Abstract
Climate forcing, in combination with local anthropogenic pressures, has drastically modified the physical and chemical properties of lakes worldwide, affecting the abundance and diversity of fish populations. In the context of these combined changes, understanding the interactions between global and local forcing has become a major challenge for developing sustainable fisheries. We analyzed commercial landing statistics of Lake Geneva to describe the long-term changes in the abundance of exploited fish species and to identify mechanisms responsible for fish population changes. We showed a significant relationship between the decrease in phosphorus concentrations and structural changes in the composition of the fish community. Local management of reducing phosphorus loadings played a major role in the recovery of whitefish (Coregonus lavaretus) spawning areas and slowed the process toward more climate-induced percid and cyprinid communities. In addition, rising spring water temperatures have increased whitefish larval growth rates and improved whitefish recruitment. Unexpectedly, climate change and phosphorus reduction can have synergistic effects, and our results highlight the need to consider interactions between global and local anthropogenic forcing to fully understand and predict lake fish population variability in a warming world.
Introduction
Understanding the reasons behind variability in fish population dynamics constitutes an important goal for fisheries scientists. Variability in population abundance can be influenced by climate forcing, internal dynamics, and/or anthropogenic forcing (Balirwa et al. Citation2003, Eero et al. Citation2011). Anthropogenic forcing has occurred for centuries, but it has become more intense since the last century and has a significant global impact on ecosystems (Steffen et al. Citation2011, Laparidou et al. Citation2015). The shift in the relationship between humans and the global environment is so palpable that the concept of the “Anthropocene” was introduced to describe the new epoch of the natural history of the Earth driven by humankind (Steffen et al. Citation2011).
Among all the anthropogenic forcing factors with global impacts, climate change has been identified as one of the most critical threats to global biodiversity. It is now a well-established phenomenon that affects aquatic ecosystems through various ways such as changes in water temperature, lake stratification (Schneider and Hook Citation2010, Kraemer et al. Citation2015), and shifts in species abundance and phenology (Thackeray et al. Citation2010, Jeppesen et al. Citation2012). Furthermore, anthropogenic activities in watersheds contribute to modifications of the physical and chemical characteristics of lakes as well as their faunal compositions. For instance, local anthropogenic activities have resulted in various problems, including eutrophication, overfishing, introduction of alien species, and drastic changes in water levels. Anthropogenic-induced impacts are not generally limited to one trophic level, but rather may disturb the entire ecosystem through direct and indirect effects related to changes in food web structures and ecosystem functioning (Turschak et al. Citation2014). As a consequence, in industrialized countries, the majority of lakes are affected and fisheries are profoundly altered or degraded (Cowx and Gerdeaux Citation2004). Lake management practices are implemented to try to solve these problems. Management actions (fisheries regulation, fish stock enhancement, conservation, habitat manipulation, and rehabilitation) developed to improve the aquatic environment for biodiversity and allow sustainable exploitation of resources have strong impacts on the physical, chemical, and biological characteristics of the ecosystems (Cowx and Gerdeaux Citation2004, Jeppesen et al. Citation2005, Anneville et al. Citation2015).
In heavily anthropogenic-influenced lakes, global and local forcing usually co-occur, and, in a warming world, disentangling the specific effects of different stressors has become an important research topic. Nevertheless, fishing or eutrophication may affect population characteristics (Thomas and Eckman Citation2007, Laugen et al. Citation2014), and changes in population characteristics (e.g., life history traits, density dependence, and demographic structure) can modify the sensibility of a population to various stressors (Perry et al. Citation2010, Planque et al. Citation2010, Ohlberger et al. Citation2014). In those cases, the effects of different stressors are difficult to separate; instead, stressors may have antagonistic or synergistic effects. Therefore, understanding the causes of long-term variability in exploited fish populations may be challenging and require a better understanding of how the various stressors may interact and affect the recruitment success, which is closely linked to larval survival and growth rates (Cushing Citation1975).
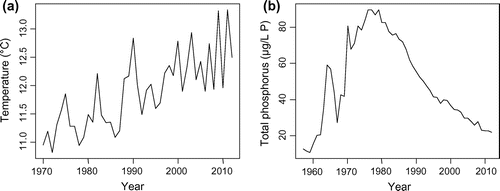
Lake Geneva is a deep peri-alpine lake under the combined influence of commercial fishing, climate variability that induces long-term changes in water temperature (Anneville et al. Citation2002, Dokulil et al. Citation2006, Molinero et al. Citation2007, Kraemer et al. Citation2015; Fig. a), and rehabilitation. Rehabilitation efforts mainly consist of reducing nutrient loadings, resulting in decreased phosphorus concentrations within the lake (Fig. b). In the mid-1990s, major changes appeared in the taxonomic composition of fisheries landings. A number of studies examined the impact of single or multiple stressors on the variability in abundance of key exploited species in Lake Geneva (Gerdeaux Citation2004, Gillet and Quétin Citation2006, Dubois et al. Citation2008, Anneville et al. Citation2009, Gerdeaux Citation2011, Caudron et al. Citation2014), providing an excellent background to interpret reasons behind interannual variability in the fish community. Previous studies, however, have been species-specific, and most ended in the early 2000s. The objectives of this study were to provide a general and updated picture of the long-term changes in the exploited fish community of Lake Geneva by considering several targeted species and to identify the drivers of changes. We (1) describe the long-term changes in the taxonomic structure of the exploited fish community and (2) focus on the most targeted species, whitefish (Coregonus lavaretus), by applying an inferring approach. In this second step, we analyze long-term changes in environmental conditions (water temperature, predator, and food abundances) and discuss how these changes may have impacted the survival and growth of the fish during their first year of life.
Figure 1. Long-term changes in (a) annual mean water temperature measured at 5 m and (b) total phosphorus concentrations (measured as P) in the water column from 0 to 309 m; modified from (Lazzarotto et al. Citation2013).
