Abstract
At the La Grande Hydroelectric Complex (Québec, Canada), total mercury (THg) levels in fish were monitored from 1978 to 2012 in more than 37 000 fish comprising 5 species: lake whitefish (Coregonus clupeaformis), longnose sucker (Catostomus catostomus), northern pike (Esox lucius), walleye (Sander vitreus), and lake trout (Salvelinus namaycush). In reservoirs, concentrations of all species increased rapidly after impoundment, peaking after 4–11 yr in nonpiscivorous species and after 9–14 yr in piscivorous species, at levels 2–8 times higher than those measured in surrounding natural lakes. In fish of standardized length, maximum levels reached 0.33–0.72 μg g−1 in nonpiscivorous species and 1.65–4.66 μg g−1 in piscivorous species. Depending on the reservoir, the return to levels equivalent (p < 0.05) to those found in fish in surrounding natural lakes was completed after 10–20 yr for all nonpiscivorous species and after 20– 31 yr in most piscivorous species, if no additional flooding occurred. These results tend to confirm the findings of other authors suggesting that the following reservoir characteristics play a major role in determining the intensity and duration of after-impoundment THg increases in fish: flooded area, annual volume of water flowing through the reservoir, filling period, water temperature, and percentage of flooded area located in the drawdown zone.
Introduction
The presence of mercury (Hg) in the environment is a concern because of potential toxicity for humans in its organic form, methylmercury (MeHg). In Canada, the main source of human exposure to MeHg is related to fish consumption (Health Canada Citation2007). Because the impoundment of hydroelectric reservoirs usually leads to a significant increase in fish Hg levels (Verta et al. Citation1986, Strange et al. Citation1991, Verdon et al. Citation1991, Porvari Citation1998, Bodaly et al. Citation2007), an extensive monitoring program was conducted for more than 30 yr at the La Grande Complex in northern Québec, Canada, to determine the extent and duration of this phenomenon (Verdon et al. Citation1991, Lucotte et al. Citation1999, Schetagne and Therrien Citation2013), as well as to provide public health agencies with the necessary data to properly manage the health risks related to fish consumption by local subsistence and sport fishers (Girard et al. Citation1996, Chevalier et al. Citation1997). This management was achieved by issuing fish consumption advisories based on Hg levels in consumption-size fish, enabling consumers to enjoy the health benefits of eating fish while insuring safe levels of Hg exposure (Schetagne et al. Citation2005).
In this region, Hg is omnipresent despite the absence of local industrial or municipal sources. It has accumulated in forest soils since the last ice age as a result of atmospheric fallout produced naturally or anthropogenically. Hg of atmospheric origin occurs mainly in the inorganic form and is not readily assimilated by living organisms. In natural aquatic environments, it is converted to MeHg by bacteria that break down organic matter. This organic form of Hg is readily assimilated by living organisms and is biomagnified along the food chain, reaching higher levels in fish and leading to human exposure by fish consumption (Jackson Citation1988, Rodgers Citation1994, Hall et al. Citation1997, Lucotte et al. Citation1999, Kidd et al. Citation2012).
The increase in Hg following the impoundment of hydroelectric reservoirs is a phenomenon studied extensively in various regions of the world (Bodaly et al. Citation1984, Citation2007, Porvari Citation1995, Citation1998, Marrugo-Negrete et al. Citation2015, Li and Xie Citation2016, Willacker et al. Citation2016). It is mainly attributable to the flooding of large quantities of labile organic matter (vegetation and soil surface layers) containing inorganic Hg. This organic matter is subject to bacterial decomposition, which transforms the inorganic Hg already present in the flooded organic matter into MeHg.
Because of its strong affinity for organic matter, much of the MeHg produced remains in the flooded soil (Thérien and Morrison Citation1999). A portion may be transferred to the food chain by the following biological and physical processes (Lucotte et al. Citation1999): (1) passive diffusion through the water column; (2) erosion of flooded organic matter in the drawdown zone, which makes fine, Hg-rich organic particles available for aquatic filter feeders; (3) active transfer of Hg by aquatic insects burrowing in flooded soil rich in MeHg; and (4) periphyton development on flooded soils and vegetation, which promotes the methylation of Hg and its active transfer to fish via aquatic insects and zooplankton feeding on it.
The MeHg thus released enters the food web through aquatic organisms at the bottom of the food chain such as zooplankton, insect larvae, or benthic organisms and is biomagnified through the food chain, reaching maximum concentrations in piscivorous fish (Tremblay et al. Citation1996b, Citation1998b, Tremblay and Lucotte Citation1997, St-Louis et al. Citation2004). Diet is consequently the principal vector of MeHg absorption for aquatic organisms (Cabana et al. Citation1994, Hall et al. Citation1997).
The increase in MeHg production is, however, temporary because readily decomposable organic matter is rapidly depleted (Lucotte et al. Citation1999, St-Louis et al. Citation2004). At the La Grande Complex, this depletion was nearly complete 10–14 yr after impoundment, depending on the reservoir, as indicated by the changes in water quality related to decomposition, such as oxygen depletion, decreasing pH, and increases in total inorganic carbon and total phosphorus (Schetagne Citation1994, Lucotte et al. Citation1999).
