?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Overgrazing is one of the major land-use impacts in Mongolia leading to habitat degradation and subsequent impairment of biological diversity. This study examined macroinvertebrate diversity among sites with different grazing intensities in Hövsgöl, Mongolia, to test whether the taxonomic and functional structure of the macroinvertebrate community differs among streams with different grazing intensity. The 14 551 total identified specimens comprised 78 genera in 27 macroinvertebrate families from the 6 study streams. Exponential Shannon index and weighted functional diversity were significantly higher in low grazing sites than in moderate and high grazing sites; no significant difference was found between moderate to high grazing intensity. Macroinvertebrate community composition was not significantly different between low and moderate or low and high grazing intensity sites. SIMPER analysis revealed the taxon with the highest contribution to dissimilarity among the levels of grazing. Thirteen trait categories from 8 traits differed significantly between sites with varying grazing pressure. The community-weighted means for 4 of these traits were filtered by high grazing intensity: dissemination, resistant form, current velocity, and saprobity. Although the other 4 traits differed significantly, they did not respond directly to grazing intensity. Further knowledge of traits, especially regarding physiological capabilities, is needed to better understand macroinvertebrate/environment relationships, but overall, these findings suggest that macroinvertebrate diversity components were affected by grazing.
Introduction
Land use is a major driver of anthropogenic change in landscapes, biogeochemical cycles, and biodiversity (Allan Citation2004, Carpenter et al. Citation2011). From local to global scales, human impacts on the environment generate a decline in freshwater biodiversity (Dudgeon et al. Citation2006) as well as cause functional homogenization due to the decline of specialized taxa with increasing stress (Mondy and Usseglio-Polatera Citation2014, de Castro et al. Citation2018).
Livestock grazing can have direct effects on watersheds, such as plant biomass reduction, alteration of plant species composition, soil erosion, soil compaction, habitat diversity reduction, increased nitrates from dung and urine, and water turbidity resulting from livestock trampling (Belsky et al. Citation1999, Reeves and Champion Citation2004). It has significant consequences for stream ecosystems (Belsky et al. Citation1999), including flow regime; changes in stream channel morphology (Knapp and Matthews Citation1996); reduction of coarse particulate organic matter (Scrimgeour and Kendall Citation2003); increased loading of fine sediments, suspended particles, and nutrients such as nitrogen and phosphorus (Quinn and Stroud Citation2002, Maasri and Gelhaus Citation2011); and alteration of stream food webs through changing light, nutrient, and organic-matter dynamics (Dolédec et al. Citation2006). Overgrazing can either negatively or positively affect densities of certain taxa associated with habitat modifications (Quinn et al. Citation1992), community structure (Scrimgeour and Kendall Citation2003), and functional attributes of macroinvertebrate communities (Dolédec et al. Citation2006). Sensitive aquatic organisms can be eliminated by eutrophication, substrate simplification, algal mat formation, bacterial pollution, acute toxicity, increased insolation, loss of riparian vegetation and other harborage for adults, dominance of burrowing taxa due to burying of solid substrate habitat, increase of suspended solids, and burial of the hyporheic zone (Strand and Merritt Citation1999).
In arid and semiarid regions across the globe, livestock grazing is a common form of land use covering >25% of the global land surface (Asner et al. Citation2004). Overgrazing is overconsumption of vegetation biomass by livestock and other grazers for long stretches of time without sufficient recovery periods (Mysterud Citation2006).
The functional trait-based approach is based on the functional differences among the species in a community associated with habitat characteristics. The method has potential to reveal changes in communities due to disturbance and its underlying mechanisms (Dolédec et al. Citation1999, Citation2006, Zhang et al. Citation2013). Townsend and Hildrew (Citation1994) made an a priori prediction (hypothesis) of expected species traits in terms of autecological interaction between organisms and their abiotic environment. The general prediction was that traits conferring population resilience (promoting refuge use and recolonization success, such as r-selected traits including many descendants per reproductive cycle, short generation time, small body size, short life span, parental care, or presence of relatively invulnerable life stages, asexual reproduction) or resistance (related to survival, such as firm attachment to substrates, high body flexibility, streamlined or flattened body form, dormancy or diapause, housing against desiccation) would be more common in temporally variable and spatially homogeneous habitats (Townsend and Hildrew Citation1994).
Since the 2000s, overgrazing has exceeded pasture carrying capacity and degraded natural habitats of Mongolian grasslands because of an almost 4-fold increase in livestock numbers over the last 80 years and a decline of traditional herding practices, particularly rotational grazing (Altanbagana and Chuluun Citation2010). With this increase in the number of livestock, >70% of pasture has exhibited some degradation and 7% is irreversibly damaged (Javzandulam et al. Citation2005). Overgrazing is one of the major land-use impacts in Mongolia leading to habitat degradation, consequently affecting the structure and function of biological communities.
The aim of this study was to examine macroinvertebrate diversity in streams with different grazing intensity along the east shore of Lake Hövsgöl, Mongolia, and to test whether the taxonomic and functional structure of the macroinvertebrate community differs among streams with different grazing intensity. Increased grazing in our study area has been shown to impact the ecosystem, resulting changes in soil temperature and moisture, plant biomass, and crane fly diversity (Batkhishig Citation2006, Lkhagva et al. Citation2013, Yadamsuren et al. Citation2015). Thus, we tested the hypothesis that macroinvertebrate diversity changes with increased grazing pressure. We predicted that (1) macroinvertebrate taxonomic and functional diversity would decrease, and (2) species traits associated with resilience and resistance such as shorter life span, high mobility, ovoviviparity, asexual reproduction, resistant form, and aerial respiration would increase with increased grazing intensity. Furthermore, considering the predicted increase in nutrient concentrations and siltation with grazing, we predicted a rise in mesotrophic and eutrophic taxa, deposit feeder and filter feeder taxa, and tolerant taxa. We sampled 3 subsites within each watershed to explore the response of instream spatial variation to grazing.