Materials and methods
Data acquisition
Lake Geneva, a deep peri-alpine lake located in the western part of the Alps, has been studied since 1974 as part of a long-term water quality and biological monitoring program. Sampling is conducted twice a month, except in winter when it occurs once a month, at a station located at the deepest point of the lake. Water temperature is measured using multiparameter probes (Sharma et al. Citation2015), and water samples for nutrient measurements are collected at discrete depths from the surface to the bottom of the lake. The analytical methods used are detailed in an International Commission for the Protection of Lake Geneva (CIPEL) annual report (Monod et al. Citation1984) and are available on the website dedicated to the observatory of alpine lakes (http://www6.inra.fr/soere-ola). Zooplankton was sampled from a depth of 50 m to the surface using a 200 μm-mesh plankton net. Samples were preserved in a 5% buffered formaldehyde solution. Zooplankton taxa were counted and identified to species and developmental stage in one subsample using a dissecting microscope.
French and Swiss professional fishermen share resources and target different fish species. Depending on the species, Swiss landings may contribute 20–75% of the total landing (Anneville et al. Citation2009, Hofmann and Raymond Citation2014). Swiss and French catches of the species analyzed in this study are strongly correlated (O. Anneville, J.-C. Raymond, unpubl. data). Despite a strong fisheries activity, no long-term scientific monitoring of fish populations exists for Lake Geneva. Fisheries management policies are based on information provided by Swiss and French catch statistics. These data are useful to identify changes over decades in the fisheries, but they may not reflect the abundance of the population in recent years (Hofmann and Raymond Citation2014). The use of catch per unit effort (CPUE) as an index of fish abundance has been widely debated (Campbell Citation2004, Maunder et al. Citation2006, Tian et al. Citation2010) but is often used for fisheries management purposes due to a lack of more accurate methods (Harley et al. Citation2001, Ye and Dennis Citation2009, Gerdeaux and Janjua, 2009). Consequently, we focused our analysis on CPUE based on French commercial landings (Gerdeaux Citation2004, Anneville et al. Citation2009) and excluded Swiss catch statistics because information on fishing effort and number of fishermen were not available. Fish abundances from French commercial landing statistics were compiled annually by the Haute-Savoie’s Direction Départementale des Territoires. French commercial landings date back to 1950 for whitefish, perch (Perca fluviatilis), Arctic charr (Salvelinus alpinus), brown trout (Salmo trutta), pike (Esox lucius), and roach (Rutilus rutilus). Data on fishing activities have been recorded for the last few years, and data on the number of French professional fishermen are available from 1979. The available data allowed the computation of annual CPUE in kilograms per fisherman per species (kg/fisherman) from 1979 to 2012 for all species and more precise CPUEs (kg/m2) of set net per year for whitefish from 2002 to 2009.
The age structure of fish populations caught by fishermen provides information about year class strength, an index important to the management of inland fisheries. Its temporal variation is attributed to variability in the recruitment success, which makes this index useful for inferring environmental factors responsible for interannual changes in recruitment success. Although the validity of the scale method has been challenged for aging slow-growing populations, scales can provide reliable age information for short-lived, heavily exploited, fast-growing fishes, such as whitefish in Lake Geneva (Gerdeaux D., INRA, 1992, pers. comm.). Accordingly, scales have historically been the primary structure used to estimate adult whitefish ages in Lake Geneva (Perga and Gerdeaux Citation2003). Furthermore, scales are easy to collect and process, and cost less to use than otoliths. To determine the age distribution, fish scales were sampled every month from 20 randomly sampled fish caught by fishermen with gillnets during the fishing period (Feb–Oct). Scales were taken from beneath the dorsal-fin above the lateral line and stored air-dried in scale envelopes. Individuals were aged by counting summer and winter annuli using a binocular (1.2×).
Analyses
Prior to further statistical analysis, the reliability of CPUEs in kg/fisherman as an index of abundance was assessed based on the Pearson correlation coefficient between the finest information available (i.e., whitefish CPUEs in kg/m² of set net/year) and the down-scaled information (i.e., annual whitefish CPUEs in kg/fisherman). The correlation between annual whitefish CPUEs in kg/fisherman and kg/m² of net set/year reached 0.93 (p < 0.001, n = 9) and validated the use of CPUEs as an index of abundance for whitefish. Regarding the other commercially exploited fish species, we assumed that the number of fishermen could also be used as a proxy of fishing effort to compute the CPUE.
Possible interactions (synergistic or antagonistic effects) between environmental forcing factors and changes in the correlational profile between variables, which may be positively coupled for long periods and can spontaneously become decoupled or anti-correlated (Sugihara et al. Citation2012), influence the reliability of using correlation to infer causation and understand fluctuations in fish abundances. For that reason, we proposed a descriptive approach adapted to our dataset and that allowed us to infer and discuss the possible mechanisms responsible for the observed changes in population abundance. Statistical analyses were performed using the “ade4” library (Chessel et al. Citation2004) in R (R Core Team Citation2014). First, a principal component analysis (PCA) was used to describe the long-term changes in the harvested fish community from 1979 to 2012. A centered PCA was run on fish species CPUE, which were previously log-transformed to reduce the variability and obtain normal distributions. Long-term changes in the log-CPUE of whitefish were then analyzed to identify the mechanisms responsible for the observed changes in abundance.
Because year-class strength is set early in life, the second analysis focused on the environmental conditions during the larval and juvenile stages of whitefish from 1974 to 2012. The prevailing environmental conditions during different sub-periods were compared using between-group PCA (Dolédec and Chessel Citation1989, Boulesteix Citation2005) to identify the environmental variable that presented important interannual changes, which would likely explain the observed changes in fish abundance. Between-group PCA consists of running a PCA on a dataset where observations are gathered by user-defined groups to emphasize the between-group variability in the analysis process. The method consisted of running a PCA on the group-weighted mean values of the variables. The weight of each group was computed during the analysis and was proportional to the number of samples in the group. Changes in the abundance of whitefish were considered to be explained by the number of stocked larvae (number of released equivalent larvae); abundance of perch whose predatory behavior may influence coregonids fry (Caranhac and Gerdeaux Citation1998, Haakana et al. Citation2007); phosphorus concentration, which was expected to influence the reproductive success (Müller Citation1992); and water temperature and food abundance, which may impact recruitment success (Eckmann and Pusch Citation1989, Rellstab et al. Citation2004). Because the recruitment success is established early in life, but not necessarily shortly after hatching or at first feeding (Eckmann and Pusch Citation1991), water temperature and food abundance were considered during the larval and juvenile periods (spring and summer, respectively): water temperature in spring and summer; Daphnia abundance in spring and in summer; the date of spring maximum Daphnia abundance; and carnivorous cladocerans Leptodora kindtii and Bythotrephes longimanus abundance in summer. Based on previous studies that showed important changes in whitefish population characteristics during the period of study (1974–2012), 3 sub-periods and a reference year were identified. Shifts were observed in the density-dependence relationship (Anneville et al. Citation2009) and age structure of landings in the late 1990s and late 2000s, respectively. Accordingly, the studied period was divided into the 3 sub-periods: P1 (1974–1992), P2 (1993–2005), and P3 (2007–2009). Based on the age structure of fish landings determined from scale readings, year-class 2006 was strong and was thus considered in the analysis as the reference year that consisted of environmental conditions associated with high recruitment success.