No other study has monitored fish Hg levels in northern hydroelectric developments as intensively and for such a long a period in boreal reservoirs as that conducted at the La Grande Complex. The only other similar monitoring program, but somewhat less extensive, was in northern Manitoba, Canada (Bodaly et al. Citation2007). The present paper summarizes 35 yr of fish Hg monitoring in 7 large boreal reservoirs. It establishes the duration and intensity of increased fish Hg levels and identifies the main factors explaining these changes.
Study site
The La Grande Hydroelectric Complex is located in northern Québec, Canada, between the 51st and 55th parallels of north latitude in the Canadian Shield (Fig. ). It was built in 3 phases between 1973 and 2013 and called for the creation of large reservoirs, the diversion of several rivers, and a reduction in their flows (Table ). Phases 1 and 2 of this complex comprise 8 reservoirs, impounded between 1978 and 1993, with a cumulative flooded land area of 10 769 km2 for the 7 largest reservoirs covered by this study. Fish Hg levels were monitored from 1978 to 2012 in both natural and modified environments.
Figure 1. La Grande Hydroelectric Complex, including lakes and reservoirs in the East and West sectors.
Table 1. Characteristics of La Grande Complex reservoirs with post-impoundment fish total mercury (THg) level increase factors.
With the exception of brackish waters at the mouth of the La Grande Rivière, the whole complex is a freshwater system spanning 3 separate zones made up of deposits of silty clay and fine deltaic sands with extensive peatlands to the west, a middle plateau with numerous lakes, and a hilly area with glacial deposits to the east. Waters are relatively transparent, well oxygenated, and slightly acidic with a pH between 5.9 and 6.9 (Verdon et al. Citation1991, Schetagne Citation1994). The hydrological regime is governed by rain and snow, and the ice-cover period lasts from November to early May in the West Sector and to early June in the East Sector. The climate is a cold-continental type characteristic of the humid subarctic zone, with a mean annual temperature of −4 °C (SEBJ Citation1988, Lucotte et al. Citation1999), and the area is characterized by boreal forest (Roy et al. Citation1986).
Although this area is far from any major industrial activity, background fish Hg levels are relatively high (Schetagne and Therrien Citation2013, Bilodeau et al. Citation2016). Overall mean total Hg (THg) levels in nonpiscivorous species vary from 0.13 μg g−1 in longnose sucker (Catostomus catostomus) to 0.16 μg g−1 in lake whitefish (Coregonus clupeaformis). Correspondent levels in piscivorous species range from 0.59 μg g−1 in northern pike (Esox lucius) and walleye (Sander vitreus) to 0.93 μg g−1 in lake trout (Salvelinus namaycush). Although no particular geographic trend is usually observed, fish THg levels vary greatly from one lake to another, often by factors of as much as 3 to 4, as for example, from 0.30 to 1.02 μg g−1 in walleye. Hg levels in natural lakes of the area are described and discussed in Supplemental Table S1 and accompanying text. Regular monitoring of THg levels in fish of a few natural lakes from 1984 to 2012 showed no time trends, although year-to-year values may sometimes vary significantly (p < 0.05). Temporal variations in natural lakes are presented in Supplemental Fig. S1.
Methods
Sampling
The fish species targeted for THg level monitoring were those frequently consumed by indigenous peoples and were well distributed throughout the area before and after the reservoirs’ impoundments. The target species were 2 nonpiscivorous species: lake whitefish and longnose sucker; and 3 piscivorous species: northern pike, walleye, and lake trout.
Many stations were sampled in natural lakes (26) and in modified environments (56), including reservoirs, water diversion routes, and rivers with reduced flow. Of these, 41 stations were monitored regularly from 1978 to 2003. In 2004, the number of stations was reduced from 41 to 16 based on statistical analyses. Mean THg concentrations obtained with the reduced number of stations were not significantly different (p < 0.05) from those measured with all sampling stations (Therrien and Schetagne Citation2005).
For logistical reasons, the area was separated into 2 sectors, referred to as the East (Caniapiscau region) and West (Robert-Bourassa and Opinaca region) sectors, with the dividing line located between the La Grande 3 and La Grande 4 reservoirs (Fig. ). Fish populations differ slightly between the 2 sectors because dwarf lake whitefish are found only in the East Sector, whereas lake cisco (Coregonus artedi) and walleye are present only in the West Sector. In general, the sectors were sampled alternately every 2 yr.
At each station, a maximum fishing effort of 24 net-days was applied using multiple-mesh gill nets (mesh 2.5–10.2 cm) and single-mesh nets (mesh 2.5, 7.5, and 10.2 cm) 46 m long and 2.4 m high. The goal was to harvest 30 specimens of each target species at each station, evenly distributed in 5 or 6 predetermined size classes. This approach offered greater accuracy in establishing the THg–length relationship (Tremblay et al. Citation1996a).
All specimens caught were identified, counted, measured, and weighed, and their sexual maturity was determined according to the Bückmann classification (Citation1929). Bone structures were also removed to determine fish age. A portion of 20–50 g of muscle tissue, free of skin and abdominal spine, was sampled from each selected fish for THg analysis. All muscle tissues were kept frozen until laboratory analysis.
