Abstract
Alpine lakes and ponds are unique and vulnerable aquatic habitats inhabited by specific species assemblages. In contrast to alpine streams, the genetic diversity and population structure of the organisms found in alpine standing waters are almost completely unknown. Here we present a population genetic study of a macroinvertebrate species from alpine lakes, the diving beetle Agabus bipustulatus (Linnaeus, 1767). We used partial cytochrome b (Cyt b) mtDNA gene of 560 specimens from 43 lakes and ponds distributed across 20 mountain valleys of the Tatra Mountains (Western Carpathians) and their foothills. The aims of this study were to provide detailed genetic metapopulation analysis of A. bipustulatus within a geographically small mountain system, to explore the role of ridges in isolating lake populations, and to test for the applicability of Cyt b in insect population genetic studies. We detected high genetic variability (42 haplotypes), comparable to many large spatial scale studies. There was a star-shaped genetic structure with few dominant haplotypes, which confirmed recent origin, rapid expansion of metapopulation, and multiple colonization of the Tatra Mountain region by A. bipustulatus. The populations within the mountain system differed from each other, suggesting that the Tatra Mountain lakes and ponds were colonized through multiple colonization events, and also that colonization of the region and evolution of metapopulations are to a certain extent influenced by ridge barriers.
Introduction
Quaternary glaciations formed the landscape of the central European mountain ranges and influenced the distribution, diversity, and genetic structure of alpine biota (Hewitt Citation2004). As remnants of the last glaciation, numerous lakes and ponds are now scattered over the arctic and alpine regions (Hantke Citation1978). Alpine lakes are often isolated from each other by mountain ridges, representing heterogeneous island-like biotopes (Hamerlík et al. Citation2014). Inhabited by specific cold-adapted invertebrate assemblages (Lencioni Citation2004), these remote biotopes react sensitively to environmental fluctuations (Skjelkvåle and Wright Citation1998, Fjellheim et al. Citation2000) and can serve as “early-response” indicators of global environmental changes (Korhola et al. Citation2002, Marchetto and Rogora Citation2004). The population structure of these ecosystems, however, must be understood to learn how these systems will respond to change.
Although studied from various aspects (e.g., Catalan et al. Citation2009, Khamis et al. Citation2014), information on population-genetic structure of alpine lake-dwellers is still limited. Other than scarce data on fish (Brunner et al. Citation1998, Citation2001), bacteria (Bosshard et al. Citation2000), crustaceans (Meyran and Taberlet Citation1998), and zooplankton (Petrusek et al. Citation2007, Ventura et al. Citation2014), the communities of alpine lakes and ponds are still almost unknown (Bilton Citation1992, Pauls et al. Citation2014, Young et al. Citation2014). The existing high-altitude aquatic phylogeographic studies were conducted mostly on a large geographic scale (e.g., Carpathians, Central Europe, Pyrenees), dealing mainly with stream biota across Central Europe (e.g., Brändle et al. Citation2007, Engelhardt et al. Citation2011, Theissinger et al. Citation2013). Some small-scale population genetic studies exist but are focused on dendritic stream networks and/or alpine lake outlet fauna (e.g., Alp et al. Citation2012, Elbrecht et al. Citation2014, Macher et al. Citation2015).
Recent population genetic studies, including those of mountain freshwater species, frequently use barcoding of the mtDNA COI gene in molecular analyses (e.g., Meyran and Taberlet Citation1998, Lehrian et al. Citation2009). The populations studied herein come from closely located sites, and hence applying a more variable marker is appropriate. Because microsatellites for the studied species are not yet available, we chose cytochrome b (Cyt b) for its higher rate of molecular evolution in Adephagan beetles (Andújar et al. Citation2012). Cyt b is commonly used in studies of vertebrates (e.g., Babik et al. Citation2004), and we hypothesized that it could provide useful information even within small-scale insect population studies.
The main aim of this study was to provide detailed genetic metapopulation analysis of alpine lake insect species within a geographically small mountain system (Tatra Mountains). The Tatra Mountain region comprises ~120 glacial lakes and many smaller ponds within <780 km2. The area has highly rugged relief with numerous natural barriers (mountain ridges up to 2655 m) that separate the waterbodies into discrete mountain valleys (Kopáček et al. Citation2006); therefore, the next aim was to test for a possible role of the ridges in isolation of the lake populations.