Results
Long-term changes in the fish community
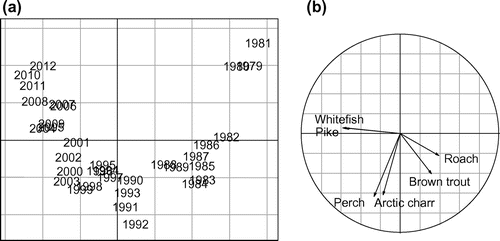
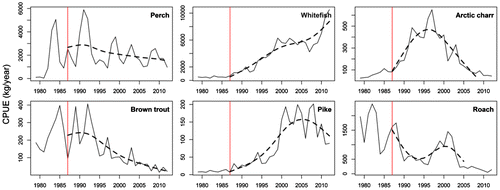
Years were chronologically distributed within the plan composed of the first and second principal components (PC1 and PC2) that accounted for 56.9% and 24.5% of the total variance, respectively (Fig. a). The 1980s were clearly separated from the 2000s and 2010s along PC1. The projection of each variable on the factorial map showed the ecological meaning of the map (Fig. b). The main contributor species to the formation of PC1 were roach, brown trout, pike, and whitefish. These species presented the highest variability during the studied period (1979–2012). While the maximum abundances of roach and brown trout occurred during the 1980s (Fig. ), pike and whitefish were associated with recent years (Fig. ). Therefore, PC1 depicted a progressive change of the Lake Geneva fish community, consisting of a decrease in roach and brown trout abundances that paralleled an increase in pike and whitefish abundances. PC1 revealed a trajectory that significantly correlated with the annual total phosphorus concentration (Pearson coefficient; r = 0.98, p < 0.01), annual 0–20 m average water temperature (r = 0.98, p < 0.01), and average of Daphnia abundance from March to August (r = 0.98, p < 0.01). All of these environmental parameters were strongly correlated with one another. PC2 was associated with perch and Arctic charr, which were both characteristic of the 1990s. No correlation was observed between PC2 and the environmental variables.
Interannual variability in abundance and the age structure of whitefish stock
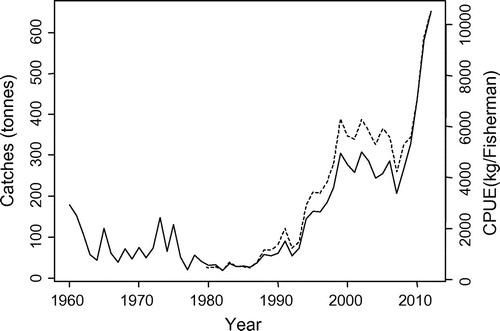
Whitefish catches and CPUE presented comparable interannual trends (Fig. ). Whitefish catches slightly decreased in the 1960s and 1970s, maintained low values during the 1980s, and started to increase beginning in the 1990s. The population reached a plateau at the end of the 1990s and maintained high values until a sharp increase in 2009.
Figure 4. Historical records of whitefish catch (solid line) and abundances via CPUE (dashed line) in Lake Geneva.
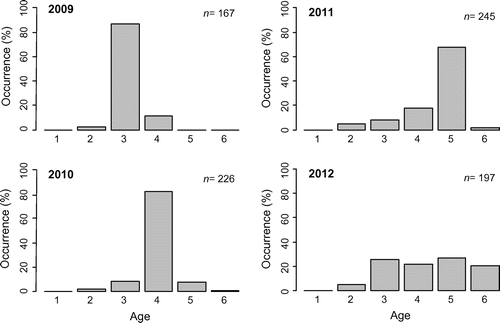
In 2009, the age classes caught by fishermen ranged from 2 to 4 years with the majority being 3 years old (Fig. ). The 2006 cohort that dominated the landings in 2009 prevailed during the following years (2010 and 2011). Consequently, in 2011, landings were essentially composed of 5-year-old fish. In 2012, the 2006 cohort had similar contributions to the 2007, 2008, and 2009 cohorts.
Identifying factors responsible for long-term change in whitefish abundance
The environmental conditions during the larval and juvenile stages were highly different between periods (Fig. a). The between-group PCA showed a strong segregation of P1 from the other years on PC1, explaining 88% of the between-group variability, indicating that the shift from low to high abundance in whitefish was associated with drastic changes in the environmental conditions. The conditions that changed most between P1 and the other periods were phosphorus concentrations, Daphnia abundance in summer, day of spring maximum abundance, and spring water temperatures (Fig. b). Although P1 was characterized by a high Daphnia abundance, high phosphorus concentration, low spring water temperature, and late spring maximum Daphnia abundance, the recent periods showed a lower Daphnia abundance, lower phosphorus concentrations, higher water temperatures in spring, and an earlier spring growth of Daphnia (Fig. ).
Figure 6. (a) Distribution of the center of gravity of periods (P1: 1974–1992; P2: 1993–2005; and P3: 2007–2009) within the first plane defined by axes I and II from the between-group PCA. (b) Ecological meaning of axes I and II: number of stocked larvae (Stocking), phosphorus concentration (Ptot), abundance of perch (Perch), water temperature in spring (T_Sp) and summer (T_S), Daphnia abundance in spring (D_Sp) and in summer (D_S), the date of spring maximum Daphnia abundance (D_MaxD), and L. kindtii and B. longimanus abundance in Summer (CClad_S). 2006 is represented separately as the reference year that consists of environmental conditions associated with high recruitment success.