Analysis of total mercury
Several studies show Hg accumulation in fish is mainly in MeHg form, ranging in proportion from 80% to 100%, depending on the species (Lindqvist et al. Citation1991, Bloom Citation1992, Watras et al. Citation1994, Lasorsa and Allen-Gil Citation1995, Wiener et al. Citation2003). THg measurements in the fish can therefore slightly overestimate actual MeHg levels. THg analyses were performed using cold vapor atomic absorption spectrophotometry, as recommended by Environment Canada (Environment Canada Citation1979), and were measured in >35 000 fish, expressed in μg g−1 (wet weight).
From 1979 to 2012, the limit of detection (LOD) of the THg method was reduced considerably from 0.05 to 0.003 μg g−1. To perform rigorously comparable temporal statistical analyses, the data <0.05 μg g−1 measured since 1998 were automatically considered to be at this threshold.
Quality control during analyzes included method blanks, spike samples, laboratory triplicates, blind triplicates, and certified standards. The yearly average coefficient of variation (standard deviation/mean × 100) for accuracy ranged from 3.1% to 7.6% for all samples from 1986 to 2012, with an overall mean of 5.5%. Average yearly coefficients of variation for the blind triplicate samples varied from 4.1% to 9.6%, from 1990 to 2012, with an overall mean of 6.3%. Additional information concerning quality control is presented in the Supplemental Materials.
Statistical analyses
The statistical analyses (p < 0.05) of temporal and spatial trends were performed using polynomial regression analysis with indicator variables using a backward stepwise procedure (Tremblay et al. Citation1998a).
Because fish size affects Hg levels (Scott Citation1974, MacCrimmon et al. Citation1983, Porvari Citation1995), the comparisons of THg levels were made using a standardized length for each species. This length corresponds approximately to the mean lengths of the catches: 400 mm for lake whitefish, walleye, and longnose sucker and 700 mm for northern pike and lake trout.
The mean THg concentration for a standardized length of a species was considered to have returned to a level equivalent to that found in a region’s natural lakes when it fell within the range of values recorded in natural lakes or when it did not differ significantly (p > 0.05) from the levels of at least one natural lake in the region. This “background condition” was determined with fish THg data from 31 unaffected natural lakes in the La Grande Complex region (21 and 10 from the West and East sectors, respectively), including pre-flood data from lakes and rivers flooded during the impoundment of most of the reservoirs.
Results
The impoundment of all the reservoirs in the La Grande Complex caused significant but temporary increases in THg levels in all fish species (Fig. ). In the nonpiscivorous lake whitefish of standardized length, significant increases (p < 0.05) in mean THg levels were observed in most of the reservoirs following impoundment (Fig. a and b). Depending on the reservoir, mean THg concentrations reached maximum values ranging from 0.33 to 0.53 μg g−1 5–8 yr after impoundment, corresponding to increase factors of 2–5 relative to natural lake values. The return to levels equivalent to those of natural lakes occurred 10–15 yr after impoundment. Only the maximum THg levels measured in La Grande 4 and Laforge 1 reservoirs did not differ significantly (p > 0.05) from those obtained in certain natural lakes of the region (Fig. b), reaching values of only 0.33 and 0.36 μg g−1, respectively.
Figure 2. Temporal evolution of mean concentrations of total mercury (μg g−1 wet weight, ±95% CI) at standardized lengths of the main fish species in reservoirs in the La Grande Complex: (a and b) lake whitefish; (c and d) longnose sucker; (e and f) northern pike, in West and East Sector reservoirs, respectively; (g) walleye in West Sector; and (h) lake trout in East Sector.
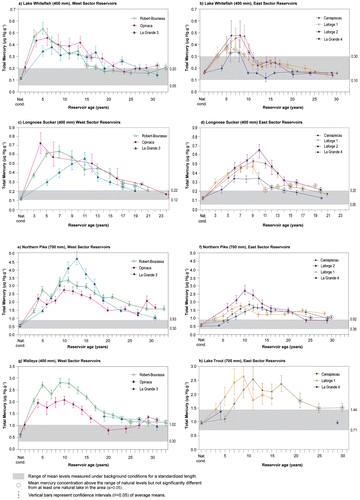
Significant increases (p < 0.05) in mean THg levels at standardized length were also observed in the nonpiscivorous longnose sucker in all the reservoirs (Fig. c and d), with a pattern of change comparable to that in lake whitefish. The mean THg concentrations reached slightly higher levels (0.34–0.72 μg g−1) than those of lake whitefish and occurred 4–11 yr after impoundment. These maximum values correspond to increase factors of 3 to 6. The time required for a return to levels equivalent to those of natural lakes was 11–20 yr after reservoir impoundment.
