ABSTRACT
Background
A substantial proportion of individuals with COPD have never smoked, and it is implied to be more common than previously anticipated but poorly studied.
Aim
To describe the process of recruitment of never-smokers with COPD from a population-based cohort (n = 30 154).
Methods
We recruited never-smokers with COPD, aged 50–75 years, from six University Hospitals, based on: 1) post broncho-dilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) < 0.70 and 2) FEV1 50–100% of predicted value and 3) being never-smokers (self-reported). In total 862 SCAPIS participants were identified, of which 652 were reachable and agreed to a first screening by telephone. Altogether 128 (20%) were excluded due to previous smoking or declined participation. We also applied a lower limit of normal (LLN) of FEV1/FVC (z-score<-1.64) according to the Global Lung Initiative to ensure a stricter definition of airflow obstruction.
Results
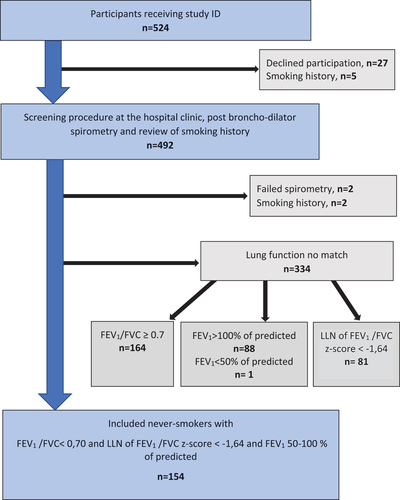
Data on respiratory symptoms, health status, and medical history were collected from 492 individuals, since 32 were excluded at a second data review (declined or previous smoking), prior to the first visit. Due to not matching the required lung function criteria at a second spirometry, an additional 334 (68%) were excluded. These exclusions were by reason of: FEV1/FVC ≥0.7 (49%), FEV1 > 100% of predicted (26%) or z-score ≥ −1,64 (24%). Finally, 154 never-smokers with COPD were included: 56 (36%) women, (mean) age 60 years, FEV1 84% of predicted, FEV1/FVC: 0.6, z-score: −2.2, Oxygen saturation: 97% and BMI: 26.8 kg/m2.
Conclusions
The challenges of a recruitment process of never-smokers with COPD were shown, including the importance of correct spirometry testing and strict inclusion criteria. Our findings highlight the importance of repeated spirometry assessments for improved accuracy in diagnosing COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major public health problem, characterized by persistent airflow limitation and respiratory symptoms [Citation1]. Risk factors for COPD are multiple, but tobacco smoking is the major one [Citation2,Citation3]. The global prevalence of COPD in the adult population is estimated at approximately 10% [Citation3]. According to the recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, COPD is used as an umbrella term and suggested to be classified in six different etiotypes, where environmental COPD, including tobacco smoking, is one [Citation1]. However, a substantial proportion of individuals with COPD have never smoked. In a recent review, the prevalence of COPD in never-smokers was 4–16% worldwide and the prevalence of never-smokers among people with COPD, was 7–51% [Citation4]. In the revised GOLD guidelines, several non-smoking COPD etiotypes are suggested, for example, genetics, abnormal lung development, infections, asthma, and unknown causes [Citation1]. These etiotypes need better characterization in terms of clinical behavior, causes, molecular mechanisms, and prognosis.
The Swedish CardioPulmonary BioImage Study (SCAPIS) is a collaborative project between six Swedish Universities and University hospitals, designed as a cross-sectional study. The study consists of a randomly selected sample of 30,154 women and men from the general population, aged 50–64 years. The project was aiming to study cardiovascular disorders, pulmonary disorders, and metabolic disorders, all to increase the knowledge of basal mechanisms and improve risk prediction [Citation5].
Since COPD in never-smokers is implied to be more common than previously anticipated but poorly studied, we used the broad population-based SCAPIS cohort to filter out a sub-cohort for specific investigations of involved characteristics, risk factors, and disease mechanisms. The aim of this manuscript is to describe the process of recruitment of this population with COPD. Disclosing challenges and pitfalls in the recruitment process can provide valuable general insights into how research cohorts are defined and get valuable perspectives on the representativeness of study samples.