The diving beetle Agabus bipustulatus (Coleoptera: Dytiscidae) has a West Palaearctic distribution (Nilsson and Hájek Citation2014) and inhabits alpine lakes in most European mountain districts (e.g., Füreder et al. Citation2006, de Mendoza et al. Citation2012). Within the (sub)alpine region of the Tatra Mountains, it prefers larger oligotrophic lakes up to 2000 m a.s.l., with the optimum between 1500 and 1800 m a.s.l. (Čiamporová-Zaťovičová and Čiampor Citation2011). A. bipustulatus was selected for this study because it is a frequent inhabitant of alpine lakes and is representative of a common group of aquatic invertebrates with good dispersal ability. In the future, its population genetic structure and diversity could be compared with that of less mobile species, for example the co-occurring Agabus guttatus (Paykull, 1798). This ability for comparison would improve predicted responses of alpine lake communities to possible future environmental changes. The most important benefit of this study is that it provides a detailed picture of the genetic structure and diversity of the study species compared to prior studies of alpine aquatic insects (Lehrian et al. Citation2009, Kubow et al. Citation2010) and produces valuable data on whole alpine lake ecosystems that until now have been overlooked and understudied.
Study site
The Tatra Mountains (49°1′–49°06′N, 19°36′–20°20′E; 778 km2) are the highest and northernmost alpine-type range in the Carpathian chain situated on the Slovak–Polish border (Zasadni and Kłapyta Citation2014), forming the European watershed between the Baltic and Black seas (Fig. ). The studied lakes and ponds were situated between 940 and 2124 m a.s.l. in several well-delineated mountain valleys (Table ). For more detailed lake characteristics see Kopáček et al. (Citation2004), Gregor and Pacl (Citation2005), and Bitušík et al. (Citation2006).
Figure 1. Study area and detailed lake locations within Europe (for abbreviations and coordinates see Table ).

Table 1. Characteristics of populations (lakes), number of haplotypes, and haplotype distributions in populations. WeT = Western Tatras, HiT = High Tatras, PL = Polish side of the Tatra Mts, nHT = number of haplotypes recorded for respective population, Hap (n) = codes of haplotypes (number of samples representing respective haplotype); private haplotypes are in bold, n = sample size, * = sites outside the Tatra Mountains.
Material and methods
Sampling
Samples of the A. bipustulatus were taken between 2009 and 2014 from 43 lakes and ponds using kick nets. The specimens were sorted and then stored in pure ethanol at −25 °C. Identification of adults followed Nilsson and Holmen (Citation1995) and Galewski (Citation1980). Larvae were assigned to relevant species a posteriori, based on uncorrected molecular distance. Each lake (except a few closely located sites that were pooled together) was considered one population (Table ), whereas all populations together were considered a metapopulation (following Young et al. Citation2014). Two populations outside the Tatra Mountains (JE, DD; Fig. ) were also used in the analyses because they are geographically close and thus can be included in the studied metapopulation.
Genotyping, alignments, and genetic variation
Genomic DNA was extracted from thoracic muscles or legs using extraction kits (DNeasy tissue kit, Qiagen; ReliaPrep gDNA Tissue Miniprep System, Promega) following manufacturer’s protocols. Partial Cyt b (343 bp fragment of mtDNA) was used for analyses and amplified using primers CB3 and CB4 (Simon et al. Citation1994). Reactions were conducted in 25 μL total volume using a GoTaq Flexi PCR kit (Promega), containing 1–2 μL of DNA template, set up as follows: 2 min initial denaturation at 95 °C, 35 cycles (94 °C for 30 s, 48 °C for 40 s, and 72 °C for 40 s), 10 min final extension at 72 °C. Polymerase chain reaction (PCR) products were checked with 1% agarose gel electrophoresis, and purification and sequencing were performed in a commercial laboratory (Macrogen Europe Inc., Amsterdam, Netherlands). Sequences were edited in Sequencher 5.1 (Gene Codes). All variable positions were checked on chromatographs, and unreliable samples were discarded. Alignments, final matrix, and uncorrected p-distances were completed using Mega 6.06 (Tamura et al. Citation2013). The sequences of A. bipustulatus were separated from other congeneric species by uncorrected pairwise p-distances. Haplotype frequencies and standard indices of genetic variation were calculated in Arlequin 3.5.1.3. (Excoffier and Lischer Citation2010). The haplotype sequences were sent to GenBank and have accession numbers KX214697-KX214738; voucher specimens are deposited at the authors’ institution.