Mean THg levels in the piscivorous northern pike also increased significantly (p < 0.05) in all reservoirs (Fig. e and f), reaching maximum values ranging from 1.65 to 4.66 μg g−1 at standardized length 9–14 yr after impoundment, for increase factors of 3 to 8 relative to those of natural lakes. In Caniapiscau, Laforge 2, La Grande 4, and La Grande 3 reservoirs, impounded only once, mean THg concentrations then declined significantly (p < 0.05), becoming once again equivalent to background levels 24–31 yr after impoundment. For reservoirs impounded once, only mean THg levels in northern pike in Robert-Bourassa and Opinaca reservoirs remained significantly (p < 0.05) higher than background levels after more than 30 yr. Indeed, a mean THg level of 1.61 μg g−1 was obtained after 33 yr in Robert-Bourassa Reservoir, whereas a corresponding value of 1.70 μg g−1 was measured after 31 yr in Opinaca Reservoir.
Mean THg levels in northern pike in Laforge 1 Reservoir had almost completely returned to levels in the region’s natural lakes 13 yr after the reservoir’s first impoundment (1.21 μg g−1). Subsequently, another increase was observed caused by a second impoundment in 1993, flooding an additional 798 km2 of forest soils. A mean THg of 1.27 μg g−1, still higher than background levels (p < 0.05), was obtained 28 yr after the first impoundment.
In the piscivorous walleye, a significant increase (p < 0.05) in mean THg levels was observed in all reservoirs where the species was found and where sample size permitted a statistical analysis (Fig. g). The maximum values reached at standardized length ranged from 2.07 to 2.82 μg g−1 9–10 yr after impoundment, for increase factors of 4 to 5 relative to those of natural lakes. The return to values equivalent to those of natural lakes took 20–25 yr depending on the reservoir. A second significant increase (p < 0.05) was observed in Opinaca Reservoir in 2009, but in 2011 mean THg concentrations had returned to values equivalent to background levels.
The Caniapiscau Reservoir is the only reservoir impounded once that contains an abundant piscivorous lake trout population (Fig. h). For lake trout in this reservoir, mean THg levels at standardized length increased significantly (p < 0.05), reaching a maximum value of 2.53 μg g−1 11 yr after impoundment, for an increase factor of 3. Mean THg concentration returned to values equivalent to those of natural lakes 21 yr after impoundment.
In Laforge 1 Reservoir, lake trout mean THg levels reached 2.63 μg g−1 at standardized length 9 yr after the first impoundment, corresponding to an increase factor of 3, followed by a plateau in THg levels from 1993 to 1999. Monitoring campaigns conducted after 1999 in Laforge 1 Reservoir and between 1989 and 2007 in La Grande 4 Reservoir did not yield sufficient catches of lake trout to allow calculation of a statistically suitable mean THg concentration.
Discussion
Following reservoir impoundment, mean THg levels in all fish species increased significantly but temporarily. The return to mean THg levels representative of natural lakes was complete 10–20 yr after flooding in nonpiscivorous species and generally complete after 20–31 yr in piscivorous species, if no additional flooding occurred.
The analysis of fish-year classes suggests that this pattern of change reflects a situation in which the first-year classes of fish to hatch after impoundment populated the reservoirs when bacterial decomposition, methylation, and bioavailability of Hg were all at peak levels; consequently, their Hg accumulation rate would also have been maximized. Because of the depletion of readily decomposable organic matter, however, year classes born a decade or so after impoundment would have grown in reservoirs where Hg methylation and bioavailability had returned to the levels of natural lakes. Most nonpiscivorous fish reach the standardized length used to calculate mean Hg levels at roughly age 10 according to Bilodeau et al. (Citation2016), which explains why their Hg levels were no higher than those in fish of natural lakes about 20 yr after impoundment. The longer return time for large predatory fish may reflect the time necessary for later year classes of prey fish to become the main food source.
The return times recorded for La Grande reservoirs correspond well to those reported for other reservoirs in boreal regions. Indeed, a return time of 10–15 yr was observed for lake whitefish in the La Grande Complex reservoirs, comparable to that observed for other reservoirs in Québec such as Outardes 2 (<14 yr), Outardes 4 (<24 yr), Manic 1 (<18 yr), and Manic 5 (<21 yr; Verdon et al. Citation1991, Lucotte et al. Citation1999). As observed by Anderson (Citation2011), lake whitefish of 300 mm (fork length) in Smallwood Reservoir in Labrador required <16 yr to return to values measured in Labrador headwater lakes. For Manitoba reservoirs, Bodaly et al. (Citation2007) also demonstrated that concentration in lake whitefish of 350 mm (fork length) generally peaked 6 yr after impoundment and took 10–20 yr to return to values measured in surrounding natural lakes.
For northern pike and walleye, the abundant piscivorous species in the La Grande Complex, an observed resorption period usually varying 20–31 yr is also comparable to results obtained for other boreal reservoirs. For northern pike, the data gathered at other reservoirs in Québec (standardized length, 700 mm), Labrador (600 mm), and Finland (500 mm) also suggest a return to values within the range observed in natural lakes 25–30 yr after flooding (Verta et al. Citation1986, Lucotte et al. Citation1999, Anderson Citation2011, Schetagne and Therrien Citation2013). For Manitoba reservoirs, mean concentrations in northern pike at 550 mm and walleye at 400 mm (fork length) peaked 2–8 yr after impoundment, returning to levels equivalent to natural lakes 10–23 yr after flooding (Bodaly et al. Citation2007).
