2]: 25–50%, third quartile [Q3]: 50–75%, fourth quartile [Q4] 75–100%). Logistic regression was employed to estimate the association of NLR in different quartiles and the risk of bronchiectasis exacerbation requiring hospitalization during the 4-year follow-up period. To assess the association between risk of hospital-requiring bronchiectasis exacerbation and NLR, Cox-regression was used to estimate the time to first hospital-requiring bronchiectasis exacerbation. Kaplan – Meier method was used to estimate the cumulative event risks and the stratified log-rank statistic to assess the NLR in quartiles with respect to the composite end point. E-FACED score, gender, age, and smoking status, which are important clinical parameters that could affect the future risk of hospital-requiring bronchiectasis exacerbation, as well as other parameters that were significantly different at baseline were adjusted as potential confounders. Age, E-FACED score, and presence of co-existing chronic obstructive pulmonary disease were statistically different in the two groups so were adjusted in multivariate analysis. E-FACED is also demonstrated to be a reliable scoring system to predict bronchiectasis exacerbation [Citation27] hence it should also be adjusted as the primary outcome is bronchiectasis exacerbation. Gender and smoking status, although not statistically significantly different in the two groups, were adjusted as they were important baseline demographic data. The development of bronchiectasis in relation to gender and smoking history is also reported [Citation28]. Sub-analysis was performed using the previously reported NLR cut-off at 2.92. Sensitivity analysis was performed among patients age <65 years. Statistical significance was determined at the level of p = 0.05. All statistical analyses were performed using the 26th version of SPSS® IBM® Statistics.
Results
A total of 473 Chinese patients with non-CF bronchiectasis managed and followed-up at QMH were included, of whom 97 developed bronchiectasis exacerbation that required hospitalization during the 4-year follow-up.
Baseline characteristics
The mean age was 68.1 ± 12.0 years. There were more females (67.7%) than males and never-smokers (80.1%) than smokers. A total of 96 (20.3%) patients had Pseudomonas aeruginosa colonization in their sputum. The mean FEV1 was 1.69 ± 0.64 L (84.5 ± 24.1%). Multi-lobar involvement, defined as disease affecting ≥3 lobes, was evident in 189 (40%) patients. The median NLR was 2.25 (Inter-quartile range = 1.60–3.42). The results are summarized in . The patients were divided into four groups according to NLR quartile: Q1: NLR < 1.60, Q2: NLR = 1.60–2.24, Q3: NLR = 2.25–3.42, Q4: NLR > 3.42. Of the 94 patients who developed hospital-requiring bronchiectasis exacerbation during the 4-year follow-up, 58 had one episode, 15 had two episodes and 21 had at least 3 episodes.
Table 1. Baseline demographic and clinical characteristics.
Risk of hospital-requiring bronchiectasis exacerbation and NLR
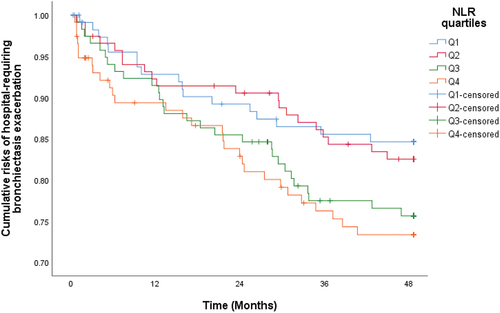
Univariate logistic regression revealed that compared with patients in Q1 (lowest quartile), those in NLR Q4 (highest quartile) were at increased risk of hospital-requiring bronchiectasis exacerbation with odds ratio (OR) of 1.94 (95% confidence interval [CI] = 1.01–3.77, p = 0.048). The OR for NLR Q3 (second highest quartile) and NLR Q2 (second lowest quartile) were 1.83 (95% CI = 0.94–3.57, p = 0.08) and 1.20 (95% CI = 0.59–2.43, p = 0.61), respectively. In multi-variable logistic regression adjusted for age, sex, smoking status, E-FACED score, and presence of co-existing chronic obstructive pulmonary disease (COPD), patients in NLR Q4 (highest quartile) had an increased risk of hospital-requiring bronchiectasis exacerbation with adjusted OR (aOR) of 2.02 (95% CI = 1.00–4.12, p = 0.05) when compared with patients in NLR Q1 (lowest quartile), while the aOR were 1.76 (0.86–3.56, p = 0.12) and 1.53 (95 CI = 0.73–3.22, p = 0.26) for NLR Q3 (second highest quartile) and NLR Q2 (second lowest quartile), respectively. This suggested a statistically significant increased risk of requiring hospitalization for bronchiectasis exacerbation associated with increasing NLR when the highest and lowest quartiles were compared. The results are summarized in .