Population genetic structure
To examine population genetic structure, a median-joining network (MJ) was constructed in Network 4.6.1.2. The origin of each specimen carrying a given haplotype was colour-coded to illustrate haplotype distribution among the mountain valleys. The genetic structure was evaluated by means of the combination of haplotype network and F-statistics. The overall population genetic differentiation between lakes (i.e., populations) and between valleys was described by pairwise ΦST (significance level was Bonferroni corrected) and exact tests of population differentiation (ETPD; Raymond and Rousset Citation1995) in Arlequin. ETPD was performed with default settings in 15 000 steps (5000 were discarded). Spatial analysis of molecular variance was done in SAMOVA 1.0 (Dupanloup et al. Citation2002) with k = 2–21 groups. Five populations included only one specimen; hence, SAMOVA was performed once with all populations (43) and once with a reduced set (38). Statistical variance was evaluated with 1000 permutations. Hierarchical analysis of molecular variance (AMOVA; Excoffier et al. Citation1992) was conducted in Arlequin (with complete and reduced set as in SAMOVA) to evaluate geographic division of the A. bipustulatus metapopulation in the Tatra Mountains. The grouping design was based on the distribution of lakes in the major mountain valleys (Table ).
Statistical significance was assessed by 1000 permutations. Isolation by distance (IBD) and the effect of altitude and natural landscape barriers between populations were tested by simple and partial Mantel tests (Mantel Citation1967) with 10 000 permutations in Arlequin. Several models combining various measures of genetic and geographic distance and natural topographic barriers were tested. Three measures of geographic distance (G) between pairs of populations were used: straight-line distance (Ga; calculated in Geographic Distance Matrix Generator; Ersts Citation2008), the shortest distance following the terrain (Gb), and (because A. bipustulatus are able to fly between lakes) the shortest air distance subsuming flight over the highest geographic barrier (mountain peak) on the transect (Gc). Differences in elevation and existence of barriers between the population pairs were expressed as presence/absence (B) and number of barriers (absolute/categorical; BNo) on the transect, maximum altitude within transect (AMax), and elevation of barrier (EBar). Barrier elevation was calculated as height of the barrier (difference between maximum altitude on the transect and the average altitude of both populations), maximum height of the barrier (difference between maximum altitude on the transect and altitude of the lower population), or the sum of differences between maximum altitude on the transect and altitudes of both populations considered. The geographical measures are based on outputs from the Google Earth Pro (Google Inc.). We used ΦST and its derivations (Slatkin’s linearized ΦST, log ΦST) as measures of genetic distance. Populations with one sample were excluded from analyses.
Demographic history
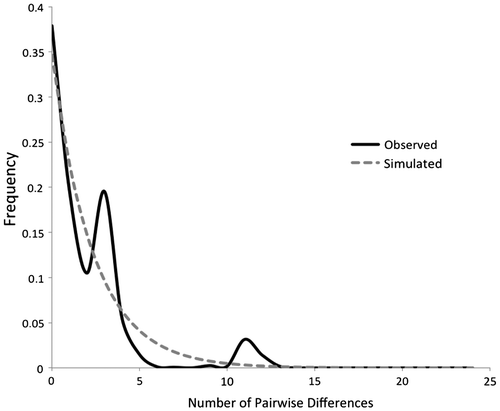
To determine historical demographic patterns and to test for a recent demographic expansion of A. bipustulatus in the Tatra Mountains, we evaluated whether Cyt b sequences have evolved under neutrality by applying Tajima’s D test (Tajima Citation1989) and Fu’s FS test (Fu Citation1997) in Arlequin with default settings (significance evaluated with 1000 permutations). The distribution of pairwise nucleotide differences (mismatch distribution) under the expectations of a sudden-expansion model (Rogers and Harpending Citation1992) was computed in Arlequin. The model validity was assessed by the sum of squared deviations (SSD) between observed and expected mismatches. A degree of approximation of the sequence data between observed mismatch distribution and the distribution expected under population growth was tested using Harpending’s raggedness statistic (Hri; Harpending Citation1994).