At the La Grande Complex, the pattern of change in THg levels was also similar for most species and reservoirs (Fig. ); the few exceptions seem to be related to reservoir characteristics and operation, building schedule, export of Hg from upstream reservoirs, or by fish diet. THg levels in northern pike in Robert-Bourassa Reservoir remaining significantly (p < 0.05) higher than background levels after >30 yr can be explained by a change in diet. Stomach content analyses conducted several years after impoundment revealed that nearly 60% of the diet of northern pike of this reservoir consisted of piscivorous fish, namely northern pike, walleye, and burbot (Lota lota; Doyon et al. Citation1996). This type of “super-predator behavior” increases with size in this species and causes a substantial increase in Hg levels by the addition of a link (or trophic level) to the food chain because Hg concentrations typically increase by a factor of 3 from one trophic level to the next (Meili Citation1991, Cabana et al. Citation1994, Kidd et al. Citation1995). This change in diet would contribute to a delay in the period of return to levels representative of natural lakes, probably for as long as such behavior persists.
For Opinaca Reservoir, the impoundment of Eastmain 1 Reservoir located immediately upstream seems to be responsible for a second significant increase (p < 0.05) in THg levels in northern pike and walleye from 2005 to 2006. Several studies have shown that Hg is exported downstream from reservoirs, thus increasing Hg levels in downstream fish (Brouard et al. Citation1994, Schetagne and Verdon Citation1999, Anderson Citation2011). A mass balance study conducted downstream from the Caniapiscau Reservoir indicated that Hg would mainly be transferred via particulate matter and zooplankton (Schetagne et al. Citation2000).
In Laforge 1 Reservoir, northern pike and lake trout THg levels peaked 9 yr after impoundment but did not show the typical gradual return to background levels; concentrations either showed a plateau from 1993 to 1999 (lake trout) or a second increase after 1993 (northern pike). This discrepancy may clearly be explained by a second impoundment occurring 13 yr after the first, flooding an additional 798 km2 of forest soils. This second impoundment would also explain, via the export of Hg downstream, why the return to background levels in northern pike was delayed in La Grande 4 Reservoir located immediately downstream of Laforge 1.
Although THg levels in the main species of fish showed the same overall pattern of change in most La Grande reservoirs, peak THg levels varied greatly from one reservoir to another. For example, for lake whitefish and northern pike, the average increase factor between peak and background levels varied from 2.5 in La Grande 4 to 5.6 in La Grande 3 Reservoir (Table ).
A better understanding of these differences is critical to improving models used to predict Hg levels in fish of planned reservoirs (Schetagne et al. Citation2009) as well as identifying potential mitigation methods (Mailman et al. Citation2006). Accordingly, numerous authors have suggested a number of key factors that would regulate after-impoundment fish Hg levels in reservoirs. The following seem to apply to the La Grande Complex reservoirs.
The land area flooded would be an indicator of the quantity of organic matter stimulating bacterial methylation of inorganic Hg (Jones et al. Citation1986, Johnston et al. Citation1991, Verdon et al. Citation1991, Kelly et al. Citation1997). The annual water volume passing through the reservoir would be an indicator of the dilution of the Hg released in the water column and would play a part in the degree of dissolved oxygen depletion (Schetagne and Verdon Citation1999), which influences the rate of Hg methylation (Gilmour and Henry Citation1991). Water temperature is deemed important because the methylation of inorganic Hg is essentially a bacterial-driven process regulated by temperature (Morrison and Thérien Citation1991, Bodaly et al. Citation1993). The reservoir filling time would contribute to determining the rate of MeHg production because most of the overall amount of MeHg produced can be reached during just 1 or 2 high temperature seasons (summer) in the case of a short filling time (Kelly et al. Citation1997, St-Louis et al. Citation2004) or during several summers in the case of a long filling time (Morrison and Thérien Citation1991). The percentage of flooded land located in the drawdown zone would indicate the rate of active transfer of MeHg to fish by periphyton and benthic organisms. Indeed, wave action in the drawdown zone rapidly breaks down the thin boreal soils, removing organic matter from the upper layers of the reservoir, thus reducing active transfer by benthic organisms. Furthermore, the removed organic material is deposited in deeper and colder waters less conductive to methylation (Lucotte et al. Citation1999). Finally, as Hg is exported downstream from reservoirs, the presence of a large reservoir immediately upstream may determine the downstream fish-Hg increases (Brouard et al. Citation1994, Schetagne et al. Citation2000, Bodaly et al. Citation2007).
Because all these factors are in play simultaneously in large boreal reservoirs, pinpointing the controlling factors is difficult. By relating the physical characteristics of the reservoirs to the measured averaged fish-Hg increase factors, however, certain factors or combination of factors can be singled out (Table ). Robert-Bourassa and La Grande 3 reservoirs showed similar average fish-Hg increase factors (5.2–5.6), with similar values for flooded land area to annual water volume ratio (31–32), for similar proportions of flooded land in the drawdown zone (29%) and for similar water temperature (1600 degree C-days for the first 10 m during the ice-free period; Table ). Opinaca Reservoir showed a similar fish-Hg increase factor (4.5), with a similar proportion of flooded land in drawdown zone (28%) but a lower flooded land area to annual water volume ratio (23) that would have been compensated by higher water temperatures (1950 degree C-days). Caniapiscau Reservoir fish showed a lower THg increase factor (3.4), despite a higher flooded land area to annual water volume ratio (115), but with a much higher proportion of flooded land in the drawdown zone (61%) and colder water temperatures (1350 degree C-days).