Materials and methods
Study area and sampling design
Lake Hövsgöl, a graben lake of the Baikal Rift System located on the southern border of the Siberian taiga, is the largest lake in terms of the freshwater volume in Mongolia. It is the fourth deepest lake in Central Asia and the world’s 14th largest source of freshwater. Lake Hövsgöl is also important because of its largely undisturbed watershed and a diverse and interesting biota (Kozhova et al. Citation1989, Goulden et al. Citation2006). The climate of the region is distinctly continental with a maximum air temperature 35 °C, a minimum temperature of –45 °C, and a mean annual precipitation of 300 mm a year (Nandintsetseg et al. Citation2007).
Six valleys along the northeastern shore of the lake were selected for study by the Hövsgöl Global Environmental Facility (GEF) project (). Grazing pressure in these valleys increases from south to north and includes the northern valleys of Turag and Shagnuul (heavy grazing pressure), the middle valleys of Noyon and Sevsuul (moderate grazing), and the southern valleys of Dalbay and Borsog (little or no grazing; Goulden et al. Citation2005). The upper sections of the rivers are mountainous and the river channels are poorly developed. The middle and lower reaches of each river have a well-defined water channel and are surrounded by steppe-type landscapes ().
Figure 1. The Hövsgöl region (top left) and north central Mongolia (bottom left). Filled colored triangles indicate upper, middle, and lower sampling sites along the 6 streams in this study (right). Colour version available online.
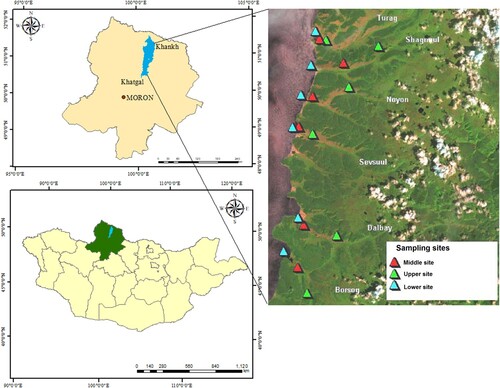
Table 1. Livestock number and descriptive characteristics of study streams in the Hövsgöl region, north central Mongolia (modified from Yadamsuren et al. Citation2015). A 3-year average estimate of stream discharge, width, depth, and velocity are included (mean [standard deviation]).
Livestock number was variable, ranging from ∼3000 to 50 livestock in sheep units between 2003 and 2005, with the highest density in the Turag and Shagnuul valleys and lowest in Borsog valleys (). Sheep units are used to give the same weight for each type of livestock. For example, 1 horse is equal to 6 sheep units, 1 cow is equal to 5 sheep units, and 1 goat is equal to 0.9 sheep units (Lkhagva et al. Citation2013). The grazing density was 45 livestock/km2 in sheep units in Turag, 67 in Shagnuul, 8 in Noyon, and 10 in Sevsuul (Lkhagva et al. Citation2013). Most nomad summer camps are located in the middle reach of each valley, and they move only 4–5 km to their winter camp; thus, grazing is almost year-round in the study valleys (Bayasgalan Citation2005). Carrying capacity is the maximum number of animals (usually expressed as a standardized livestock unit) that an area of land can support on a sustainable basis. Carrying capacity can be estimated by calculating the total amount of forage at the end of the growing season divided by the average daily feed requirements of a livestock unit (Hocking and Mattick Citation1993). A sheep livestock unit utilizes 2 kg of plants per day during summer in the Khangai region of Mongolia (Jigjidsuren and Johnson Citation2003). Pasture carrying capacity estimates indicated that the 2 northern valleys were overgrazed, the middle 2 valleys were beyond their carrying capacity, and the southern 2 valleys had low to no grazing pressure (Bayasgalan Citation2005, Puntsag et al. Citation2010).
Sampling design
Macroinvertebrates were sampled at the upper, middle, and lower reaches in the study streams with 1–3 replications from June to August using a kick sampling protocol modified from the US EPA Rapid Bioassessment Protocol (RBPs; Barbour et al. Citation1999; ). Sites were sampled over a 3-year period (2002–2005) from the different microhabitats (riffle, run, and pool) by their percent representation within a reach using kick nets (500 µm mesh size). To reduce time and effort for sorting and identification, we used a fixed-count approach, the preferred subsampling method for RBPs (Barbour et al. Citation1999). Subsamples of 200 organisms were picked from a composite of kick nets at each site; 104 total samples were collected from study sites.
Identification
Samples were fixed in 80% ethanol, and macroinvertebrates were identified to the genus level using regional identification keys (Wiederholm Citation1983, Morse et al. Citation1994, Tsalolkhin Citation1997, Citation2001, Citation2004, Zaika Citation2000, Judson and Nelson Citation2012). Chironomidae identifications were cross-checked using updated keys in Andersen et al. (Citation2013) and Ferrington and Berg (Citation2019) (Supplemental Table S1).
Macroinvertebrate identifications were made in the laboratories of the Institute of Meteorology and Hydrology, Institute of Geo-Ecology, Hövsgöl GEF offices, Ulaanbaatar, Mongolia, and Wayne State College, Wayne, Nebraska, USA. Voucher collections of macroinvertebrates were deposited in the Laboratory at the Institute of Meteorology and Hydrology and Mongolian Academy of Sciences, Ulaanbaatar.