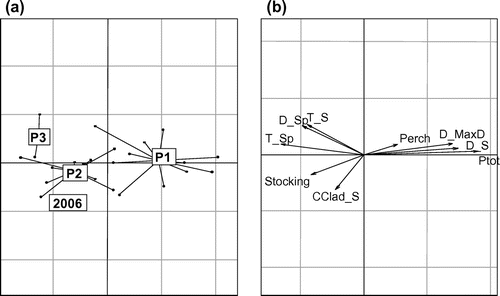
Figure 7. Environmental parameters considered to explain long-term change in whitefish abundance. Boxes and whiskers, respectively, represent the 25th–75th and 5th–95th percentiles. 2006 is represented separately as the reference year consisting of environmental conditions associated with high recruitment success.
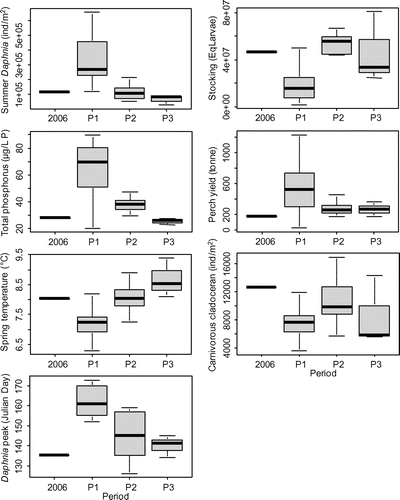
Variables, such as carnivorous cladoceran in summer, perch, and stocking had a low contribution to the formation of PC1. Instead, they explained PC2, which accounts for 8% of the between-group variability and highlights the differences between P3 and the reference year 2006 (Fig. ). In 2006, stocking was higher than in P1 and was in the range of variability of P2 and P3 (Fig. ). By contrast, the abundance of carnivorous cladoceran and perch was higher and lower, respectively, in 2006 than in P2 and P3 (Fig. ).
Discussion
Long-term changes in the fish community
Lake Geneva is suffering from high anthropogenic forcing, which we hypothesized would influence the species composition of fish communities. Our study confirmed this hypothesis. We found that from 1979 to 2012, the taxonomic structure of the exploited fish community progressively shifted from a community characterized by roach and brown trout to a community characterized by whitefish and pike. Annual phosphorus concentrations co-varied with water temperature and food abundance. All 3 explanatory variables were correlated with PC1 and may have contributed to the shift in species composition; however, the ecological requirements of the species that contributed to this trajectory emphasized the role of the phosphorus decrease in the shifting species composition.
Pike is known to spawn in macrophyte beds of the littoral zone (Gillet Citation2001). In Lake Geneva, the expansion of aquatic plants induced by the improvement of the trophic status and water visibility have provided more spawning areas for pike and favored their population increase (C. Gillet, INRA, 2003, pers. comm.). Whitefish reproductive success depends on the oxygen conditions in the spawning area (Müller Citation1992); consequently, its abundance is generally strongly interconnected with lake trophic state (Gerdeaux et al. Citation2006). Although reduced egg survival in spawning areas limits the abundance of whitefish stocks in eutrophic lakes, oligotrophic and mesotrophic lakes provide favorable conditions for whitefish (Gillet Citation2001). For those reasons, as already suggested by other authors, the improvement of the trophic status in Lake Geneva, where whitefish spawn in the littoral zone, is likely to promote the expansion of the whitefish stock (Gerdeaux Citation2004, Anneville et al. Citation2009). Roach are generally more abundant in eutrophic than in oligotrophic lakes because they may be indirectly favored by high phosphorus concentrations (Massol et al. Citation2007). Furthermore, because roach and whitefish feed on the same zooplankton prey during summer (Ponton and Gerdeaux Citation1988), in eutrophic lakes, roach benefit from reduced interspecific competition with coregonids whose recruitments are low (Gerdeaux et al. Citation2006). By contrast, during the re-oligotrophication of Lake Geneva, the increase in whitefish abundance enhanced competition between whitefish and roach for a sharply declining food resource (Jacquet et al. Citation2014). Similarly, because young of the year (YOY) perch eat zooplankton in the pelagic zone (Masson et al. Citation2001, Guillard et al. Citation2006, Janjua and Gerdeaux Citation2011), competition between whitefish and young perch was probably amplified by the increase in the whitefish stock. According to the PCA results (Fig. ), 1979 and the early 1980s were characterized by low perch CPUE. Because of low recruitment induced by unfavorable meteorological conditions, unusually low catches were recorded in the late 1970s (Dubois et al. Citation2008). By contrast, in the mid-1970s, perch catches were high, and the stock was probably more abundant than in recent years, signifying an overall decrease in perch abundance (Jacquet et al. Citation2014).
This trend is consistent with expectations in response to an enhanced trophic status (Gerdeaux et al. Citation2006). In Lake Geneva, the decreasing abundance of perch was mainly driven by the decrease in abundance of zooplankton (Dubois et al. Citation2008). Brown trout were more abundant during the late 1980s and early 1990s than in recent years. The reproductive success of brown trout was not directly affected by lake eutrophication because this species spawns in lake tributaries (Champigneulle et al. Citation1990a, Citation1990b). By contrast, long-term changes in the abundance of this species in the lake may be more related to stocking strategies, forage-fish availability, and changes in the environmental conditions of the tributaries, such as climate-induced stream warming. Warming causes a reduction in thermal habitat and an increase in the occurrence of proliferative kidney disease (PKD), which probably caused the long-term overall decrease in the catch of brown trout populations in many rivers in Switzerland (Hari et al. Citation2006). Finally, Arctic charr was expected to be favored by a decrease in phosphorus concentrations, but in Lake Geneva, the yield of this species remained low due to multiple factors (Caudron et al. Citation2014), including the recent warming of Lake Geneva (Gerdeaux Citation2011).