The much lower fish-Hg increase factors measured for La Grande 4 and Laforge 1 reservoirs (2.5–2.7) would mainly be attributable to much lower flooded land area to annual water volume ratios (4–14). For the Laforge 2 Reservoir, the similar increase factor to that of the Caniapiscau fish-Hg observed (3.8 vs. 3.4), despite a low flooded land area to annual water volume ratio (7), would clearly result from the export of Hg from Caniapiscau because 98% of the annual water volume flowing through the Laforge 2 Reservoir comes from Caniapiscau (Schetagne and Verdon Citation1999, Schetagne et al. Citation2000).
Conclusion
This study presenting >3 decades of monitoring of THg levels in fish of the La Grande Hydroelectric development helps to better understand the evolution of THg levels in fish of large boreal reservoirs and clearly establishes the extent and duration of elevated fish Hg levels.
Following reservoir impoundment, mean THg levels in all fish species increased significantly, but temporarily. In piscivorous species, mean THg levels were up to 8 times higher than those observed in natural lakes. The return to mean THg levels representative of natural lakes was complete 10–20 yr after flooding in nonpiscivorous species and generally complete after 20–31 yr in piscivorous species, if there was no additional flooding. The pattern of change in THg levels was similar for all species and reservoirs. Based on the findings of other studies as well as our own, the variations observed would be explained by physical and hydrologic characteristics such as land area flooded, annual volume of water flowing through the reservoir, filling period, water temperature, and percentage of flooded area located in the drawdown zone.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
The supplemental data for this article can be accessed here.
Funding
This work was supported by the James Bay Mercury Agreements.
Supplemental Material
Download MS Word (1.5 MB)Supplemental Fig.S1
Download JPEG Image (2.2 MB)Acknowledgements
This study was made possible by the Mercury Agreement signed by the Crees of the Baie-James region, Hydro-Québec, and the Québec government.
References
- Anderson MR. 2011. Duration and extent of elevated mercury levels in downstream fish following reservoir creation. River Syst. 19:167–176.
- Bilodeau F, Schetagne R, Therrien J, Verdon R. 2016. Absence of noticeable mercury effects on fish populations in boreal reservoirs despite threefold to sevenfold increases in mercury concentration. Can J Fish Aquat Sci. 73:1104–1125.
- Bloom NS. 1992. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 49:1010–1017.
- Bodaly RAD, Hecky RE, Fudge RJP. 1984. Increase in fish mercury levels in lakes flooded by Churchill River diversion, northern Manitoba. Can J Fish Aquat Sci. 41:682–691.
- Bodaly RAD, Jansen WA, Majewski AR, Fudge RJP, Strange NE, Derksen AJ, Green GJ. 2007. Post impoundment time course of increased mercury concentrations in fish in hydroelectric reservoirs of northern Manitoba. Canada. Arch Environ Con Tox. 53:379–389.
- Bodaly RAD, Rudd JWM, Fudge RJP, Kelly CA. 1993. Mercury concentrations in fish related to size of remote Canadian Shield lakes. Can J Fish Aquat Sci. 50:980–987.
- Brouard D, Doyon J-F, Schetagne R. 1994. Amplification of mercury concentration in lake whitefish (Coregonus clupeaformis) downstream from Robert-Bourassa reservoir, James Bay, Québec. In: Watras C, Huckabee JW, editors. Proceedings of the International Conference on Mercury Pollution: integration and synthesis. Boca Raton (FL): Lewis Publishers, CRC Press; p. 369–380.
- Bückmann A. 1929. Translated from Die methodik fishereibiologischer untersuchunsen an meeressischen. Abderhalden, handbuch der biologischen arbeidsmethoden [Methods of fishery biological investigations on marine fishes]. Berlin: Urban and Schwarsenberg, vol. 9; 194 p.
- Cabana GA, Tremblay A, Kalff J, Rasmussen JB. 1994. Pelagic food chain structure in Ontario lakes: a determinant of mercury levels in lake trout (Salvelinus namaycush). Can J Fish Aquat Sci. 51:381–389.
- Chevalier G, Dumont C-L, Penn A. 1997. Mercury in northern Québec: role of the mercury agreement and status of research and monitoring. Water Air Soil Pollut. 97:75–84.
- Doyon J-F, Tremblay A, Proulx M. 1996. Régime alimentaire des poissons du complexe La Grande et teneurs en mercure dans leurs proies (1993−1994) [La Grande complex fish diet and mercury levels in their prey]. Report by Groupe conseil GENIVAR Inc. for Hydro-Québec. 105 p. and appendices.
- Environment Canada. 1979. Analytical methods manual. Ottawa (ON): Inland Waters Directorate.
- Gilmour CG, Henry EA. 1991. Mercury methylation in aquatic systems affected by acid deposition. Environ Pollut. 71:131–169.
- Girard M, Noël F, Dumont C. 1996. Varying mercury exposure with varying food source in a James Bay Cree community. Arct Med Res. 55:69–74.