Results
mtDNA sequence diversity
Of the 591 total Agabus sequences analyzed, 11 were discarded due to ambiguities and 20 belonged to different Agabus species, leaving 560 used in the analyses. In the dataset, 42 unique haplotypes (HT) were recovered (Table ). Genetic distance among HTs ranged from 0.3% to 4.1%, overall haplotype diversity (Hd) was 0.621 (SD 0.022), and nucleotide diversity (π) was 0.0055 (SD 0.0004). For particular populations see Supplemental Table S1.
Haplotype network and population-genetic structure
The MJ network showed a star-shaped genetic structure (Fig. ) characteristic of recent and rapidly expanding populations. Two dominant HTs were assigned to almost three-fourths of the samples (HT1: 60%, HT3: 14%), and these HTs were also among the most numerous in samples that bordered the Tatra Mountains (JE, DD), together accounting for 26%. The remaining Tatra HTs were represented by 1–16 samples, 22 (52%) by only 1 sample. The mean uncorrected p-distance within the Tatra metapopulation was 1.2%.
Figure 2. Median-joining network of Cyt b haplotypes of the Agabus bipustulatus in the Tatra Mountains. Colored circles represent haplotypes; size is proportional to relative frequencies. Colors indicate distribution of specimens carrying a given haplotype among mountain valleys.
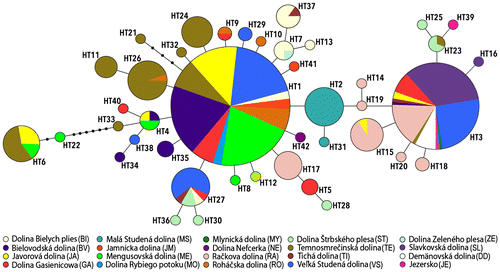
The geographic distribution of HTs (Table ) shows that the number per population varied from 1 to 6 (13 populations had only 1 HT). Only 2 HTs were scattered across most of the studied area: HT1 (23 sites) and HT3 (15 sites). Haplotype overlap between valleys in 9 other HTs was observed, indicating interconnection and/or common colonization of the valleys. Number of HTs per valley ranged from 1 to 10; 20 populations carried 1–4 private HTs. The number of private HTs per valley ranged from 0 to 5; 2 valleys (MS, NE) did not share HTs with any other valley, and the most distant haplotypes (HT6, HT21, HT22) occurred in 3 valleys (TE, ME, JA).
ETPD and pairwise ΦST values were significant in <50% of comparisons (Supplemental Table S2), supporting high levels of differentiation among populations. The results of SAMOVA did not allow unambiguous identification of the optimal k of population groups (Supplemental Table S3); therefore, the populations were grouped into particular valleys in AMOVA. The AMOVA revealed that the majority of the total variance was explained by differences within populations and among populations within valleys; 21% of the total variance accounted for variation among mountain valleys (Table ).
Table 2. Analysis of molecular variance (AMOVA) for the Agabus bipustulatus Cyt b with grouping by valleys (groups) and lakes (populations) showing % genetic variation explained by the different hierarchical levels. Significance values are based on 1000 permutations.
Simple Mantel tests (Table ) revealed no statistically significant increase of genetic differentiation with any of the geographic distance measures used. The correlations were also not significant if presence/number, altitude, or elevation of topographic barriers as an indicator matrix were considered within partial Mantel tests. Similarly, genetic distance was not significantly correlated with presence/number of barriers alone, and/or if controlled for the effect of geographic distance; however, correlations between genetic distance (ΦST) and each of the measures reflecting altitude and/or elevation of the topographical barriers (mountain ridges) were all, although slightly, significantly positive. The genetic differentiation increased when controlled by the matrices indicating number of barriers (partial Mantel tests). If any measure of geographic distance was used as an indicator matrix, the correlation coefficient decreased. The most representative results of various models correlating genetic, geographic, and barrier measures were identified and recorded (Table ).
Table 3. Results of simple and partial Mantel tests indicating relationships between genetic distance (ΦST), geographic distance (G), and natural topographic barriers among populations. Tests with low correlation coefficients and significance are not presented). Ga = straight-line geographic distance, Gc = the shortest air distance over the highest geographic barrier, B = presence/absence of barrier, BNo = number of barriers, AMax = maximum altitude, EBar = elevation of barrier; bold = significant values.