Trait selection
For 78 genera, we selected 86 trait categories for 16 biological and ecological traits including body size, life span, voltinism, aquatic stages, reproduction, dissemination, resistant form, respiration, locomotion and substrate relation, feeding habits, food, current velocity, substrate, trophic level, temperature preference, and saprobity (Supplemental Table S2), obtainable from available sources (Bis and Usseglio-Polatera Citation2004, Maasri and Gelhaus Citation2012, Schmidt-Kloiber and Hering Citation2015). These traits were associated with the resilience, resistance, and habitat optima relevant to the environmental gradients of interest.
Traits were compiled at the subfamily level for Chironomidae (Diptera) and family level for Empididae (Diptera) and Ephydridae (Diptera). No complete trait information was available for some insect genera; thus, traits were recorded at the family level for the Cinygmula (Ephemeroptera: Heptageniidae), Semblis (Trichoptera: Phryganeidae), and at the genus level for all other insect taxa.
Trait-based analytical approaches can code trait categories using fuzzy-set theory as described by Chevene et al. (Citation1994). Fuzzy coding accommodates trait variation within a species by recording intermediate affinities. Affinity scoring ranges from 0 to 3, where 0 indicates no affinity of that species to a given state and 3 indicates the species has that particular state exclusively.
Taxon-specific affinity scores for a given trait category were treated as relative scores with respect to the sum of all affinity scores a specific taxon has for all categories of that trait. In this way, affinity scores for each taxon and trait category were rescaled between 0 and 1 (Chevene et al. Citation1994):
(1)
(1) where k = a trait category,
= frequency of a trait category,
= assigned affinity of a trait category, and h = total number of categories in a given trait.
Statistical analysis
Macroinvertebrate diversity was measured using taxon richness, exponential Shannon diversity index, and functional diversity. Functional diversity was measured using weighted functional diversity (wFD) and community-weighted mean of individual traits.
The exponential Shannon’s index, a commonly used measure of diversity (Maurer and McGill Citation2011), can be decomposed as a meaningful measure of true diversity that gives an estimate equal to the number of species when species are evenly distributed (Jost Citation2006). wFD, functional diversity weighted by species abundances (Villéger et al. Citation2008), was used to quantify functional diversity within each site. The wFD index is based on a dendrogram measure proposed by Petchey and Gaston (Citation2002, Citation2006), which is sensitive to intraspecific trait variability (Albert et al. Citation2012), and modified by Pla et al. (Citation2008, Citation2012) to account for the importance of abundant species. Dendrograms were weighted with species abundances using f-diversity software (Casanoves et al. Citation2011).
Individual trait category differences for each trait value were determined by a community-weighted mean (CWM), a mean trait value weighted by relative abundance (Díaz et al. Citation2007). CWM represents the expected functional value of a random community sample (Casanoves et al. Citation2011):
(2)
(2) where
= relative abundance of ith species, and
= trait value of ith species. The f-diversity software was used to compute a CWM for each trait and pairwise comparison of CWM among levels of grazing and sites (Casanoves et al. Citation2011).
A 3-level nested analysis of variance (ANOVA) was conducted to test whether diversity measures and CWM differed significantly along the levels of grazing intensity, streams within grazing intensity, and upper, middle, and lower reach subsites within streams. Pairwise post hoc comparisons were made using Fisher’s LSD test. JMP software 9.0.2 (SAS Citation2010) was used for ANOVA and post hoc comparisons. Taxon richness, Shannon diversity index, and functional diversity data met assumptions of normality and homogeneity of variance based on Shapiro-Wilk’s test of normality and Levene’s test for equality of variance.
To test for significant differences in community composition among sites with different grazing intensities, pairwise permutational multivariate analysis of variance (PERMANOVA; Anderson Citation2001) was implemented using the pairwiseadonis function in the R package vegan (Oksanen et al. Citation2007) with Bonferroni correction and 1000 permutations. An analysis of similarity percentages (SIMPER) was used to determine the contribution of each species (%) to the dissimilarity between each 2 groups of grazing levels (Clarke Citation1993). Shapiro-Wilk’s and Levene’s test, nested ANOVA, PERMANOVA, and SIMPER were performed on R software 3.0.1 using the vegan package (R Development Core Team Citation2013).
Results
In the 6 study streams, 14 551 specimens were identified that included 78 genera (Ephemeroptera: 10 genera, Plecoptera: 5, Trichoptera: 13, Diptera: 42, Coleoptera: 4, Megaloptera: 1, Amphipoda: 1, and Gastropoda: 1) from 27 macroinvertebrate families (Supplemental Table S1). The most common taxon was Baetis sp., which comprised 16% of the community in Borsog, 13% in Dalbay, 17% in Sevsuul, 14% in Noyon, 23% in Shagnuul, and 11% in Turag, and was the dominant taxon in 4 of the 6 study streams (except Noyon and Turag). The next most common taxon was Nemoura sp. (8–16%), and the third most common taxon was Ephemerella sp. (2–13%). Simulium sp. was dominant in Turag (26% of total abundance), and Micrasema sp. was dominant in Noyon (14% of total abundance).
Taxon richness differed significantly among reach subsites within high (p = 0.05) and moderate (p = 0.01) grazing, but no difference was found among reach subsites within levels of low grazing (a). High grazing sites were significantly different between lower and upper reaches. Among moderate grazing sites a significant difference was found between middle and upper reaches, but the low reach was not different from either the upper or middle reaches (a).