- Hall BD, Bodaly RA, Fudge JWM, Rosenberg DM. 1997. Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Pollut. 100:13–24.
- Health Canada. 2007. Human health risk assessment of mercury in fish and health benefits of fish consumption. Ottawa (ON): Health Canada; 70 p.
- Jackson TA. 1988. The mercury problem in recently formed reservoirs of northern Manitoba (Canada): effect of impoundment and other factors on the production of methylmercury by microorganims in sediments. Can J Fish Aquat Sci. 45:97–121.
- Johnston TA, Bodaly RA, Mathias JA. 1991. Predicting fish mercury levels from physical characteristics of boreal reservoirs. Can J Fish Aquat Sci. 48:1468–1475.
- Jones ML, Cunningham G, Marmorek DR, Stokes PM, Wren C, DeGrass P. 1986. Mercury release in hydroelectric reservoirs. Canadian Electrical Association; 156 p. and appendices.
- Kelly CA, Rudd JWM, Bodaly RA, Roulet NP, St-Louis VL, Heyes A, Moore TR, Schiff S, Aravena R, Scott KJ, et al. 1997. Increases in fluxes of greenhouse gases and methylmercury following flooding of an experimental reservoir. Environ Sci Technol. 31:1334–1344.
- Kidd KA, Hesslein RH, Fudge RJP, Hallard KA. 1995. The influence of trophic level as measured by δ15N on mercury concentrations in freshwater organisms. Water Air Soil Pollut. 80:1011–1015.
- Kidd KA, Muir DCG, Evans MS, Wang X, Whittle M, Swanson HK, Johnston T, Guildford S. 2012. Biomagnification of mercury through lake trout (Salvelinus namaycush) food webs of lakes with different physical, chemical and biological characteristics. Sci Total Environ. 438:135–143.
- Lasorsa B, Allen-Gil S. 1995. The methylmercury to total mercury ratio in selected marine, freshwater and terrestrial organisms. Water Air Soil Pollut. 80:905–913.
- Li J, Xie X. 2016. Heavy metal concentrations in fish species from Three Gorges Reservoirs, China, after impoundment. Bull Environ Con Tox. doi:10.1007/s00128-016-1772-0
- Lindqvist O, Johansson K, Bringmark L, Timm B, Aastrup M, Andersson A, Hovsenius G, Hakanson L, Iverfeldt A, Meili M. 1991. Mercury in the Swedish environment; recent research on causes, consequences and corrective methods. Water Air Soil Pollut. 55:1–261.
- Lucotte M, Schetagne R, Thérien N, Langlois C, Tremblay A. 1999. Mercury in the biogeochemical cycle: natural environments and hydroelectric reservoirs of northern Québec. Berlin (Germany): Springer; 334 p.
- MacCrimmon HR, Wren CD, Gots BL. 1983. Mercury uptake by lake trout, Salvelinus namaycush, relative to age, growth, and diet in Tadenac Lake with comparative data from other pre-Cambrian shield lakes. Can J Fish Aquat Sci. 40:114–120.
- Mailman M, Stepnuk L, Cicek N, Bodaly RAD. 2006. Strategies to lower methyl mercury concentrations in hydroelectric reservoirs and lakes: a review. Sci Total Environ. 368:224–235.
- Marrugo-Negrete J, Navarro-Frometa A, Ruiz-Guzman J. 2015. Total mercury concentrations in fish from Urra reservoir (Sinu River, Colombia). Six years of monitoring. Revista MVZ Cordoba. 20:4754–4765.
- Meili M. 1991. In situ assessment of trophic levels and transfer rates in aquatic food webs, using chronic (Hg) and pulsed (Chernobyl 137Cs) contaminants. Verh Internat Verein Limnol. 24:2970–2975.
- Morrison K, Thérien N. 1991. Experimental evolution of mercury release from flooded vegetation and soil. Water Air Soil Pollut. 56:607–619.
- Porvari P. 1995. Mercury levels of fish in Tucuruì hydroelectric reservoir and in River Mojù in Amazonia, in the state of Parà. Brazil. Sci Total Environ. 175:109–117.
- Porvari P. 1998. Development of fish mercury concentrations in Finnish reservoirs from 1979 to 1994. Sci Total Environ. 213:279–290.
- Rodgers DW. 1994. You are what you eat and a little bit more: bioenergetics-based models of methylmercury accumulation in fish revisited. In: Watras CJ, Huckabee JW, editors. Mercury as a global pollutant. Boca Raton (FL): Lewis Publishers; p. 427–439.
- Roy D, Boudreault J, Boucher R, Schetagne R, Thérien N. 1986. Réseau de surveillance écologique du complexe La Grande 1978−1984. Synthèse des observations [La Grande complex environmental monitoring network 1978–1984. Synthesis of observations]. Société d’énergie de la Baie James; 74 p.
- Schetagne R. 1994. Water quality modifications after impoundment of some large northern reservoirs. Arch Hydrobiol-Adv Lim. 40:223–229.
- Schetagne R, Doyon J-F, Fournier J-J. 2000. Export of mercury downstream from reservoirs. Sci Total Environ. 260:135–145.