Methods
Recruitment of never-smokers with COPD
The present study was a multicenter study, with a cross-sectional design, where data were collected from 2017 to 2023. Recruitment of never-smokers with COPD was based on the following three criteria: 1) post-broncho dilatory forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC), < 0.70 and 2) FEV1 50–100% of predicted value [Citation6] and 3) an answer ‘No, I have never smoked’ to the question ‘Do you smoke?’ Information on all subjects from SCAPIS project who were categorized as ‘never-smokers with COPD’ according to the above definition were received from the SCAPIS office.
Altogether, 59,909 persons from the Swedish population register were invited by letter to participate in the SCAPIS project. The positive response rate was 50.3% resulting in 30,154 participants included [Citation7]. Out of them, 862 never-smoking participants with COPD according to the definition above were eligible (see flow chart in ). To include participants in the present study, several steps were undertaken. A list of all presumptive participants was received from the SCAPIS database. They were contacted by regular post with brief information about the present study (termed the Broncho-SCAPIS study) and with a request to reply to the responsible researcher at each study site. The presumptive participants had the option to respond by regular post, e-mail, or by telephone. The non-responders were contacted by telephone by a research nurse. If no reply remained after three attempts on separate occasions, the person was considered unreachable. In total 200 of the individuals were unreachable. There were ten drop-outs due to death or having moved beyond reach of the study site area.
Figure 1. Flowchart for the recruitment of never-smokers with chronic obstructive pulmonary disease (COPD) from the SCAPIS project.
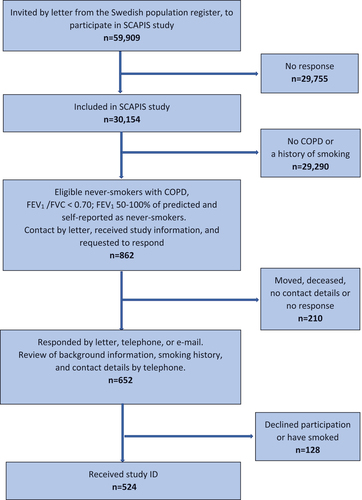
All the responding participants received additional information on study procedures, according to a pre-specified checklist. Background information, including smoking history, age, current medication, and recent exacerbations, was assessed and if participants matched inclusion criteria, an appointment for the screening procedure at the University hospital lung clinic was scheduled. All study procedures followed standard operating procedures (SOPs). Altogether 652 positive responses resulted in a first screening by telephone, in which another 128 were excluded due to declined participation or not matching the required smoking history. Thus, 524 never-smokers with COPD were finally included and received a study ID.
In the original SCAPIS study, individuals aged 50–64 participated after invitation. As the Broncho-SCAPIS study started a few years later and was challenged by recruitment delays, partially because of the COVID-19 pandemic, individuals aged 65–75 years were also invited.
Participants categorized as ‘never-smokers’ in the present study had answered ‘no’ to the following three questions regarding their smoking history: ‘Do you smoke regularly, or have you smoked regularly? Do you smoke cannabis (or equivalent) regularly or have you regularly smoked this before? Do you smoke or have you smoked electronic cigarettes (e-cigarettes)?’ If a person answered ‘yes’ to the question ‘Are you a casual smoker, for example at parties?’ the number of cigarettes/cigarillos/pipe stops per week or per month were registered. All participants who had smoked less than 100 cigarettes or 20 cigars ever and had not been smoking at all during the last two years were also categorized as never-smokers. Self-reported information was collected from an extensive digital questionnaire (approximately 60 questions) comprising additional in-depth background information, questions on respiratory symptoms and medical history. A research nurse reviewed all the self-reported data on site, and responses could be clarified by participants, when needed.