Figure 2. Differences in (a) taxon richness, (b) exponential Shannon diversity index, and (c) functional diversity indices among the sites within 3 levels of grazing intensity. Lines in boxes are medians, and box ends are quartiles. Whiskers indicate maximum and minimum values (excluding outliers), and dots represent outliers. Capital letters indicate significant differences among the categories of grazing, and lowercase letters denote significant differences among sites within grazing gradient.
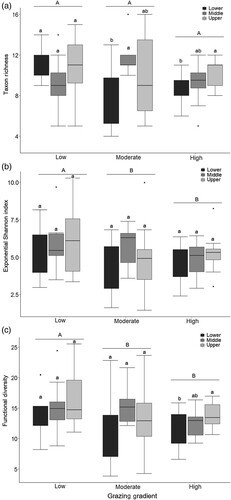
Exponential Shannon index (exp H′; p = 0.03) and functional diversity (wFD; p < 0.008) were significantly higher in low grazing than in moderate and high grazing sites, whereas taxon richness was not significantly different (p = 0.06) among sites (). Shannon index did not differ significantly among reach subsites (p = 0.06; b).
Functional diversity was higher at the upper reach compared to the low reach at high intensity grazing level (p = 0.04; c). All diversity measures were not significantly different among streams within levels of grazing (p = 0.8).
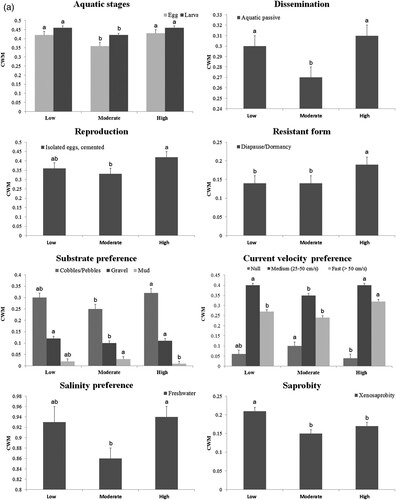
Eight traits from 13 trait categories were associated with habitat alteration due to grazing intensity (a). The CWM of these 8 traits was significantly different among the levels of grazing (a). The diapause or dormancy resistance form (p = 0.05) and preference for fast (p = 0.003) current velocity were significantly higher in sites with high grazing (a). Preference for stagnant water ( = null current velocity; p = 0.03) and fine substrates such as mud were higher in sites with moderate grazing than high grazing. No differences were found between low grazing and either moderate or high grazing (a). Aquatic life stage traits, including eggs (p = 0.03) and larva (p = 0.02), the aquatic passive dissemination trait (p = 0.02), preference for gravel substrate (p = 0.01), and medium (p = 0.02) current velocity, were lowest in moderate grazing sites (a). In addition, isolated cemented egg reproduction (p = 0.03) and preference for coarse substrates, such as cobble/pebble (p = 0.03), and freshwater (p = 0.05) were lower in moderate than in highly grazed sites. Xenosaprobic taxa were significantly lower (p = 0.01) in sites with moderate and high grazing sites (a).
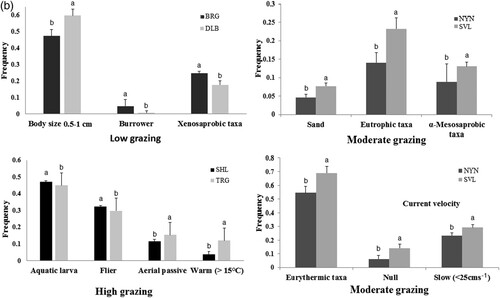
Results of the pairwise, post hoc analysis of individual traits indicated that streams with low grazing level had greater frequency of burrowers (p = 0.02) and xenosaprobic taxa (p = 0.05) in Borsog relative to the Dalbay River, whereas medium body-sized organisms (0.5–1 cm; p = 0.05) were less frequent in Borsog (b). Streams with a moderate grazing level had a greater frequency of sandy substrate (p = 0.02), eutrophic (p = 0.03), α-mesosaprobic (p = 0.02), and eurythermic (p = 0.04) taxa and null and slow current velocity (p = 0.05) in Sevsuul relative to the Noyon River (b). Streams with a high grazing level had a greater frequency of aquatic larva (p = 0.05) and flier dissemination (p = 0.01) and less aerial passive dissemination (p = 0.05) and warm water preference (p = 0.002) in Shagnuul relative to the Turag River (b). At the reach level, only brackish water preference (p = 0.02) was significantly higher in low reaches than in upper and middle reaches in the Sevsuul River (c).
PERMANOVA showed significant differences in composition between communities with moderate and high grazing (p = 0.03) but no significant difference between low and moderate or low and high grazing intensity sites. No differences were found between streams within grazing levels and among reaches along a stream. SIMPER analysis revealed the taxa with the highest contribution to dissimilarity among the levels of grazing (). The moderate grazing sites differed from high grazing sites, with Baetis, Simulium, Ephemerella, Nemoura, Rhitrogena, Triznaka, Cinygmula, Rhyacophila, and Micracema accounting for >70% of dissimilarity.
Table 2. Summary of SIMPER results among levels of grazing: average abundance of discriminating taxon in each pair of grazing levels, their contribution (%) to the dissimilarity between communities, and cumulative total (%) of contributions (∼70% cut-off).