To summarize, in Lake Geneva from 1979 to 2012, we observed a decrease in the abundance of the eurythermal species, roach and perch, and a sharp increase in the abundance of whitefish, a more cold-stenothermic species. This change in the fish community was in accordance with the projected changes induced by re-oligotrophication (Gerdeaux et al. Citation2006, Massol et al. Citation2007), and the increase in whitefish did not fit the predictions in response to climate warming that was expected to favor eurythermal species (Jeppesen et al. Citation2012). Although climate change and eutrophication are expected to operate concurrently and in the same direction, our results indicate that nutrient reduction can change and slow the process toward more climate-induced percid and cyprinid communities during our studied period.
The exact role of climate cannot be evaluated through correlations of principal components without taking into account its impacts on recruitment success, however. Meteorological variability seemed to be an important driver to explain the year-class strength and recruitment variations in several large lakes (Straile et al. Citation2007, Pritt et al. Citation2014), but the effect of climate change on fish recruitment might be complex and species-specific because it results from a large number of mechanisms, including perturbations in trophic relationships due to the disruption in temporal synchronization (i.e., mismatch) between fish and their prey (Edwards and Richardson Citation2004, Durant et al. Citation2007). The second part of the analysis was therefore oriented to better understand the possible interactions between climate and other local stressors on the long-term changes in the abundance of one species, whitefish, which is highly exploited and displayed strong interannual variability in Lake Geneva.
Implication of climate variability and fisheries management in the long-term changes of whitefish abundance
The interannual changes in whitefish abundances were characterized by 2 major increases (Fig. ), the first beginning in the mid-1990s and the second in 2009. Our results showed that stocking, as well as the abundance of perch and carnivorous cladoceran in summer that contributed to PC2 of the between-group-PCA, may have contributed to the shift from P1 toward P2 and P3. They alone, however, cannot explain the higher abundances observed since the late 1990s.
According to Gerdeaux (Citation2004), stocking probably contributed to the recovery of the whitefish stock, but the first increase and the maintenance of a strong recruitment during P2 was attributed to the improvement and extension of spawning areas following lake rehabilitation and a shift in the regulatory mechanisms of the population (Anneville et al. Citation2009). Anneville et al. (Citation2009) hypothesized that warmer temperatures and a climate-induced better match between the larvae and zooplankton prey in spring sped up larval growth and thus reduced mortality caused by predation or starvation. Accordingly, our results showed that the phosphorus concentration, water temperature, and date of maximum prey abundance differed significantly between periods of low and high recruitment success. As demonstrated for whitefish populations in Lake Constance (Eckmann and Pusch Citation1991), we expected a positive impact of spring water temperatures that controlled the whitefish larval growth rate (Perrier et al. Citation2012) and may have enhanced the recruitment successes. Furthermore, when whitefish larvae are large enough, their diet shifts from copepods to Daphnia (Anneville et al. Citation2007, Pothoven et al. Citation2014). This prey is larger and more easily caught by whitefish larvae than more evasive copepods (Drenner et al. Citation1978, Anneville et al. Citation2011); thus, Daphnia may require less energy from their predators to be captured.
The date of ontogenic change in the larvae diet depends on Daphnia’s availability (Anneville et al. Citation2007, Pothoven et al. Citation2014), which presents a marked annual dynamic strongly influenced by weather conditions (Anneville et al. Citation2010). In Lake Geneva, warmer springs recorded since the end of the 1980s have modified the phenology of this taxa and induced an earlier spring increase in Daphnia abundance (Anneville et al. Citation2002), as has also been recorded in several European lakes (Straile Citation2000, Citation2002). These observations imply that after the 1980s, an earlier spring increase in Daphnia abundance resulted in a better match between larvae and their prey (Anneville et al. Citation2009) and improved food availability for whitefish larvae. In addition, warmer conditions may have increased whitefish larvae’s growth rate and reduced larval mortality by predation. As a consequence, the improvement in both reproductive success and recruitment success was likely responsible for the observed increase in whitefish abundance in Lake Geneva.
The second increase was a consequence of a strong cohort in 2006 resulting from environmental conditions favorable for reproduction and larval survival. In addition, in 2006, large cladocerans were more abundant in summer and perch were less abundant. More abundant high quality food and lower predation pressure constituted additional conditions beneficial for whitefish larvae and juveniles in 2006. Fish hatched in 2006 produced a high quantity of offspring in 2009 that, unlike the previous cohorts, were not fully harvested in the first or second entry year to the fishery. Relatively low fishing, compared to the available stock, allowed the 2006 cohort to maintain until 2012, thus allowing this cohort to spawn several times. Furthermore, older and larger females often produce larger eggs that maybe more viable (Gillet Citation2001). Because egg size may affect larval size (Kamler Citation1992) and small differences in the hatching size may significantly affect the larvae fitness through various mechanisms such as decreased predator vulnerability, more effective feeding, greater tolerance to starvation (Kamler Citation2005), and higher survival of larger individuals resulting from larger eggs has been demonstrated for several species (Kamler Citation1992). The presence of older individuals in the spawning stock likely has a positive impact on offspring survival and recruitment at the population level (Venturelli et al. Citation2010). As a consequence, the second increase in whitefish abundance observed in 2009 may have resulted from favorable environmental conditions and a fishing pressure that has facilitated the maintenance of individuals with potentially high reproductive value.
Conclusions
Our study supported the hypothesis that the decreasing phosphorus concentration was an important driver of the fish community modifications and, to date, was able to slow the unwanted process toward more percid- and cyprinid-dominated communities, a stage expected to be induced by climate warming. When identifying the mechanisms responsible for the long-term changes in fish abundances, however, the effects of rehabilitation, fisheries, and climate variability seemed to be strongly interconnected and difficult to separate. Stocking may have sped the restoration of the whitefish population, the decreasing phosphorus concentration has improved the hatching success, and climate-induced change in water temperature has improved the recruitment success. Because of the complexity of the interactions between management practices and climate forcing, our results illustrate the urgent need to better understand the combined nature of the interactions among stressors (Perry et al. Citation2010, Planque et al. Citation2010).