Data collected () on site at the University hospital clinics included vital signs: heart rate, systolic and diastolic blood pressure, and oxygen saturation. Routine blood tests and biobank samples including urine, plasma, serum, and blood cells were also collected at inclusion. Some of the participants performed a fractional exhaled nitric oxide (FeNO) test, including at least one exhalation at 50 mL/s (EcoMedics CLD88 or NIOX Vero). Spirometry (Jaeger Masterscope, Jaeger Masterscreen, Medikro, or Welch-Allyn) included a bronchodilator test; thus, measurements pre- and post-bronchodilator (salbutamol 0.1 mg/dose × 4 doses administrated via a spacer) with at least three forced expiratory curves, reproducible to within 150 ml, obtained from the participant, all according to GOLD [Citation1]. Participants were all in a sitting position, using a nose clip, and they were coached by an experienced research nurse or biomedical scientist. Participants were recruited from all six SCAPIS study sites, Gothenburg, Linköping, Lund, Stockholm, Umeå, and Uppsala (). In the present study, we included never-smokers with COPD defined as a post-bronchodilator FEV1/FVC <0.70 and FEV1 50–100% of predicted value, using the European Community of Coal and Steel (ECCS) reference values [Citation6] together with FEV1/FVC < lower limit of normal (LLN) (z-score < −1.64), according to Global Lung Initiative (GLI) [Citation8]. The calculations were performed at European Respiratory Society (ERS) online spirometry equation tool [Citation9]. The employment of both the LLN and the fixed ratio was made to ensure that only participants with airflow obstruction were included.
Table 1. Data collection procedures for participants with study ID (n = 524).
Table 2. Recruitment of never-smokers with chronic obstructive pulmonary disease (COPD) presented by study site in the present study.
Recruitment of controls
To secure age and sex-matched controls, four additional groups were recruited, defined as follows: never-smokers with normal lung function, smokers with normal lung function, ex-smokers with COPD and current smokers with COPD. () Ex-smokers were defined as having a history of at least 10 pack years of smoking, and more than two years since smoking cessation. Participants with a smoking history of at least 10 pack years and regular smoking of at least 10 cigarettes per day during the last year were defined as ‘current smokers’. Smoking electronic cigarettes (e-cigarettes) was an exclusion criterium.
Table 3. Five study groups recruited. Controls (n = 536) and never-smokers with obstructive pulmonary disease (COPD)(n = 154), grouped according to smoking history and airflow limitation.
Data handling and data protection
All data handling procedures adhered to the guidelines by the General Data Protection Regulation (GDPR). Data from the SCAPIS project were managed by the national coordinator of the present study, all in accordance with instructions from the Information technology (IT) Department at Karolinska Institutet, Stockholm, Sweden. All collected data was located on the Research Electronic Data Capture (REDCap) platform which could only be reached from a digitally secured web link, together with a personal code. The collected data were handled as sensitive data, in accordance with the personal data processing agreements between Karolinska Institutet and the other study sites.
Statistical analysis
The collected demographic data (ordinal) were summarized using descriptive quantitative statistics, the Statistical Package for the Social Sciences (SPSS, IBM, New York, USA). The statistics were presented as numbers (n), mean and standard deviation (SD), median and interquartile range (IQR), and minimum (min) and maximum (max). The significant level was set at ≤0.05. A normality test was used for normal distribution regarding (ratio data): age, body mass index (BMI), lung function, oxygen saturation, heart rate, and blood pressure.
Ethics
The Swedish ethical review authority (Dnr 2016-841-31/2, 2010–228-31 M) granted ethical approval. The study was performed according to ethical rules originating from the Declaration of Helsinki [Citation10]. All mandatory laboratory health and safety procedures have been followed. Considering the lack of data on never-smokers with COPD and the negative impact the airflow limitations may have on the individual, the study is considered to be ethically justified.
Results
Recruitment procedures at the university hospital clinics
Altogether, 524 participants were included and received a Study ID as never-smokers with COPD. Participants were excluded due to declined participation, failed spirometry, a history of smoking, but mostly never-smokers with COPD, were excluded due to lung function discrepancies between values obtained from the original broad SCAPIS study, and values from spirometry performed in the present Broncho-SCAPIS study. Finally, 154 never-smokers with COPD were included in the study ().
Figure 2. Flowchart of inclusion of never-smokers with chronic obstructive pulmonary disease (COPD), at the University hospital clinics.
Characterization of the study population
Of the 154 never-smokers with COPD included in the data analysis, 36% were women, and the mean age was 60 years. Results of post-bronchodilator spirometry values, vital signs, and health-related quality of life assessments (the St George's Respiratory Questionnaire (SGRQ) [Citation11] and the COPD Assessment Test (CAT) [Citation12], the Medical Research Council dyspnea scale (MRC) [Citation13] are shown in , together with data on participants occupation, education level and lodging conditions.