Demographic history
The mismatch distribution (Fig. ) was unimodal. Relatively low and nonsignificant values of Hri (Supplemental Table S1) support the unimodal interpretation that the mismatch distribution fits the demographic model of recent population expansion. Further, the observed distribution did not deviate significantly from the null hypothesis of population expansion under the expansion model in all groups (SSD; Supplemental Table S1). Tajima’s D and Fu’s FS neutrality tests supported the results of mismatch distribution (Supplemental Table S1).
Discussion
Recent studies on the genetic structure of alpine aquatic invertebrates at a large geographical scale have usually revealed clear structuring and visible interregional relationships (e.g., Theissinger et al. Citation2013). Small-scale studies are still rare, partly because of the expectation of low-level genetic variation, and hence difficult interpretation of results. As this study shows, these assumptions may not be grounded because reasonable variability can be achieved by more thorough sampling. Although the diversity detected in the A. bipustulatus samples from lakes and ponds of the small Tatra Mountain range was, contrary to our prior hypothesis, unexpectedly high, a higher evolutionary rate of Cyt b likely contributed to these results; nevertheless, we proved that smaller areas can also harbor a reasonable amount of the genetic diversity. Our research also showed that fine-scale studies (i.e., more samples from smaller areas) are important because under-sampling within larger-scale surveys might omit HTs found elsewhere (Provan and Bennett Citation2008). The haplotype diversity (42 HTs) of A. bipustulatus within the small mountain range clearly showed that the genetic diversity estimates based on a small dataset could be underestimated and that undersampling (i.e., selecting one or few HTs that subsequently represented the whole area) can bias haplotype relationship reconstructions.
The star-shaped haplotype network, as well as mismatch distribution and additional tests, indicate a recent demographic expansion of the A. bipustulatus metapopulation in the Tatra lakes and ponds, similar to findings for other alpine species (e.g., Engelhardt et al. Citation2008). The presence of 2 HTs (HT1 and HT3) with high relative frequency suggests that the Tatra metapopulation has developed from more (at least 2) independent sources, as shown for planktonic Crustacea from the same region (Petrusek et al. Citation2007). The remaining HTs are derived from HT1 and HT3 by a maximum of 3 mutation events. Only 3 HTs were more distant and could represent older Pleistocene lineages (Drotz Citation2003) that colonized the Tatra Mountain lakes separately.
Within populations of recently expanding insect species, high levels of genetic variation have often been detected (Conord et al. Citation2006). It is questionable, however, whether the origin of the variability is autochthonous or allochthonous. The whole study area was completely glaciated during the last glacial maximum (LGM), ~25 000 to 18 000 years BP, which means that all sampled lakes and ponds must have been formed and subsequently colonized after the LGM. Most HTs (31), however, were private, and only 11 occurred in more than one valley, so it is unlikely that most populations were colonized from a different refuge. Drotz (Citation2003) suggested that A. bipustulatus originates from the Mediterranean and that its distribution expanded/contracted through Pleistocene glacial cycles. There is evidence however, that the area around the Carpathians functioned as northern refuge (Provan and Bennett Citation2008); thus, the high genetic variability found in the Tatra lakes is likely a synergistic result of migration and survival (Stewart and Lister Citation2001) and suggests that, similar to findings by Krebes et al. (Citation2010), at least some of the private HTs could have evolved in the Tatra lakes after the LGM.
Our results also revealed that Cyt b could be a useful and informative mtDNA marker in invertebrate population genetic studies. When compared with the studies of aquatic invertebrates using COI (Lehrian et al. Citation2009, Engelhardt et al. Citation2011), Cyt b revealed higher diversity, although sampled in a much smaller geographic area. The expectation that local and recent populations of an agile species would have limited genetic variability was not supported. Mantel tests confirmed a weak but significant influence of mountain ridges as dispersal barriers, implying that topographic isolation of the lake populations likely contribute to the high genetic diversity. This finding was also supported by the AMOVA results, where a statistically significant proportion of the total variance was explained by the variation among mountain valleys.
This study was conducted in the Tatra Mountains, the highest and formerly one of the most glaciated massifs in the Carpathian mountain chain. The Carpathians are a European biodiversity hotspot (e.g. Bálint et al. Citation2011) and belong to the important extra-Mediterranean Pleistocene periglacial refugia and diversification centers for European fauna (Provan and Bennett Citation2008, Pauls et al. Citation2009). Although aquatic invertebrates are not the only fauna represented within the Carpathian biota, they play an important role in the lake ecosystems and could provide useful information for estimating biodiversity changes over time. We conclude that a small spatial scale genetic analysis can provide a more accurate image of the local population structure and improve our understanding of the species genetic diversity in general. In the future, this work can be expanded to include more co-occurring species with different ecological traits in the analysis to improve conservation efforts in the studied region and subsequent application of the results in different parts of the Carpathians or other mountain ranges.