Currently in Lake Geneva, climate change benefits whitefish, a cold stenothermic species; however, whitefish is already living at the upper end of its thermal tolerance range (Gillet Citation2001). Empirical observations indicated that the spawning phenology of whitefish was closely related to water temperature (Numann Citation1970), and the ovulation of whitefish did not occur when temperatures were >10 °C (C. Gillet, INRA, 2003, pers. comm.). Furthermore, experimental studies have demonstrated the negative effects of temperatures >7 °C on the success of fertilization, viability of eggs, and survival of embryos (Cingi et al. Citation2010). During the spawning season, water temperatures exceeding 8 °C, as are expected due to climate change, will likely cause a decline in whitefish reproductive success (Gillet Citation1991, Anneville et al. Citation2013). In that case, changes in interspecific competition would be expected that obviously benefit other species such as perch and roach (Gerdeaux et al. Citation2006, Massol et al. Citation2007), which are already experiencing changes in spawning phenology because of warmer water temperatures (Gillet and Quétin Citation2006, Anneville et al. Citation2013). These observed changes in fish community emphasize the need to continue monitoring environmental parameters and fisheries to gather more accurate data to explain the fish community succession and better understand interactions between global and local anthropogenic forcing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
Data are from SOERE OLA-IS, INRA Thonon-les-Bains, CIPEL, and the Direction Départementale des Territoires de Haute-Savoie. The authors thank all the technicians, engineers, and researchers who have been involved in the environmental monitoring of Lake Geneva and contributed to producing the data, which are now stored in the OLA database (http://si-ola.inra.fr).
References
- Anneville O, Beniston M, Gallina N, Gillet C, Jacquet S, Lazzarotto J, Perroud M. 2013. L’empreinte du changement climatique sur le Léman. Arch Sci. 66:157–172.
- Anneville O, Berthon V, Glippa O, Mahjoub M-S, Molinero JC, Souissi S. 2011. Ontogenetic dietary changes of whitefish larvae: insights from field and experimental observations. Environ Biol Fish. 91:27–38.
- Anneville O, Lainé L, Benker S, Ponticelli A, Gerdeaux D. 2007. Food habits and ontogenetic changes in the diet of whitefish larvae in Lake Annecy. Bull Fr Pêche Piscic. 387:21–33.
- Anneville O, Lasne E, Guillard J, Eckmann R, Stockwell JD, Gillet C, Yule DL. 2015. Impact of fishing and stocking practices on coregonid diversity. Food Nutr Sci. 6:1045–1055.
- Anneville O, Molinero J-C, Souissi S, Gerdeaux D. 2010. Seasonal and interannual variability of cladoceran communities in two peri-alpine lakes: uncoupled response to the 2003 heat wave. J Plankton Res. 32:913–925.
- Anneville O, Souissi S, Ginot V, Ibanez F, Druart J-C, Angeli N. 2002. Temporal mapping of phytoplankton assemblages in Lake Geneva: annual and interannual changes in their patterns of succession. Limnol Oceanogr. 47:1355–1366.
- Anneville O, Souissi S, Molinero J-C, Gerdeaux D. 2009. Influences of human activity and climate on the stock-recruitment dynamics of whitefish, Coregonus lavaretus, in Lake Geneva. Fish Manag Ecol. 16:492–500.
- Balirwa JS, Chapman CA, Chapman LJ, Cowx IG, Geheb K, Kaufman L, Lowe-McConnell RH, Seehousen O, Wanink JH, Welcomme RL, Witte F. 2003. Biodiversity and fishery sustainability in the Lake Victoria basin: an unexpected marriage? BioScience. 53:703–715.
- Boulesteix A-L. 2005. A note on between-group PCA. Int J Pure Appl Math. 19:359–366.
- Campbell R. 2004. CPUE standardisation and the construction of indices of stock abundance in a spatially varying fishery using general linear models. Fish Res. 70:209–227.
- Caranhac F, Gerdeaux D. 1998. Analysis of fluctuations in whitefish (Coregonus lavaretus) abundance in Lake Geneva. Arch Hydrobiol Spec Issues Adv Limnol. 50:197–206.
- Caudron A, Lasne E, Gillet C, Guillard J, Champigneulle A. 2014. Thirty years of reoligotrophication do not contribute to restore self-sustaining fisheries of Arctic charr, Salvelinus alpinus, in Lake Geneva. Fish Res. 154:165–171.
- Champigneulle A, Melhaoui M, Gerdeaux D, Rojas-Beltran R, Gillet C, Guillard J. 1990a. La truite commune (Salmo trutta L.) dans le Redon, un petit affluent du lac Léman: 1 Caractéristiques de la population en place (1983–1987) et premières données sur l’impact des relâchers d’alevins nourris. Bull Fr Pêche Piscic. 319:181–196.
- Champigneulle A, Melhaoui M, Gerdeaux D, Rojas-Beltran R, Gillet C, Guillard J, Moille J-P. 1990b. La truite commune (Salmo trutta L.) dans le Redon, un petit affluent du lac Léman: 2 Caractéristiques des géniteurs de truite de lac (1983–1987) et premières données sur l’impact des relâchers d’alevins nourris. Bull Fr Pêche Piscic. 319:197–212.
- Chessel D, Dufour AB, Thioulouse J. 2004. The ade4 package-I-One-table methods. R News. 4:5–10.
- Cingi S, Keinanen M, Vuorinen PJ. 2010. Elevated water temperature impairs fertilization and embryonic development of whitefish Coregonus lavaretus. J Fish Biol. 76:502–521.
- Cowx IG, Gerdeaux D. 2004. The effects of fisheries management practices on freshwater ecosystems. Fish Manag Ecol. 11:145–151.
- Cushing DH. 1975. Marine ecology and fisheries. Cambridge (UK): Cambridge University Press.
- Dokulil M, Jagsch A, George GD, Anneville O, Jankowski T, Wahl B, Lenhart B, Blenckner T, Teubner K. 2006. Twenty years of spatially coherent deepwater warming in lakes across Europe related to the North Atlantic Oscillation. Limnol Oceanogr. 51:2787–2793.
- Dolédec S, Chessel D. 1989. Rythmes saisonniers et composantes stationnelles en milieu aquatique II - Prise en compte et élimination d’effets dans un tableau faunistique. Acta Oecol-Oec Gen. 10:207–232.
- Drenner R, Strickler JR, O’Brien WJ. 1978. Capture probability: the role of zooplankter escape in the selective feeding of planktivorous fish. J Fish Res Board Can. 35:1370–1373.