Table 2. Hospital-requiring bronchiectasis exacerbation risk among patients with NLR at different quartiles, when compared with Q1 (lowest quartile).
NLR and time to first hospital-requiring bronchiectasis exacerbation
Time to first hospitalization for bronchiectasis exacerbation was significantly shorter for patients in NLR Q4 (highest quartile) with a hazard ratio (HR) of 1.86 (95% CI = 1.02–3.38), p = 0.042, when compared with NLR Q1 (lowest quartile). The HR for NLR Q3 (second highest quartile) and NLR Q2 (second lowest quartile) were 1.65 (95% CI = 0.90–3.01, p = 0.10) and 1.13 (95% CI = 0.59–2.15, p = 0.72), respectively. The adjusted HR (aHR) was 1.83 (95% CI = 1.00–3.36), p = 0.050, after adjustment for age, sex, smoking status, E-FACED score, and presence of co-existing COPD (). The aHR for NLR Q3 (second highest quartile) and NLR Q2 (second lowest quartile) were 1.67 (95% CI = 0.91–3.08, p = 0.10) and 1.32 (95% CI = 0.69–2.52, p = 0.40), respectively.
NLR and correlation with other parameters of bronchiectasis severity
NLR had a weak negative association with FEV1 as well as percentage predicted, with Pearson coefficient of −0.096 (p-value = 0.016) and −0.0142 (p-value <0.001), respectively. NLR was not associated with the extent of bronchiectasis, FACED score or E-FACED score.
Sub-analysis
Sub-analysis was performed using the previously reported NLR cut-off at 2.92. In univariate logistic regression, patients with high NLR (NLR > 2.92) had an increased risk of hospital-requiring bronchiectasis exacerbation with OR of 1.81 (95% CI = 1.15–2.85), p = 0.011. The aOR was 1.48 (95% CI = 0.90–2.43), p = 0.12, after adjustment for age, sex, smoking status, E-FACED score, and presence of co-existing COPD.
Sensitivity analysis
Sensitivity analysis was performed among 169 patients aged <65 years. The results were consistent with the primary analysis by multi-variable logistic regression adjusted for sex, age, smoking status, E-FACED score, and presence of co-existing COPD where patients in NLR Q4 (highest quartile) were compared with patients in NLR Q1 (lowest quartile) with OR of 5.36 (95% CI = 1.36–21.2, p = 0.016) and aOR of 5.41 (95% CI = 1.21–24.2, p = 0.027). The OR and aOR for NLR Q3 (second highest quartile) when compared with NLR Q1 (lowest quartile) were 2.05 (95% CI = 0.48–8.83, p = 0.33) and 2.55 (95% CI = 0.54–11.97, p = 0.24), respectively.
The OR and aOR for NLR Q2 (second lowest quartile) when compared with NLR Q1 (lowest quartile) Q2 (second lowest quartile) were 2.16 (95% CI = 0.52–8.97, p = 0.29) and 3.30 (95% CI = 0.73–14.94, p = 0.12), respectively.
Discussion
In this single-center study, NLR was found to be a biomarker that could predict the risk of hospital-requiring bronchiectasis exacerbation. The study was performed in an exclusively Chinese population with predominant post-infective and idiopathic bronchiectasis. Our findings, together with others in the literature, support the use of this simple and readily available marker in prognostication of bronchiectasis, especially for the need for hospitalization for bronchiectasis exacerbation.