Funding
This work was supported by the VEGA 02/0081/13 and the project ITMS 26240220049 funded by ERDF.
Supplemental data
Supplemental data for this article can be accessed here. http://dx.doi.org/10.1080/20442041.2017.1294361.
Supplementary Table 3
Download PDF (194.6 KB)Supplementary Table 2
Download PDF (190.1 KB)Supplementary Table 1
Download PDF (56.7 KB)Acknowledgements
We thank Darina Šípošová and colleagues from FEE TU Zvolen and FNS UMB Banská Bystrica for helping us with field and laboratory work. For valuable comments and suggestions, we thank Ignacio Ribera, Tomasz Mamos, and Milada Čiamporová. All anonymous reviewers are also acknowledged for their comments.
References
- Alp M, Keller I, Westram AM, Robinson CT. 2012. How river structure and biological traits influence gene flow: a population genetic study of two stream invertebrates with differing dispersal abilities. Freshwater Biol. 57:969–981.
- Andújar C, Serrano J, Gómez-Zurita J. 2012. Winding up the molecular clock in the genus Carabus (Coleoptera: Carabidae): assessment of methodological decisions on rate and node age estimation. BMC Evol Biol. 12:40.
- Babik W, Branicki W, Sandera M, Litvinchuk S, Borkin LJ, Irwin JT, Rafinski J. 2004. Mitochondrial phylogeography of the moor frog. Rana arvalis. Mol Ecol. 13:1469–1480.
- Bálint M, Ujvárosi L, Theissinger K, Lehrian S, Mészáros N, Pauls SU. 2011. The Carpathians as a major diversity hotspot in Europe. In: Habel JC, editor. Biodiversity hotspots in Europe. Berlin (Germany): Springer; p. 189–205.
- Bilton DT. 1992. Genetic population structure of the postglacial relict diving beetle Hydroporus glabriusculus Aubé (Coleoptera: Dytiscidae). Heredity. 69:503–511.
- Bitušík P, Kopáček J, Stuchlík E, Šporka F, editors. 2006. Limnology of lakes in the Tatra Mountains. In: Biologia Bratislava, Vol. 61( Suppl. 18). 222 p.
- Bosshard PP, Santini Y, Grüter D, Stettler R, Bachofen R. 2000. Bacterial diversity and community composition in the chemocline of the meromictic alpine Lake Cadagno as revealed by 16S rDNA analysis. FEMS Microbiol Ecol. 31:173–182.
- Brändle M, Heuser R, Marten A, Brandl R. 2007. Population structure of the freshwater flatworm Crenobia alpina (Dana): old lineages and low gene flow. J Biogeogr. 34:1183–1192.
- Brunner PC, Douglas MR, Bernatchez L. 1998. Microsatellite and mitochondrial DNA assessment of population structure and stocking effects in arctic charr Salvelinus alpinus (Teleostei: Salmonidae) from central Alpine lakes. Mol Ecol. 7:209–223.
- Brunner PC, Douglas MR, Osinov A, Wilson CC, Bernatchez L. 2001. Holarctic phylogeography of Arctic charr (Salvelinus alpinus L.) inferred from mitochondrial DNA sequences. Evolution. 55:573–586.
- Catalan J, Curtis CJ, Kernan M. 2009. Remote European mountain lake ecosystems: regionalisation and ecological status. Freshwater Biol. 54:2419–2432.
- Čiamporová-Zaťovičová Z, Čiampor F Jr. 2011. Aquatic beetles of the alpine lakes: diversity, ecology and small-scale population genetics. Knowl Manag Aquat Ec. 402:10.
- Conord C, Lempérière G, Taberlet P, Després L. 2006. Genetic structure of the forest pest Hylobius abietis on conifer plantations at different spatial scales in Europe. Heredity. 97:46–55.
- de Mendoza G, Rico E, Catalan J. 2012. Predation by introduced fish constrains the thermal distribution of aquatic Coleoptera in mountain lakes. Freshwater Biol. 57:803–814.