Table 4. Sociodemographic and disease-related characteristics of the included never-smokers with chronic obstructive pulmonary disease, COPD.
Recruitment of controls
The recruitment of age and sex-matched controls according to the inclusion criteria resulted in 536 participants. They were grouped into never-smokers with normal lung function (52%), smokers with normal lung function (18%), ex-smokers with COPD (19%), and smokers with COPD (10%) (). Altogether 129 participants originally screened as ‘never-smokers with COPD’, were shown to have either normal lung function (n = 127) or were included as ‘ex-smoker with COPD’ or as ‘smoker with COPD.’
Recruitment over time
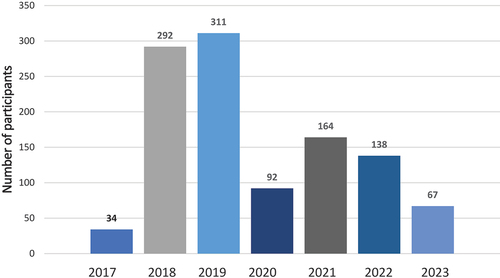
The recruitment of participants resulted in a total of 1098 participants receiving a study ID. The recruitment process started in June 2017 and most participants were included in 2018 and 2019. Due to the COVID-19 pandemic, the recruitment pace slowed down in 2020. The inclusion was ended in June 2023. ()
Discussion
In the recently revised COPD criteria presented by GOLD [Citation1], the term COPD is used to describe both participants with and without a history of tobacco smoking and the requirement of having a smoking history is toned down. Due to the well-known association between COPD and tobacco smoking, many studies only focus on individuals with a history of smoking, but the burden of never-smoking COPD has been shown to be higher than previously assumed. About half of all COPD cases in the world are due to non-tobacco-related risk factors [Citation4], but still there is, to our knowledge, just a limited number of large population-based study on never-smokers with COPD. Nevertheless, it has been shown that a prior diagnosis of asthma, higher age, and, among women, lower education levels are associated with an increased risk for COPD among never smokers [Citation14]. They have also been observed to have milder respiratory symptoms and less airflow limitation [Citation4,Citation15,Citation16]. This was also shown in the present study, where never-smokers with COPD had normal oxygen saturation at rest, they reported low impact on mMRC dyspnea score, and reported low impact on health-related quality of life assessed with CAT and SGRQ (). The majority of never-smokers with COPD in this study were men, a finding that is in agreement with previous observations [Citation17], while another study showed men and women to be equally affected [Citation4]. The mean age was 60 years, which could explain why participants were mostly still working. The education level was high, also in line with some previous studies [Citation15,Citation16]. Never-smokers with COPD also have an increased risk of exacerbations [Citation4] which altogether underscores the importance of further investigations in order to develop a knowledge base for the management of this group.
The major finding of the present study was the importance of a robust recruitment procedure to ensure that all the participants met the detailed inclusion criteria. Included participants were thoroughly examined to make the results from this study group as reliable as possible and to focus on how to increase the knowledge on conducting similar large clinical studies. The present study approach incorporated many pitfalls regarding contact with participants, meeting inclusion criteria and assessing study procedures.
We received 862 eligible self-reported never-smokers with COPD from the SCAPIS cohort. Finally, 154 participants fulfilled the inclusion criteria, a total of 708 dropouts and excluded participants (82%). The high exclusion rate was mainly due to no contact with the presumptive participants, ‘no match’ in lung function inclusion criteria, together with a history of smoking or declined participation. Currently, there is no specified value for what represents an adequate response rate, as response rates across all survey approaches have declined [Citation18]. The inability to contact presumptive participants or them declining without explanation was a common reason for not participating both in the SCAPIS study [Citation5], and in this study. These non-participants were also a concern as they incorporated a risk of selection bias into the present study. Still, it is important to report on response rate and provide information on study survey processes including, for example, participants qualifications, the length of the survey, the cultural and national context [Citation19].