Various blood and sputum inflammatory markers have been evaluated for their role in bronchiectasis. Nonetheless, not all markers are readily available. For example, sputum cytokines need fresh sputum specimens that are properly handled and stored. They also require special kits to process and these are costly and not readily available outside research settings or selected tertiary centers. Their role in predicting bronchiectasis exacerbation risks is limited. Other blood inflammatory biomarkers such as CRP and hs-CRP can be easily ascertained from blood sampling but incur a higher than normal processing cost compared with that for a complete blood count. Previous studies revealed NLR to be closely related to bronchiectasis severity with a stronger correlation with multidimensional scores (BSI, FACED score, and E-FACED score) than other blood inflammatory markers such as CRP, fibrinogen, or platelet count. NLR was also shown to be associated with worse clinical, functional, and quality-of-life outcomes. It was also shown to be associated with the probability of Pseudomonas aeruginosa infection. NLR had a good prognostic value to predict incident exacerbations [Citation17]. In our study, the association of NLR with hospital-requiring bronchiectasis exacerbation risk was again demonstrated over a prolonged 4year follow-up. Previous studies have reviewed only the one-year exacerbation risks. Our findings reinforce the prognostic role of NLR, independent of E-FACED score, in predicting hospital-requiring bronchiectasis exacerbation risk. The optimal cut-off for NLR warrants further assessment in a separate study with larger sample size, followed by validation in a separate cohort.
As a readily available and low-cost biomarker, the potential of NLR in exacerbation prediction is demonstrated in our study. NLR can be easily calculated from a peripheral blood complete blood count and is easily repeatable with serial measurements feasible. The normal NLR in an adult, non-geriatric, population in good health is 0.78 and 3.53 [Citation29]. Factors that affect NLR value include race, age, gender, and smoking status [Citation30]. Whether this value is valid for bronchiectasis and the older population is yet to be defined, but the above reference range can serve as a reference. The association of NLR with hospitalization for bronchiectasis exacerbation is likely to be related to the pathophysiology of bronchiectasis. Higher NLR can be attributed to increased neutrophil count and reduced lymphocyte count. Increased neutrophil count is due to the neutrophilic inflammation in bronchiectasis and has been well demonstrated in previous literature. Increased blood neutrophil count reflects airway neutrophilic inflammation and increased sputum neutrophil counts. A reduced lymphocyte count may be related to nutritional deprivation due to a chronic inflammatory state. This is also reflected by lower serum albumin in the group with exacerbation, who are also those with more severe inflammation and worse nutritional status. NLR can take account of these two factors and reflect the end result of chronic airway inflammation in bronchiectasis that is associated with increased hospital-requiring bronchiectasis exacerbation risks.
Unlike other reported studies, our study involved a longer follow-up, and we focused on bronchiectasis exacerbations requiring hospital admission as the primary outcome, the latter more specific than all exacerbations. Patients were assessed by a clinician who determined whether the exacerbation mandated hospitalization. All patients underwent chest radiograph, blood test and sputum testing for microbiology, with antibiotics prescribed. The selection of bronchiectasis exacerbation necessitating hospital admission as the primary outcome can increase the reliability of the study finding by using a more specific outcome.
NLR has also been studied in other respiratory diseases. It is increased among patients with COPD compared with healthy subjects [Citation31] and has a significant positive correlation with smoking index, COPD stage, and dyspnea severity. It is also significantly correlated with various COPD-related clinical parameters such as FEV1, BODE (body mass index (BMI), airflow obstruction, dyspnea, exercise capacity) index, emphysematous changes as represented by percentage of low-attenuation area, fat-free mass index, BMI, nutritional status and severity of COPD, 6-min walk test, and the modified Medical Research Council dyspnea scale (mMRC) score [Citation32]. NLR is also a predictor of COPD exacerbation and prognosis [Citation33–35]. NLR significantly correlates with lung function in idiopathic pulmonary fibrosis and can predict outcomes in IPF [Citation36].