Discussion
Macroinvertebrate communities at low grazing sites were more diverse (Shannon index and wFD; ) than at sites with moderate and high levels of grazing intensity, indicating an impact of grazing on aquatic macroinvertebrate taxonomic and functional trait diversity. In addition to overall grazing level, functional diversity differed significantly between reach subsites only within high grazing sites. Our results corroborate research showing that overgrazing by livestock has driven changes in terrestrial and aquatic ecosystems along the 6 streams of this study (Batkhishig Citation2006, Nandintsetseg et al. Citation2007, Sharkhuu et al. Citation2007, Otgonsuren et al. Citation2008, Puntsag et al. Citation2010, Lkhagva et al. Citation2013, Yadamsuren et al. Citation2015). Furthermore, livestock grazing in Mongolia has an indirect effect on macroinvertebrate diversity by modifying suitable habitat (e.g., increase in nutrient loading and sedimentation; Hayford and Gelhaus Citation2010, Yadamsuren et al. Citation2015). Habitat destruction and degradation leads to diversity decline, extirpation, and possibly extinction (Lande et al. Citation1999, Dudgeon et al. Citation2006). Functional diversity loss in biological communities and altered species composition in macroinvertebrate communities is associated with higher land-use intensity (Buendia et al. Citation2013, Colzani et al. Citation2013, de Castro et al. Citation2018). Subsequently, declining biodiversity may degrade ecosystem function (Naeem et al. Citation1999, Covich et al. Citation2004, Giller et al. Citation2004, Vaughn Citation2010).
Shannon diversity and functional diversity index scores were highest in the low grazing intensity sites compared to moderate and high grazing intensity sites, although taxon richness was not significantly different (). Genus-level taxonomic resolution may not have been sufficient to show a strong response in variation between different grazing intensities. Researchers have debated the impact of taxonomic resolution when studying the response of biodiversity to anthropogenic impacts, and, in some cases, species-level data are more responsive to environmental conditions than higher-level taxonomic resolution (e.g., Caradima et al. Citation2020). Conversely, the Shannon index was responsive to changes in grazing intensity, indicating that numerical dominance of groups such as Baetis sp. may have driven some of the observed responses. Baetis sp. was the most abundant taxon in this study and the numerically dominant taxon in 4 of the 6 study streams. Tolerance values are different among Ephemeroptera species, and Baetis sp. is relatively tolerant of pollution, with medium tolerance values (TV = 6; Merritt and Cummins Citation1996). Simulium sp. is relatively tolerant (TV = 6; Merritt and Cummins Citation1996), and this taxon was dominant in one of the high land-use intensity streams (Turag). The dominance of collector-gatherers (Baetis sp.) and collector-filterers (Simulium sp.; Merritt and Cummins Citation1996) can be related to increased fine sediment loading (Dolédec et al. Citation2006) and were the biggest contributors in characterizing the dissimilarity between the community composition of moderate and high grazing sites, as confirmed by SIMPER analysis ().
The River Continuum Concept (RCC) describes changes along a river from its headwater to mouth in terms of physical properties such as decreased substrate size, source of organic matter, and natural shifts in taxonomic and functional features of stream communities (Vannote et al. Citation1980). However, the diversity did not exhibit longitudinal variability in low to moderate sites, whereas the taxon richness and functional diversity showed consistent variation within the upper, middle, and lower reaches in highly grazed rivers (), likely due to the location of herding activities along each stream. In the study stream valleys, summer and spring herder campsites are typically located along the middle reaches because of cooler conditions and water accessibility. Furthermore, herder seasonal movements are limited to 4–5 km (Bayasgalan Citation2005), indicating that herding is localized along this portion of the study rivers. Stream health impairment and biodiversity decline have been documented with increased agricultural activity in the downstream direction (Delong and Brusven Citation1998, Harding et al. Citation1999). Thus, middle reach riparian grazing and trampling influences are likely to be propagated some distance downstream, creating a downstream “shadow” of upstream riparian conditions by fine sediment deposition and nutrient input (e.g., Feijó-Lima et al. Citation2018).
Generally, most traits selected for this study did not vary significantly among reaches, streams, and grazing levels, but a few traits related to habitat condition did. Southwood (Citation1977) outlined the habitat template concept, which states that ecological strategies of a species have evolved in response to the characteristics of habitat, and that these strategies are reflected in quantifiable life history and biological traits. Poff (Citation1997) described the function of trait filters across hierarchical landscape scales ranging from microhabitat to watershed or basin for a mechanistic understanding of the species–environment relationship. Only species possessing appropriate traits are likely to filter into certain environmental conditions at different scales (Poff Citation1997). In this study, 4 traits were filtered by high livestock grazing intensity: dissemination, resistant form, current velocity, and saprobity (a). For example, overgrazing has considerable consequences for stream ecosystems, including flooding, sediment loading, and nutrient input such as nitrogen and phosphate (Maasri and Gelhaus Citation2011, Hofmann et al. Citation2015). Semiarid streams in agricultural landscapes may couple natural disturbance regimes characterized by extreme floods and droughts with heavy grazing (Orr and Carling Citation2006, Pattison and Lane Citation2012). Increased flows resulting from loss of riparian vegetation buffer due to overgrazing may lead to increased numbers of taxa exhibiting preference for fast-flowing water. Higher stream velocity may filter for passive dispersers, but we also observed more passive dispersers in the low grazing sites. The Turag River is the largest river in terms of watershed area, and it had the highest discharge with maximum value of 9.92 m3/s (Oyunbaatar and Hiller Citation2005). Consistent with the expected trait filters, Blepharicera and Philorus, 2 dipteran genera highly specialized for fast-flowing water, were only sampled in the Turag River. Increased aquatic passive dissemination in low grazing sites (Dalbay) might be related to high stream discharge.