The data security policies following the GDPR, as well as ethical approvals outlined to protect participants integrity in the SCAPIS study, limited the researchers’ ways to contact the study subjects. Only presumptive participants’ personal ID numbers were transmitted to be used in the search for contact details, and no medical records were allowed to be entered. This GDPR limitation made it unclear to researchers if non-responders were diseased, had the wrong addresses or a lack of interest in participating. Ethical committee regulations, designed to protect patients, but instead found to be a barrier in the systematic inclusion strategies, were observed in this study and have been indicated previously by research staff [Citation20]. The multicenter study approach involving numerous researchers could also lead to inconsistency and lack of adherence to regulations throughout the research process. In the present study, SOPs and guidelines, were used throughout all procedures to ensure reproducibility and consistency from the six study sites, thereby verifying the reliability of the collected data [Citation21]. Even the first step of recruitment (pre receiving a Study ID) with telephone interviews followed a specified protocol.
Participants with a study ID were primarily excluded due to discrepancies in lung function inclusion criteria, i.e. ‘no match.’ The criteria were set to ensure that only participants with a defined airflow obstruction were included; this resulted in 24% of the excluded cases due to lung function discrepancies having the z-score not matching but was still seen as a strength.
Here, 164 participants recruited as never-smokers with COPD from the SCAPIS study were found to have a normal lung function (FEV1/FVC >0.7) at reinvestigation before inclusion. This variation between the two tests could have several explanations: actual improvement of the lung function since participating in the SCAPIS study, improved technique at the spirometry testing, better adherence to instructions/more appropriate instructions from research nurse, or other. The lung function testing with spirometry followed GOLD recommendations [Citation1], but still some participants had difficulties completing a FVC procedure for different reasons [Citation22]. When collecting high-quality clinical data, such as lung function measurements, these procedures are reinforced when conducted by researchers experienced in the field. In this study, data collection at each study site was administrated and reviewed by an assigned research nurse. Clinical research nurses hold important competence and knowledge of a clinical research setting as well as patient security, also reflecting the quality of a study’s results [Citation23]. A risk of diagnostic instability and diagnostic reversal has been shown previously in participants with FEV1/FVC ratios near the diagnostic threshold value of 0.7. Therefore, spirometry results should ideally be confirmed on several separate occasions [Citation24], which was the case in this study.
Another strength of the present study was the stringent inclusion criteria regarding lung function, employed to ensure only individuals with a defined airflow obstruction were included. COPD has been defined in other studies as the GOLD fixed ratio (GOLD; FEV1/FVC <0.7) together with the lower limit of normal (LLN; FEV1/FVC<LLN) [Citation3,Citation16]. In 2004, the ERS and the American Thoracic Society (ATS) made a statement encouraging the use of the LLN instead of just the fixed ratio criterion, with the main argument that a fixed ratio can lead to COPD over-diagnosis in elderly and under-diagnosis in young adults [Citation25].
In other studies, never-smokers have been defined as subjects with no history of regular smoking (one or more cigarettes a day), either before or during the study [Citation10]. To expose smoking history objectively, biomarkers as serum cotinine levels [Citation26] and/or exhaled carbon monoxide levels are often used, but they can only assess recent (<8 h) or current smoking and use of other nicotine products [Citation27]. Self-reported smoking status often leads to an underestimation of smoking prevalence and smoking behaviors [Citation28]. In this study, a pragmatic approach was used, with questions regarding smoking history repeated at the telephone interview, in the questionnaire and in person during data collection procedures at the University hospital clinic. This approach was considered as a strength, implying an increased accuracy in answers, as some participants changed their history of smoking status when asked in person. In-person interviewers have been shown to identify smoking status more correctly than just self-administered questionnaires [Citation29].
In this study, never-smokers with COPD were recruited from all six study sites, representing different areas of Sweden, considered as a strength as this might reflect any differences in the matter of living conditions and sociodemographic factors. However, as the socio-economic status in a geographic area is often reflected in participation rates, also seen in the SCAPIS study [Citation5,Citation7] and, consequently, also in this study, where the study populations’ socio-economic representativeness could be considered as a limitation. The recruitment pace of all 1,098 participants receiving a study ID from 2017 to 2023 was inhibited in 2020–2022 due to the COVID-19 pandemic. Some of the study sites were unable to recruit participants for a long time, mostly due to necessary health care priorities, leaving no staff available for research tasks. This situation prolonged the inclusion time and made it necessary to expand the participants age span at inclusion to 50–75 years. An older study population could affect the results, but the present age span was still in line with some previous studies on never-smokers with COPD [Citation15,Citation16].