There are a few limitations to our study. First, this is a registry-based cohort study so the quality of the collected data was variable. Lack of active follow-up is another limitation. The sample size was also relatively small, although the results are significant. A larger sample size, preferably with more diverse populations included, would have larger statistical power and a lower error rate, and could help detect differences in different subgroups. We noted this limitation, with the 95% CI being wide and close to 1.0 in some of the results reported. Our study involved a single center, QMH. But as a tertiary medical center in Hong Kong and a university affiliated hospital, our respiratory centre received patient referrals from different specialties in Hong Kong. A designated bronchiectasis clinic was also set up in QMH to manage all patients diagnosed with bronchiectasis. Second, spirometry was carried out at various time-points for the patients in the cohort. Despite this limitation, the results from the study are consistent with other studies. As a retrospective registry-based cohort study, the timing of blood taking for complete blood count was not standardized. For patients with multiple NLRs, we used the highest NLR in clinical stable state. Nonetheless, all blood samples were taken when patients were clinically stable and distanced from an exacerbation. A prospective study with multiple measurements of NLR using a standard protocol for blood taking and follow-up would enable a better assessment of the reported association.
Conclusion
Blood NLR can serve as a biomarker to predict the risk of bronchiectasis exacerbation necessitating hospitalization.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Chang AB, Bell SC, Byrnes CA, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. A position statement from the thoracic society of Australia and New Zealand and the Australian lung foundation. Med J Aust. 2010;193(6):356–8. doi: 10.5694/j.1326-5377.2010.tb03949.x
- Martinez-Garcia MA, Oscullo G, Gomez-Olivas JD, et al. Bronchiectasis: changes in the characterization of patients during 20 years of follow-up. Data from the Spanish bronchiectasis registries. Arch Bronconeumol. 2023;59(10):688–690. doi: 10.1016/j.arbres.2023.07.023
- Chalmers JD, Aliberti S, Polverino E, et al. The EMBARC European bronchiectasis registry: protocol for an international observational study. ERJ Open Res. 2016;2(1):00081–2015. doi: 10.1183/23120541.00081-2015
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–585. doi: 10.1164/rccm.201309-1575OC
- Chalmers JD, Smith MP, Bj M, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186(7):657–665. doi: 10.1164/rccm.201203-0487OC
- Kapur N, Masters IB, Chang AB. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis: what influences lung function stability? Chest. 2010;138(1):158–164. doi: 10.1378/chest.09-2932
- Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):50. doi: 10.1183/13993003.00629-2017
- Sheehan RE, Wells AU, Copley SJ, et al. A comparison of serial computed tomography and functional change in bronchiectasis. Eur Respir J. 2002;20(3):581–587. doi: 10.1183/09031936.02.00284602
- Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest. 2010;138(4):944–949. doi: 10.1378/chest.10-0099
- Ringshausen FC, de Roux A, Pletz MW, et al. bronchiectasis-associated hospitalizations in Germany, 2005–2011: a population-based study of disease burden and trends. PLOS ONE. 2013;8(8):e71109. doi: 10.1371/journal.pone.0071109
- McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med. 2016;4(12):969–979. doi: 10.1016/S2213-2600(16)30320-4
- Beijers RJ, van den Borst B, Newman AB, et al. A multidimensional risk score to predict all-cause hospitalization in community-dwelling older individuals with obstructive lung disease. J Am Med Dir Assoc. 2016;17(6):508–513. doi: 10.1016/j.jamda.2016.01.007
- Chan SC, Leung VO, Ip MS, et al. Shed syndecan-1 restricts neutrophil elastase from α 1 -antitrypsin in neutrophilic airway inflammation. Am J Respir Cell Mol Biol. 2009;41(5):620–628. doi: 10.1165/rcmb.2008-0185OC
- Bedi P, Davidson DJ, McHugh BJ, et al. Blood neutrophils are reprogrammed in bronchiectasis. Am J Respir Crit Care Med. 2018;198:880–890. doi: 10.1164/rccm.201712-2423OC