- Drotz MK. 2003. Speciation and mitochondrial DNA diversification of the diving beetles Agabus bipustulatus and A. wollastoni (Coleoptera, Dytiscidae) within Macaronesia. Biol J Linn Soc. 79:653–666.
- Dupanloup I, Schneider S, Excoffier L. 2002. A simulated annealing approach to define the genetic structure of populations. Mol Ecol. 11:2571–2581.
- Elbrecht V, Feld CK, Gies M, Hering D, Sondermann M, Tollrian R, Leese F. 2014. Genetic diversity and dispersal potential of the stonefly Dinocras cephalotes in a central European low mountain range. Freshwater Sci. 33:181–192.
- Engelhardt CHM, Pauls SU, Haase P. 2008. Population genetic structure of the caddisfly Rhyacophila pubescens, Pictet 1834, north of the Alps. Fund Appl Limnol. 173:165–176.
- Engelhardt CHM, Haase P, Pauls SU. 2011. From the Western Alps across Central Europe: postglacial recolonisation of the tufa stream specialist Rhyacophila pubescens (Insecta, Trichoptera). Front Zool. 8:10.
- Ersts PJ. 2008. Geographic distance matrix generator (version 1.2.3). Volume 2008-5-1 American Museum of Natural History, Center for Biodiversity and Conservation [cited 15 Jan 2016]. Available from: http://biodiversityinformatics.amnh.org/open_source/gdmg.
- Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res. 10:564–567.
- Excoffier L, Smouse PE, Quattro JM. 1992. Analysis of molecular variance from metric distance among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 131:479–491.
- Fjellheim A, Boggero A, Halvorsen GA, Nocentini AM, Rieradevall M, Raddum GG, Schnell OA. 2000. Distribution of benthic invertebrates in relation to environmental factors. A study of European remote alpine lake ecosystems. Verh Internat Verein Limnol. 27:484–488.
- Fu YX. 1997. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 147:915–925.
- Füreder L, Ettinger R, Boggero A, Thaler B, Thies H. 2006. Macroinvertebrate diversity in alpine lakes: effects of altitude and catchment properties. Hydrobiologia. 562:123–144.
- Galewski K. 1980. Third stage larvae of European species of Agabus Leach (Coleoptera, Dytiscidae). Pol Pis Entomol. 50:3–69. Polish.
- Gregor V, Pacl J. 2005. Hydrológia tatranských jazier. [Hydrology of Tatra lakes]. Acta Hydrol Slov. 6:161–187. Slovak.
- Hamerlík L, Svitok M, Novikmec M, Očadlík M, Bitušík P. 2014. Local, among-site, and regional diversity patterns of benthic macroinvertebrates in high altitude waterbodies: do ponds differ from lakes? Hydrobiologia. 723:41–52.
- Hantke R. 1978. Eiszeitalter. Thun (Switzerland): Ott Verlag. German.
- Harpending H. 1994. Signature of ancient population growth in a low resolution mitochondrial DNA distribution. Hum Biol. 66:591–600.
- Hewitt GM. 2004. Genetic consequences of climatic oscillations in the Quaternary. Phil T Roy Soc Lond B. 2004:183–195.
- Khamis K, Hannah DM, Brown LE, Tiberti R, Milner AM. 2014. The use of invertebrates as indicators of environmental change in alpine rivers and lakes. Sci Total Environ. 493:1242–1254.
- Kopáček J, Hardekopf D, Majer V, Pšenáková P, Stuchlík E. 2004. Response of alpine lakes and soils to changes in acid deposition: the MAGIC model applied to the Tatra Mountain region. Slovakia-Poland. J Limnol. 63:143–156.
- Kopáček J, Stuchlík E, Hardekopf D. 2006. Chemical composition of the Tatra Mountain lakes: recovery from acidification. Biologia. 61(Suppl. 18):S21–S33.
- Korhola A, Sorvari S, Rautio M, Appleby PG, Dearing JA, Hu Y, Rose N, Lami A, Cameron NG. 2002. A multi-proxy analysis of climate impacts on the recent development of subarctic Lake Saanajärvi in Finnish Lapland. J Paleolimnol. 28:59–77.
- Krebes L, Blank M, Jürss K, Zettler ML, Bastrop R. 2010. Glacial-driven vicariance in the amphipod Gammarus duebeni. Mol Phylogenet Evol. 54:372–385.