- Schetagne R, Plante, M, Babo S, Gagnon F. 2005. Development of health risk communication tools for fish consumers in the context of hydroelectric developments. Extended abstract #150. Proceedings of 13th International Conference on Heavy Metals in the Environment, 5–9 June 2005, Rio de Janeiro (Brazil).
- Schetagne R, Plante M, Vaillancourt G, Dumont D. 2009. Health risk assessment related to mercury exposure from the consumption of fish from a proposed hydroelectric complex. Extended abstract #128-ABS-102. Proceedings of 14th International Conference on Heavy Metals in the Environment, 16–23 Nov 2008, Taipei (Taiwan).
- Schetagne R, Therrien. J. 2013. Environmental monitoring at the La Grande complex – evolution of mercury levels in fish. GENIVAR Inc. and Hydro-Québec Production. Summary report 1978−2012; 174 p.
- Schetagne R, Verdon R. 1999. Post-impoundment evolution of fish mercury levels at the La Grande Complex, Québec, Canada (from 1978 to 1996). Mercury in the biogeochemical cycle: natural environments and hydroelectric reservoirs of Northern Québec. Berlin (Germany): Springer; p. 235–258.
- Scott DP. 1974. Mercury concentration of white muscle in relation to age, growth and condition in four species of fishes from Clay Lake. Ontario. J Fish Res Board Can. 29:1723–1729.
- [SEBJ] Société d’énergie de la Baie James. 1988. The La Grande Rivière Hydroelectric Complex: the environmental challenge; 183 p.
- St-Louis VL, Rudd JWM, Kelly CA, Bodaly DRA, Paterson MJ, Beaty KG, Hesslein RH, Heyes A, Majewski AR. 2004. The rise and fall of mercury methylation in an experimental reservoir. Environ Sci Technol. 38:1348–1358.
- Strange NE, Bodaly RA, Fudge RJP. 1991. Mercury concentrations of fish in Southern Indian Lake and Issett Lake, Manitoba 1975−1988: the effect of lake impoundment and Churchill River diversion. Can J Fish Aquat Sci. Technical Report 1824; 30 p.
- Thérien N, Morrison K. 1999. In vitro release of mercury and methylmercury from flooded organic matter. Mercury in the biogeochemical cycle: natural environments and hydroelectric reservoirs of northern Québec. Berlin (Germany): Springer; p. 147–164.
- Therrien J, Schetagne R. 2005. Réseau de suivi environnemental du complexe – Optimisation du suivi du mercure dans la chair des poissons. [Environmental monitoring network of the La Grande Complex – Optimization of mercury monitoring in fish]. Report by GENIVAR Groupe Conseil Inc. for Hydro-Québec; 43 p.
- Tremblay G, Doyon J-F, Schetagne R. 1996a. Environmental monitoring network of the La Grande Complex. Monitoring of mercury levels in fish: approach and methods. Joint report of the direction principale Communications et Environment, Hydro-Québec and Groupe-conseil Génivar Inc.; 33 p. and appendices.
- Tremblay A, Lucotte M, Meili M, Coutier L, Pichet P. 1996b. Total mercury and methylmercury contents of insects from boreal lakes: ecological, spatial and temporal patterns. Water Qual Res J Can. 31:851–873.
- Tremblay A, Lucotte M. 1997. Accumulation of total mercury and methylmercury in insect larvae of hydroelectric reservoirs. Can J Fish Aquat Sci. 54:832–841.
- Tremblay G, Legendre P, Verdon R, Doyon J-F, Schetagne R. 1998a. Polynomial regression analysis with indicator variables for the interpretation of monitoring data on mercury levels in fish. Biogeochemistry. 40:189–201.
- Tremblay A, Lucotte M, Schetagne R. 1998b. Total mercury and methylmercury accumulation in zooplankton of hydroelectric reservoirs in northern Québec (Canada). Sci Total Environ. 213:307–315.
- Verdon R, Brouard D, Demers C, Lalumière R, Laperle M, Schetagne R. 1991. Mercury evolution (1978−1988) in fishes of the La Grande Hydroelectric Complex. Québec Canada. Water Air Soil Pollut. 56:405–417.
- Verta M, Rekolainen S, Kinnunen K. 1986. Causes of increased fish mercury levels in Finnish reservoirs. Publications of the Water Research Institute of Finland. 65:44–58.
- Watras CJ, Bloom NS, Hudson RJM, Gherini S, Munson R, Claas SA, Morrison KA, Wiener JG, Fitzgerald WF, Mason R, et al. 1994. Sources and fates of mercury and methylmercury in Wisconsin lakes. In: Watras CJ, Huckabee JW, editors. Mercury pollution: integration and synthesis. Boca Raton (FL): Lewis Publishers; p. 153–177.
- Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM. 2003. Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of ecotoxicology. 2nd ed. Boca Raton (FL): CRC Press; p. 409–463.
- Willacker JJ, Eagles-Smith CA, Lutz MA, Tate MT, Lepak JM, Ackerman JT. 2016. Reservoirs and water management influence fish mercury concentrations in the western United States and Canada. Sci Total Environ. doi:10.1016/j.scitotenv.2016.03.050