Dissolved organic nitrogen and carbon concentrations tended to be higher in highly grazed sites examined in this study (Puntsag et al. Citation2010). Diapause/dormancy as a resistance strategy and saprobity responded to grazing disturbance. The presence of such resistance forms likely allows organisms to withstand the impact of environmental stress in contrast to organisms lacking such forms (Townsend and Hildrew Citation1994). According to Díaz et al. (Citation2008), agricultural land use led to greater diapause/dormancy in streams. Saprobity is based on an organism’s tolerance to organic pollution (Mackie and Mackie Citation2004). Higher numbers of xenosaprobic taxa in the low grazing sites indicate unpolluted conditions with high percentage dissolved oxygen saturation.
Overgrazing can increase sedimentation in streams (e.g., Belsky et al. Citation1999). We expected natural variation in substrates to be impacted by increased sedimentation and to drive differences in macroinvertebrate preferences for substrate types. Although substrate preference differed significantly among the 3 levels of grazing intensity, we did not observe an increase in taxa that prefer mud or other fine substrates in high grazing sites (a), as previously shown (e.g., Ladrera et al. Citation2019). Decreased frequency of cemented, isolated eggs may be explained by decreased availability of clean coarse substrates in moderate grazing sites because of the Sevsuul River’s sandy substrate (Mondy and Usseglio-Polatera Citation2013), but it is unclear why the frequency increased at the high grazing sites, which should have more embedded substrate because of higher sedimentation (O'Callaghan et al. Citation2019).
Although aquatic life stages and salinity preferences (= freshwater) were significantly lower in sites with moderate grazing intensities, no differences were found between low and high grazing sites (a). Greater abundance of aquatic larvae (Hygrotus, Chironomidae) and taxa relatively tolerant to brackish water (i.e., Cloeon, Tipula, Chironomini, and Tanytarsini) may have affected the proportion of aquatic eggs, larvae, and freshwater taxa in moderate grazing sites. In other words, a smaller proportion of freshwater taxa may have been observed because of an abundance of taxa tolerant to brackish water. Higher salinity (mean = 119.7 mg/L) was detected in the low reach of the Sevsuul River relative to the upper (85.6 mg/L) and middle (82.7 mg/L) reaches (Puntsag et al. Citation2006) and may be related to taxa with higher brackish water preference in the low reach.
Variation in individual trait categories based on the pairwise comparisons was likely due to variation in physical and chemical characteristics of the study streams. Low grazing and higher frequency of xenosaprobic taxa were probably related to higher oxygenation in Borsog (average DO = 7.6 mg/L, T = 9.5 °C; Puntsag et al. Citation2006, Citation2010) relative to the Dalbay River (DO = 7.1 mg/L, T = 12.6 °C; Puntsag et al. Citation2006, Citation2010). Low frequency of medium body size (0.5–1 cm) in Borsog could be explained by greater frequency of larger body size (1–4 cm). Also, habitat characteristics of the Sevsuul River, such as sandy stream bed, warmer water temperature (average 13.2 °CSevsuul, average 9.5 °CNoyon; p < 0.05; Puntsag et al. Citation2006, Citation2010), and lower dissolved oxygen (average DOSevsuul = 5.7 mg/L, average DONoyon = 6.9 mg/L; Puntsag et al. Citation2006, Citation2010) compared to the Noyon River were paralleled by a higher frequency of sandy substrate, warm water temperature preference, eutrophic and α-mesosaprobic taxa. Higher frequency of null to slow current velocity could be explained by less stable substrate in the Sevsuul River. Greater frequency of aerial passive dissemination in the Turag River may be related to dominance of blackflies, and warm water preference was probably related to higher water temperature (average 11.7 °C; Puntsag et al. Citation2006, Citation2010) compared to Shagnuul (average 10.3 °C; Puntsag et al. Citation2006, Citation2010).
The watersheds of the 6 rivers have exhibited differences in livestock number, plant biomass (Lkhagva et al. Citation2013), soil moisture, soil bulk density, and erosion (Batkhishig Citation2006) related to grazing, but it may take time for environmental drivers to influence the fitness of organisms and to filter less-fit taxa from the community. This explanation is likely because changes in pastoral nomadism were recent (since 1990s) in the region relative to the time of sampling (Fernandez-Gimenez and Batbuyan Citation2004). However, we are already seeing changes in the exponential Shannon index and functional diversity between streams with low and moderate grazing intensity (), which corroborates research by Hayford and Gelhaus (Citation2010) that showed early impacts of moderate grazing levels on freshwater insect biodiversity.
Overall, these findings suggest that some of the macroinvertebrate diversity components, including Shannon diversity and functional diversity of macroinvertebrate communities, were affected by grazing. At moderate levels of grazing, loss in functional diversity was observed (), suggesting that effects were seen even at intermediate levels. Despite a lack of substantial distinctions in taxonomic richness among levels of grazing, the greater Shannon diversity index and functional diversity detected in low grazing intensity sites showed that the functional trait space was occupied, and the species were sufficiently disseminated in the functional space (de Castro et al. Citation2018), while less diverse communities and taxa with traits conferring resistant capacities were observed in sites with higher grazing intensity (Townsend and Hildrew Citation1994). Declines in both taxonomic and functional diversity of biological communities have been reported for macroinvertebrates associated with local environmental condition and increased land use intensity (Quinn et al. Citation1992, Colzani et al. Citation2013, Yadamsuren et al. Citation2015, Li et al. Citation2019).
The effects of land-cover changes can have a particularly detrimental impact on watersheds in arid and semiarid areas of Mongolia. Substantial ecological degradation due to the combination of continuous land and water degradation augmented by climate change will almost certainly occur. The diversity of macroinvertebrate communities declines with increased grazing, which may result in further loss of ecological function and degradation of ecosystem services and goods. This study provides information on the impacts of grazing in Mongolia, which is crucial in developing land management policies and regulations to reduce the effects of environmental degradation.