- Kubow KB, Robinson CT, Shama LNS, Jokela J. 2010. Spatial scaling in the phylogeography of an alpine caddisfly, Allogamus uncatus, within the central European Alps. J N Am Benthol Soc. 29:1089–1099.
- Lehrian S, Pauls SU, Haase P. 2009. Contrasting patterns of population structure in the montane caddisflies Hydropsyche tenuis and Drusus discolor in the Central European highlands. Freshwater Biol. 54:283–295.
- Lencioni V. 2004. Survival strategies of freshwater insects in cold environments. J Limnol. 63(Suppl. 1):45–55.
- Macher JN, Rozenberg A, Pauls SU, Tollrian R, Wagner R, Leese F. 2015. Assessing the phylogeographic history of the montane caddisfly Thremma gallicum using mitochondrial and restriction-site-associated DNA (RAD) markers. Ecol Evol. 5:648–662.
- Mantel N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Res. 27:209.
- Marchetto A, Rogora M. 2004. Measured and modelled trends in European mountain lakes: results of fifteen years of cooperative studies. J Limnol. 63:55–62.
- Meyran JC, Taberlet P. 1998. Mitochondrial DNA polymorphism among alpine populations of Gammarus lacustris (Crustacea, Amphipoda). Freshwater Biol. 39:259–265.
- Nilsson AN, Hájek J. 2014. Catalogue of Palearctic Dytiscidae (Coleoptera) [cited 1 Jan 2014]. Available from: http://www2.emg.umu.se/projects/biginst/andersn/Cat_main.htm.
- Nilsson AN, Holmen M. 1995. The aquatic Adephaga (Coleoptera) of Fennoscandia and Denmark. II. Dytiscidae. Fauna Entomol Scand. 32. Leiden (Netherlands): Brill.
- Pauls SU, Alp M, Bálint M, Bernabò P, Čiampor F Jr, Čiamporová-Zaťovičová Z, Finn DS, Kohout J, Leese F, Lencioni V, Paz-Vinas I, Monaghan MT. 2014. Integrating molecular tools into freshwater ecology: developments and opportunities. Freshwater Biol. 59:1559–1576.
- Pauls SU, Theissinger K, Ujvarosi L, Bálint M, Haase P. 2009. Patterns of population structure in two closely related, partially sympatric caddisflies in Eastern Europe: historic introgression, limited dispersal, and cryptic diversity. J N Am Benthol Soc. 28:517–536.
- Petrusek A, Černý M, Mergeay J, Schwenk K. 2007. Daphnia in the Tatra Mountain lakes: multiple colonisation and hidden species diversity revealed by molecular markers. Fund Appl Limnol. 169:279–291.
- Provan J, Bennett KD. 2008. Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol. 23:564–571.
- Raymond M, Rousset F. 1995. An exact test for population differentiation. Evolution. 49:1280–1283.
- Rogers AR, Harpending H. 1992. Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol. 49:552–569.
- Simon C, Frati F, Beckenbach AT, Crespi B, Liu H, Flook P. 1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 87:651–701.
- Skjelkvåle BL, Wright RF. 1998. Mountain lakes: sensitivity to acid deposition and glogal climate change. AMBIO. 27:280–286.
- Stewart JR, Lister AM. 2001. Cryptic northern refugia and the origins of the modern biota. Trends Ecol Evol. 16:608–613.
- Tajima F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 123:585–595.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Theissinger K, Bálint M, Feldheim KA, Haase P, Johannesen J, Laube I, Pauls SU. 2013. Glacial survival and post-glacial recolonization of an arctic-alpine freshwater insect (Arcynopteryx dichroa, Plecoptera, Perlodidae) in Europe. J Biogeogr. 40:236–248.
- Ventura M, Petrusek A, Miro A, Hamrová E, Bunay D, de Meester L, Mergeay J. 2014. Local and regional founder effects in lake zooplankton persist after thousands of years despite high dispersal potential. Mol Ecol. 23:1014–1027.
- Young S-S, Lee Y-Y, Liu M-Y. 2014. Genetic variability and divergence of Neutrodiaptomus tumidus Kiefer 1937 (Copepoda: Calonida) among 10 subpopulations in the high mountain range of Taiwan and their phylogeographical relationships indicated by mtDNA COI gene. Zool Stud. 53:22.
- Zasadni J, Kłapyta P. 2014. The Tatra Mountains during the Last Glacial Maximum. J Maps. 10:440–456.