- Dubois J-P, Gillet C, Hilgert N, Balvay G. 2008. The impact of trophic changes over 45 years on the Eurasian perch, Perca fluviatilis, population of Lake Geneva. Aquat Living Resour. 21:401–410.
- Durant JM, Hjermann DØ, Ottersen G, Stenseth NC. 2007. Climate and the match or mismatch between predator requirements and resource availability. Climate Res. 33:271–283.
- Eckmann R, Pusch M. 1989. The influence of temperature on growth of young coregonids (Coregonus lavaretus L.) in a large prealpine lake. Rapports et procès-verbaux des réunions/Conseil permanent international pour l’exploration de la mer. 191:201–208.
- Eckmann R, Pusch M. 1991. At what life stage is year-class strength of coregonids (Coregonus lavaretus L.) in Lake Constance determined? Ver Internat Verein Limnol. 24:2465–2469.
- Edwards M, Richardson AJ. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 430:881–884.
- Eero M, MacKenzie BR, Köster FW, Gislason H. 2011. Multi-decadal responses of a cod (Gadus morhua) population to human-induced trophic changes, fishing, and climate. Ecol Appl. 21:214–226.
- Gerdeaux D. 2004. The recent restoration of the whitefish fisheries in Lake Geneva: the roles of stocking, reoligotrophication, and climate change. Ann Zool Fenn. 41:181–189.
- Gerdeaux D. 2011. Does global warming threaten the dynamics of Arctic charr in Lake Geneva. Hydrobiologia. 660:69–78.
- Gerdeaux D, Anneville O, Hefti D. 2006. Fishery changes during re-oligotrophication in 11 peri-alpine Swiss and French lakes over the past 30 years. Acta Oecol. 30:161–167.
- Gerdeaux D, Juanjua Y. 2009. Contribution of obligatory and voluntary fisheries statistics to the knowledge of whitefish population in Lake Annecy (France). Fish Res. 96:6–10.
- Gillet C. 1991. Egg production in a whitefish (Coregonus schinzi palea) brood stock: effects of photoperiod on the timing of spawning and the quality of eggs. Aquat Living Resour. 4:33–39.
- Gillet C. 2001. Le déroulement de la fraie des principaux poissons lacustres. In: Gerdeaux D, editor. Gestion piscicole des grands plans d’eaux. Paris (France): INRA; p. 241–282.
- Gillet C, Quétin P. 2006. Effect of temperature changes on the reproductive cycle of roach in Lake Geneva from 1983 to 2001. J Fish Biol. 69:518–534.
- Guillard J, Perga M-E, Colon M, Angeli N. 2006. Hydroacoustic assessment of young-of-year perch, Perca fluviatilis, population dynamics in an oligotrophic lake (Lake Annecy, France). Fish Manag Ecol. 13:319–327.
- Haakana H, Huuskonen H, Karjalainen J. 2007. Predation of perch on vendace larvae: diet composition in an oligotrophic lake and digestion time of the larvae. J Fish Biol. 70:1171–1184.
- Hari RE, Livingstone DM, Siber R, Burkhardt-holm P, Güttinger H. 2006. Consequences of climatic change for water temperature and brown trout populations in Alpine rivers and streams. Global Change Biol. 12:10–26.
- Harley SJ, Myers RA, Dunn A. 2001. Is catch-per-unit-effort proportional to abundance? Can J Fish Aquat Sci. 58:1760–1772.
- Hofmann F, Raymond J-C. 2014. Evolution de la pêche dans le Léman. Rapp Comm Int prot Eaux Léman contre pollut. Campagne 2013:163–174. French.
- Janjua MY, Gerdeaux D. 2011. Evaluation of food web and fish dietary niches in oligotrophic Lake Annecy by gut content and stable isotope analysis. Lake Reserv Manage. 27:115–127.
- Jacquet S, Domaizon I, Anneville O. 2014. The need for ecological monitoring of freshwaters in a changing world: a case study of Lakes Annecy. Bourget and Geneva. Environ Monit Assess. 186:3455–3476.
- Jeppesen E, Mehner T, Winfield IJ, Kangur K, Sarvala J, Gerdeaux D, Rask M, Malmquist HJ, Holmgren K, Volta P, et al. 2012. Impacts of climate warming on the long-term dynamics of key fish species in 24 European lakes. Hydrobiologia. 694:1–39.
- Jeppesen E, Søndergaard M, Jensen JP, Havens K, Anneville O, Carvalho L, Coveney MF, Deneke R, Dokulil M, Foy B, Gerdeaux D, et al. 2005. Lake responses to reduced nutrient loading – an analysis of contemporary data from 35 European and North American long term studies. Freshwater Biol. 50:1747–1771.
- Kamler E. 1992. Early life history of fish. An energetic approach. London (UK): Chapman & Hall.
- Kamler E. 2005. Parent-egg-progeny relationships in teleost fishes: an energetic perspective. Rev Fish Biol Fisher. 15:399–421.
- Kraemer BM, Anneville O, Chandra S, Dix M, Kuusisto E, Livingstone DM, Rimmer A, Schladow G, Silow E, Sitoki LM, et al. 2015. Morphometry and average temperature affect global lake stratification responses to climate change. Geophys Res Lett. 42:1–8.
- Laparidou S, Ramsey MN, Rosen AM. 2015. Introduction to the special issue ‘The Anthropocene in the Longue Durée’. Holocene. 25:1537–1538.
- Laugen AT, Engelhard GH, Whitlock R, Arlinghaus R, Dankel DJ, Dunlop ES, Eikeset AM, Enberg K, Jorgensen C, Matsumura S, et al. 2014. Evolutionary impact assessment: accounting for evolutionary consequences of fishing in an ecosystem approach to fisheries management. Fish Fisheries. 15:65–96.
- Lazzarotto J, Quétin P, Klein A. 2013. Evolution physico-chimique des eaux du Léman (éléments majeurs) et données météorologiques. Rapp Comm Int Prot Eaux Léman contre pollut. Campagne 2012:16–46. French.
- Massol F, David P, Gerdeaux D, Jarne P. 2007. The influence of trophic status and large-scale climatic change on the structure of fish communities in Perialpine lakes. J Anim Ecol. 76:538–551.