Supplemental Material
Download MS Word (47.8 KB)Acknowledgements
We thank all project implementation units and all researchers who assisted with these projects. Also thanks to office of Research and Innovation at the Mongolian National University of Education for additional support. Chironomidae were identified under the auspices of Wayne State College, Nebraska, USA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Albert CH, de Bello F, Boulangeat I, Pellet G, Lavorel S, Thuiller W. 2012. On the importance of intraspecific variability for the quantification of functional diversity. Oikos. 121(1):116–126.
- Allan JD. 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol Syst. 35:257–284.
- Altanbagana M, Chuluun T. 2010. Vulnerability assessment of Mongolian social–ecological systems. In: Renchin T, editor. Proceedings of the 4th International and National Workshop: Applications of geo-informatics for natural resources and the environment. Ulaanbaatar (Mongolia): National University of Mongolia; p. 1–11.
- Andersen T, Cranston PS, Epler JE, editors. 2013. The larvae of Chironomidae (Diptera) of the Holarctic Region—keys and diagnoses. Lund (Sweden): Insect Syst Evol (Suppl 66). 577 p.
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26:32–46.
- Asner GP, Elmore AJ, Olander LP, Martin RE, Harris AT. 2004. Grazing systems, ecosystem responses, and global change. Annu Rev Environ Resour. 29:261–299.
- Barbour MT, Gerritsen J, Snyder BD, Stribling JB. 1999. Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish. Washington (DC): US Environmental Protection Agency, Office of Water. Vol. 339.
- Batkhishig O. 2006. Soils of the Lake Hövsgöl area and its watershed. In: Goulden CE, Sitnikova T, Gelhaus J, Boldgiv B, editors. The geology, biodiversity and ecology of Lake Hövsgöl (Mongolia). Leiden (Netherlands): Backhuys Publishers; p. 93–114.
- Bayasgalan A. 2005. Nomad land use and livestock grazing impact. In: Goulden CE, Tsogtbaatar J, Mendsaikhan B, editors. The dynamics of biodiversity loss and permafrost melt in Lake Hövsgöl National Park. Ulaanbaatar (Mongolia): Mongolian Academy of Sciences, Institute of Geoecology; p. 53–80.
- Belsky AJ, Matzke A, Uselman S. 1999. Survey of livestock influences on stream and riparian ecosystems in the western United States. J Soil Water Conserv. 54(1):419–431.
- Bis B, Usseglio-Polatera P. 2004. Species trait analysis. Star Deliverable. 2:1–138.
- Buendia C, Gibbins CN, Vericat D, Batalla RJ, Douglas A. 2013. Detecting the structural and functional impacts of fine sediment on stream invertebrates. Ecol Indic. 25:184–196.
- Caradima B, Reichert P, Schuwirth N. 2020. Effects of site selection and taxonomic resolution on the inference of stream invertebrate responses to environmental conditions. Freshw Sci. 39(3):415–432.
- Carpenter SR, Stanley EH, Vander Zanden MJ. 2011. State of the world’s freshwater ecosystems: physical, chemical, and biological changes. Annu Rev Environ Resour. 36:75–99.
- Casanoves F, Pla L, Di Rienzo JA, Díaz S. 2011. F Diversity: a software package for the integrated analysis of functional diversity. Methods Ecol Evol. 2(3):233–237.
- Chevene F, Dolédec S, Chessel D. 1994. A fuzzy coding approach for the analysis of long-term ecological data. Freshw Biol. 31(3):295–309.
- Clarke KR. 1993. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 18:117–143.
- Colzani E, Siqueira T, Suriano MT, Roque FO. 2013. Responses of aquatic insect functional diversity to landscape change in Atlantic forest. Biotropica. 45(3):343–350.
- Covich AP, Austen MC, Bärlocher F, Chauvet E, Cardinale BJ, Biles CL, Inchausti P, Dangles O, Solan M, Gessner MO, et al. 2004. The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. BioScience. 54(8):767–775.
- de Castro DMP, Dolédec S, Callisto M. 2018. Land cover disturbance homogenizes aquatic insect functional structure in neotropical savanna streams. Ecol Indic. 84:573–582.
- Delong MD, Brusven MA. 1998. Macroinvertebrate community structure along the longitudinal gradient of an agriculturally impacted stream. Environ Manag. 22(3):445–457.
- Díaz AM, Alonso ML, Gutiérrez MR. 2008. Biological traits of stream macroinvertebrates from a semi-arid catchment: patterns along complex environmental gradients. Freshw Biol. 53(1):1–21.
- Díaz S, Lavorel S, de Bello F, Quétier F, Grigulis K, Robson TM. 2007. Incorporating plant functional diversity effects in ecosystem service assessments. Proc Natl Acad Sci USA. 104(52):20684–20689.
- Dolédec S, Phillips N, Scarsbrook M, Riley RH, Townsend CR. 2006. Comparison of structural and functional approaches to determining land-use effects on grassland stream invertebrate communities. J North Am Benthol Soc. 25(1):44–60.
- Dolédec S, Statzner B, Bournard M. 1999. Species traits for future biomonitoring across ecoregions: patterns along a human-impacted river. Freshw Biol. 42(4):737–758.
- Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, et al. 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev. 81(2):163–182.
- Feijó-Lima R, Mcleay SM, Silva-Junior EF, Tromboni F, Moulton TP, Zandonà E, Thomas SA. 2018. Quantitatively describing the downstream effects of an abrupt land cover transition: buffering effects of a forest remnant on a stream impacted by cattle grazing. Inland Waters. 8(3):294–311.
- Fernandez-Gimenez ME, Batbuyan B. 2004. Law and disorder: local implementation of Mongolia’s land law. Dev Change. 35(1):141–166.