The digital questionnaire was a comprehensive battery of questions with approximately 60 questions, depending on the participant’s responses which could generate complementary (up to 71) questions. The impact of a questionnaire content could affect the response rate [Citation30], as well as its length, as a long questionnaire reduces the response rate compared to a shorter [Citation31]. However, a strength with the methodology used here was enabling the research nurse to clarify questions if further information was needed and to check for blank entries in the questionnaire. Altogether, this procedure led to few missing data.
There were 536 participants in the four control groups in this study. Out of them, 129 were recruited from the never-smokers with COPD group. They had a change in status (lung function or smoking) and were instead matching the inclusion criteria for controls. This inclusion strategy was seen as a strength and ethically correct since they had already conducted the inclusion procedures.
Conclusion
This study describes the challenges of a recruitment process of never-smokers with COPD, with both drop-outs and exclusion of participants. The strict, but necessary inclusion criteria regarding COPD definition, resulted in the exclusion of 68% of the participants and 26% were instead included as controls, but also the inclusion of 154 participants being unquestionable never-smokers with COPD. Our findings highlight the importance of repeated lung function assessments for improved accuracy in diagnosing COPD, and thereby to enable correct interpretation of study results. We believe the information and the raised awareness from this description may help others in future research, especially when it comes to planning population-based clinical studies. Establishing a correct diagnosis of never-smoking COPD is important to enable further investigation of characteristics and to personalize care in COPD.
Acknowledgments
We would like to acknowledge the SCAPIS research team, and the individuals involved in the collection, organization, and maintenance of data in the Broncho-SCAPIS project: Maria Ahlsén, Benita Engvall, Monika Ezerskyte, Margitha Dahl, Susanne Schedin, Emma Sjöström, Stockholm. Jaro Ankerst, Linnea Jarenbäck, Karina Kanzenbach, Abir Nasr, Emma Pettersson, Anna Sikesjö, Lund. Martin Larsson, Janos Tamas Padra, Gothenburg. Frida Holmström, Annika Johansson, Jamshid Pourazar, Umeå. Annemo Frid, Ozren Kricka, Apostolos Sioutas, Linda Vainikka, Linköping. Abigayle Dalby, Tor Halle, Gunilla Hägg, Katarina Nisser, Annette Nordin, Svetlana Popova, Uppsala.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. doi: 10.1183/13993003.00239-2023
- Diaz-Guzman E, Mannino DM. Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):7–11. doi: 10.1016/j.ccm.2013.10.002
- Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. The Lancet Respir Med. 2022;10(5):447–458. doi: 10.1016/S2213-2600(21)00511-7
- Yang IA, Jenkins CR, Salvi SS. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. The Lancet Respir Med. 2022;10(5):497–511. doi: 10.1016/S2213-2600(21)00506-3
- Bergström G, Berglund G, Blomberg A, et al. The Swedish CArdioPulmonary BioImage study: objectives and design. J Intern Med. 2015;278(6):645–659. doi: 10.1111/joim.12384
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official statement of the European respiratory society. Eur Respir J. 1993;6(Suppl 16):5–40. doi: 10.1183/09041950.005s1693
- Bonander C, Nilsson A, Björk J, et al. The value of combining individual and small area sociodemographic data for assessing and handling selective participation in cohort studies: evidence from the Swedish cardiopulmonary bioimage study. PLOS ONE. 2022;17(3):e0265088. doi: 10.1371/journal.pone.0265088
- Stanojevic S, Quanjer P, Miller MR, et al. The global lung function initiative: dispelling some myths of lung function test interpretation. Breathe. 2013;9(6):462–474. doi: 10.1183/20734735.012113