- Finch S, Shoemark A, Dicker AJ, et al. Pregnancy zone protein is associated with airway infection, neutrophil extracellular trap formation, and disease severity in bronchiectasis. Am J Respir Crit Care Med. 2019;200(8):992–1001. doi: 10.1164/rccm.201812-2351OC
- Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med. 2021;9(8):873–884. doi: 10.1016/S2213-2600(20)30504-X
- Martinez-Garcia MA, Olveira C, Giron R, et al. Peripheral neutrophil-to-lymphocyte ratio in bronchiectasis: a marker of disease severity. Biomolecules. 2022;12(10):12. doi: 10.3390/biom12101399
- Coban H, Gungen AC. Is there a correlation between new scoring systems and systemic inflammation in stable bronchiectasis? Can Respir J. 2017;2017:9874068. doi: 10.1155/2017/9874068
- Georgakopoulou VE, Trakas N, Damaskos C, et al. Neutrophils to lymphocyte ratio as a biomarker in bronchiectasis exacerbation: a retrospective study. Cureus. 2020;12:e9728. doi: 10.7759/cureus.9728
- Nacaroglu HT, Erdem SB, Karaman S, et al. Can mean platelet volume and neutrophil-to-lymphocyte ratio be biomarkers of acute exacerbation of bronchiectasis in children? Cent Eur J Immunol. 2017;42(4):358–362. doi: 10.5114/ceji.2017.72808
- Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013–2017: a nationwide population-based cohort study. Respir Res. 2022;23(1):111. doi: 10.1186/s12931-022-02023-8
- Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009–2017: a population-based cohort study. Eur Respir J. 2019;54(6):54. doi: 10.1183/13993003.00499-2019
- Aliberti S, Goeminne PC, O’Donnell AE, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10(3):298–306. doi: 10.1016/S2213-2600(21)00277-0
- Cheung KS, Leung WK, Seto WK. Application of big data analysis in gastrointestinal research. World J Gastroenterol. 2019;25(24):2990–3008. doi: 10.3748/wjg.v25.i24.2990
- Doring G, Conway SP, Heijerman HG, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000;16(4):749–767. doi: 10.1034/j.1399-3003.2000.16d30.x
- Ip MS, Ko FW, Lau AC, et al. Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization. Chest. 2006;129(2):384–392. doi: 10.1378/chest.129.2.384
- Martinez-Garcia MA, Athanazio RA, Giron R, et al. Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J Chron Obstruct Pulmon Dis. 2017;12:275–284. doi: 10.2147/COPD.S121943
- Yang B, Han K, Kim B, et al. Association between smoking status and incident non-cystic fibrosis bronchiectasis in young adults: a nationwide population-based study. J Pers Med. 2022;12(5):12. doi: 10.3390/jpm12050691
- Forget P, Khalifa C, Defour JP, et al. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. doi: 10.1186/s13104-016-2335-5
- Calixte R, Ye Z, Haq R, et al. Demographic and social patterns of the mean values of inflammatory markers in U.S. Adults: a 2009–2016 NHANES analysis. Diseases. 2023;11(1):11. doi: 10.3390/diseases11010014
- El-Gazzar AG, Kamel MH, Elbahnasy OKM, et al. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Review of Respiratory Medicine. 2020;14(1):111–116. doi: 10.1080/17476348.2019.1675517
- Furutate R, Ishii T, Motegi T, et al. The neutrophil to lymphocyte ratio is related to disease severity and exacerbation in patients with chronic obstructive pulmonary disease. Intern Med. 2016;55(3):223–229. doi: 10.2169/internalmedicine.55.5772
- Karauda T, Kornicki K, Jarri A, et al. Eosinopenia and neutrophil-to-lymphocyte count ratio as prognostic factors in exacerbation of COPD. Sci Rep. 2021;11(1):4804. doi: 10.1038/s41598-021-84439-8
- Lu FY, Chen R, Li N, et al. Neutrophil-to-lymphocyte ratio predicts clinical outcome of severe acute exacerbation of COPD in frequent exacerbators. Int J Chron Obstruct Pulmon Dis. 2021;16:341–349. doi: 10.2147/COPD.S290422
- Zinellu A, Zinellu E, Pau MC, et al. A comprehensive systematic review and meta-analysis of the association between the neutrophil-to-lymphocyte ratio and adverse outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. J Clin Med. 2022;11(12):11. doi: 10.3390/jcm11123365
- Mikolasch TA, George PM, Sahota J, et al. Multi-center evaluation of baseline neutrophil-to-lymphocyte (NLR) ratio as an independent predictor of mortality and clinical risk stratifier in idiopathic pulmonary fibrosis. EClinicalMedicine. 2023;55:101758. doi: 10.1016/j.eclinm.2022.101758