- Masson S, Angeli N, Guillard J, Pinel-Alloul B. 2001. Diel vertical and horizontal distribution of crustacean zooplankton and young of the year fish in a sub-alpine lake: an approach based on high frequency sampling. J Plankton Res. 23:1041–1060.
- Maunder MN, Sibert JR, Fonteneau A, Hampton J, Kleiber P, Harley SJ. 2006. Interpreting catch per unit effort data to assess the status of individual stocks and communities. ICES J Mar Sci. 63:1373–1385.
- Molinero JC, Anneville O, Souissi S, Lainé L, Gerdeaux D. 2007. Decadal changes in water temperature and ecological time-series in Lake Geneva, Europe - relationship to subtropical Atlantic climate variability. Climate Res. 34:15–23.
- Monod R, Blanc P, Corvi C. 1984. Le régime thermique du Léman. In: CIPEL, editor. Le Léman synthèse 1957–1982. Lausanne; p. 75–88.
- Müller R. 1992. Trophic state and its implications for natural reproduction of salmonid fish. Hydrobiologia. 243(244):261–268.
- Numann W. 1970. The Blaufelchen of Lake Constance, Coregonus wartmani under negative and positive influence of man. In: Lindsey CC, Wood CS, editors. Biology of coregonid fishes. University of Manitoba Press; p. 531–552.
- Ohlberger J, Thackeray SJ, Winfield IJ, Maberly SC, Vollestad LA. 2014. When phenology matters: age-size truncation alters population response to trophic mismatch. P R Soc Lond B Biol Sci. 281:20140938.
- Perrier C, Molinero JC, Gerdeaux D, Anneville O. 2012. Effects of temperature and food supply on the growth of whitefish (Coregonus lavaretus) larvae in an oligotrophic peri-alpine lake. J Fish Biol. 81:1501–1513.
- Perry RI, Cury P, Brander K, Jennings S, Möllmann C, Planque B. 2010. Sensitivity of marine systems to climate and fishing: concepts, issues and management responses. J Mar Syst. 79:427–435.
- Perga M-E, Gerdeaux D. 2003. Using the δ13C and δ15N of whitefish scales for retrospective ecological studies: changes in isotope signatures during the restoration of Lake Geneva, 1980–2001. J Fish Biol. 63:1197–1207.
- Planque B, Fromentin J-M, Cury P, Drinkwater KF, Jenning S, Perry RI, Kifani S. 2010. How does fishing alter marine populations and ecosystems sensitivity to climate? J Mar Syst. 79:403–417.
- Ponton D, Gerdeaux D. 1988. Quelques aspects de l’alimentation de deux poissons planctonophages du lac Léman: le corégone (Coregonus Schinzii palea cuv. et val.) et le gardon (Rutilus rutilus (L.)). Bull Fr Pêche Piscic. 308:11–23.
- Pothoven SA, Hook TO, Roswell CR. 2014. Feeding ecology of age-0 lake whitefish in Saginaw Bay. Lake Huron. J Great Lakes Res. 40:148–155.
- Pritt JJ, Roseman EF, O’Brien TP. 2014. Mechanisms driving recruitment variability in fish: comparisons between the Laurentian Great Lakes and marine systems. ICES J Mar Sci. 71:2252–2267.
- R Core Team. 2014. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Rellstab C, Bürgi HR, Müller R. 2004. Population regulation in coregonids: the significance of zooplankton concentration for larval mortality. Ann Zool Fenn. 41:281–290.
- Schneider P, Hook SJ. 2010. Space observations of inland water bodies show rapid surface warming since 1985. Geophys Res Lett. 37:L22405. DOI:10.1029/2010GL045059.
- Sharma S, Gray DK, Read JS, O’Reilly CM, Schneider P, Qudrat A, Gries C, Stefanoff S, Hampton SE, Hook S, et al. 2015. A global database of lake surface temperatures collected by in situ and satellite methods from 1985–2009. Sci Data. 2:150008. DOI:10.1038/sdata.2015.8.
- Steffen W, Grinevald J, Crutzen P, McNeill J. 2011. The Anthropocene: conceptual and historical perspectives. Philos T Roy Soc A. 369:842–867.
- Straile D. 2000. Meteorological forcing of plankton dynamics in a large and deep continental European lake. Oecologia. 122:44–50.
- Straile D. 2002. North Atlantic Oscillation synchronizes food-web interactions in central European lakes. P R Soc Lond B Biol Sci. 269:391–395.
- Straile D, Eckmann R, Jüngling T, Thomas G, Löffler H. 2007. Influence of climate variability on whitefish (Coregonus lavaretus) year-class strength in deep, warm monomictic lake. Oecologia. 151:521–529.
- Sugihara G, May R, Ye H, Hsieh C-H, Deyle E, Fogarty M, Munch S. 2012. Detecting causality in complex ecosystems. Science. 338:496–500.
- Thackeray SJ, Sparks TH, Frederiksen M, Burthe S, Bacon PJ, Bell JR, Botham MS, Brereton TM, Bright PW, Carvalho L, et al. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Global Change Biol. 16:3304–3313.
- Thomas G, Eckmann R. 2007. The influence of eutrophicaton and population biomass on common whitefish (Coregonus lavaretus) growth – the Lake Constance example revisited. Can J Fish Aquat Sci. 64:402–410.
- Tian S, Chen Y, Chen X, Xu L, Dai X. 2010. Impacts of spatial scales of fisheries and environmental data on catch per unit effort standardization. Mar Freshw Res. 60:1273–1284.
- Turschak BA, Bunnell D, Czesny S, Höök TO, Janssen J, Warner D, Bootsma HA. 2014. Nearshore energy subsidies support Lake Michigan fishes and invertebrates following major changes in food web structure. Ecology. 95:1243–1252.
- Venturelli PA, Murphy CA, Shuter B, Johnston TA, Van Coeverden De Groot PJ, Boag PT, Casselman JM, Montgomerie R, Wiegand MD, Leggett WC. 2010. Maternal influences on population dynamics: evidence from exploited freshwater fish. Ecology. 91:2003–2012.
- Ye Y, Dennis D. 2009. How reliable are the abundance indices derived from commercial catch-effort standardization? Can J Fish Aquat Sci. 66:1169–1178.