- ERS. The Global Lung Function Initiative GLI, calculators for Spirometry, TLCO and Lung volume. ERS European Respiratory Society; 2021 [ 2021 Version 2.0] https://gli-calculator.Ersnet.org/index.html
- Forster HP, Emanuel E, Grady C. The 2000 revision of the declaration of Helsinki: a step forward or more confusion? Lancet (London, England). The Lancet. 2001;358(9291):1449–1453. doi: 10.1016/S0140-6736(01)06534-5
- Jones PW, Quirk FH, Baveystock CM. The St george’s respiratory Questionnaire. Respir Med. 1991;85:25–31; discussion 3-7.doi: 10.1016/S0954-6111(06)80166-6
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581
- Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–763. doi: 10.1378/chest.10-1253
- Lindberg A, Linder R, Backman H, et al. From COPD epidemiology to studies of pathophysiological disease mechanisms: challenges with regard to study design and recruitment process: respiratory and cardiovascular effects in COPD (KOLIN). Eur Clin Respir J. 2017;4(1):1415095. doi: 10.1080/20018525.2017.1415095
- Çolak Y, Afzal S, Nordestgaard BG, et al. Majority of never-smokers with airflow limitation do not have asthma: the Copenhagen General Population Study. Thorax. 2016;71(7):614–623. doi: 10.1136/thoraxjnl-2015-208178
- Terzikhan N, Verhamme KMC, Hofman A, et al. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol. 2016;31(8):785–792. doi: 10.1007/s10654-016-0132-z
- Sammut R, Griscti O, Norman IJ. Strategies to improve response rates to web surveys: a literature review. Int J Nurs Stud. 2021;123:104058. doi: 10.1016/j.ijnurstu.2021.104058
- Holtom B, Baruch Y, Aguinis H, et al. Survey response rates: trends and a validity assessment framework. Hum Relat. 2022;75(8):1560–1584. doi: 10.1177/00187267211070769
- Adams M, Caffrey L, McKevitt C. Barriers and opportunities for enhancing patient recruitment and retention in clinical research: findings from an interview study in an NHS academic health science centre. Health Res Policy Sys. 2015;13(1):8. doi: 10.1186/1478-4505-13-8
- Sajdak R, Trembath L, Thomas KS. The importance of standard operating procedures in clinical trials. J Nucl Med Technol. 2013;41(3):231–233. doi: 10.2967/jnmt.113.121467
- Bhatt SP, Y-I K, Wells JM, et al. FEV 1 /FEV 6 to diagnose airflow obstruction. comparisons with computed tomography and morbidity Indices. Ann Am Thorac Soc. 2014;11(3):335–341. doi: 10.1513/AnnalsATS.201308-251OC
- Lönn BB, Hörnsten Å, Styrke J, et al. Transitioning to the clinical research nurse role - a qualitative descriptive study. J Adv Nurs. 2022;78(11):3817–3829. doi: 10.1111/jan.15397
- Aaron SD, Tan WC, Bourbeau J, et al. Diagnostic instability and reversals of chronic obstructive pulmonary disease diagnosis in individuals with mild to moderate airflow obstruction. Am J Respir Crit Care Med. 2017;196(3):306–314. doi: 10.1164/rccm.201612-2531OC
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205
- Seccareccia F, Zuccaro P, Pacifici R, et al. Serum cotinine as a marker of environmental tobacco smoke exposure in epidemiological studies: the experience of the MATISS project. Eur J Epidemiol. 2003;18(6):487–492. doi: 10.1023/A:1024672522802
- Sandberg A, Sköld CM, Grunewald J, et al. Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLOS ONE. 2011;6(12):e28864. doi: 10.1371/journal.pone.0028864
- Williams J, Rakovac I, Loyola E, et al. A comparison of self-reported to cotinine-detected smoking status among adults in Georgia. Eur J Public Health. 2020;30(5):1007–1012. doi: 10.1093/eurpub/ckaa093
- Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–1093. doi: 10.2105/AJPH.84.7.1086
- Rolstad S, Adler J, Rydén A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health. 2011;14(8):1101–1108. doi: 10.1016/j.jval.2011.06.003
- Iglesias C, Torgerson D. Does length of questionnaire matter? A randomised trial of response rates to a mailed questionnaire. J Health Serv Res & Policy. 2000;5(4):219–221. doi: 10.1177/135581960000500406