- Ferrington LC Jr, Berg MB. 2019. Chironomidae. In: Merritt R, Cummins K, Berg MB, editors. An introduction to aquatic insects of North America, 5th ed. Dubuque (IA): Kendall Hunt; p. 1119–1074.
- Giller PS, Hillebrand H, Berninger UG, Gessner MO, Hawkins S, Inchausti P, Inglis C, Leslie H, Malmqvist B, Monaghan MT, et al. 2004. Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos. 104(3):423–436.
- Goulden CE, Etzelmuller B, Ariuntsetseg L. 2005. Permafrost thermal properties and thaw and its relationship to soil and plant cover, Lake Hövsgöl, Mongolia. EOS Trans. AGU 86(52). Fall Meet Suppl, Abstr. C31A-1121.
- Goulden CE, Sitnikova T, Boldgiv B, editors. 2006. The geology, biodiversity, and ecology of Lake Hövsgöl, Mongolia. Leiden (Netherlands): Backhuys Publishers; 525 p.
- Harding JS, Young RG, Hayes JW, Shearer KA, Stark JD. 1999. Changes in agricultural intensity and river health along a river continuum. Freshw Biol. 42(2):345–357.
- Hayford B, Gelhaus J. 2010. The relationship between grazing, erosion and adult aquatic insects in streams in Mongolia. Mong J Biol Sci. 8(1):27–39.
- Hocking D, Mattick A. 1993. Dynamic carrying capacity analysis as tool for conceptualizing and planning range management improvements, with a case study from India. London (UK): Overseas Development Institute.
- Hofmann J, Karthe D, Ibisch R, Schäffer M, Avlyush S, Heldt S, Kaus A. 2015. Initial characterization and water quality assessment of stream landscapes in northern Mongolia. Water. 7(7):3166–3205.
- Javzandulam T, Tateishi R, Sanjaa T. 2005. Analysis of vegetation indices for monitoring vegetation degradation in semi-arid and arid areas of Mongolia. Int J Environ Stud. 62(2):215–225.
- Jigjidsuren S, Johnson DA. 2003. Forage Plants in Mongolia. Ulaanbaatar (Mongolia): Admon Press.
- Jost L. 2006. Entropy and diversity. Oikos. 113(2):363–375.
- Judson SW, Nelson CR. 2012. A guide to Mongolian stoneflies (Insecta: Plecoptera). Zootaxa. 3541(1):1–18.
- Knapp RA, Matthews KR. 1996. Livestock grazing, golden trout, and streams in the Golden Trout Wilderness, California: impacts and management implications. N Am J Fish Manag. 16(4):805–820.
- Kozhova OM, Shagdarsuren O, Dashdorzh A, Sodnom N, Bogoyavlenskii BA, Martinov VP, Batchargal B. 1989. Atlas of Lake Hövsgöl. Moscow, Irkutsk University and the Mongolian National University. Institute of Geography of Siberian Branch of the USSR Academy of Sciences.
- Ladrera R, Belmar O, Tomás R, Prat N, Cañedo-Argüelles M. 2019. Agricultural impacts on streams near nitrate vulnerable zones: a case study in the Ebro Basin, Northern Spain. PloS One. 14(11):e0218582.
- Lande RU, Landweber LF, Dobson AP. 1999. Extinction risks from anthropogenic, ecological and genetic factors, genetics and the extinction of species: DNA and the conservation of biodiversity. Princeton (NJ): Princeton University Press; p. 1–22.
- Li Z, Wang J, Liu Z, Meng X, Heino J, Jiang X, Xiong X, Jiang X, Xie Z. 2019. Different responses of taxonomic and functional structures of stream macroinvertebrate communities to local stressors and regional factors in a subtropical biodiversity hotspot. Sci Total Environ. 655:1288–1300.
- Lkhagva A, Boldgiv B, Goulden CE, Yadamsuren O, Lauenroth WK. 2013. Effects of grazing on plant community structure and aboveground net primary production of semiarid boreal steppe of northern Mongolia. Grassl Sci. 59(3):135–145.
- Maasri A, Gelhaus J. 2011. The new era of livestock production in Mongolia: consequences on streams of the Great Lakes Depression. Sci Total Environ. 409(22):4841–4846.
- Maasri A, Gelhaus J. 2012. Stream invertebrate communities of Mongolia: current structure and expected changes due to climate change. Aquat Biosyst. 8(1):1–13.
- Mackie GL, Mackie GL. 2004. Water quality assessment techniques: applied aquatic ecosystem concepts. Dubuque (IA): Kendall Hunt; p. 419–510.
- Maurer BA, McGill BJ. 2011. Measurement of species diversity. In: Magurran AE, McGill BJ, editors. Biological diversity. Oxford (UK): Oxford University Press; p. 55–65.
- MEC. 2014. Lake Hovsgol Conservancy. Mongol Ecology Center. [accessed 2021 Jul 7]. http://www.mongolec.org/lake/en/4/m/63/aboutus.html
- Merritt RW, Cummins KW. 1996. An introduction to the aquatic insects of North America. 3rd ed. Dubuque (IA): Kendall Hunt.
- Mondy CP, Usseglio-Polatera P. 2013. Using conditional tree forests and life history traits to assess specific risks of stream degradation under multiple pressure scenario. Sci Total Environ. 461–462:750–760.
- Mondy CP, Usseglio-Polatera P. 2014. Using fuzzy-coded traits to elucidate the non-random role of anthropogenic stress in the functional homogenisation of invertebrate assemblages. Freshw Biol. 59(3):584–600.
- Morse JC, Lianfang Y, Lixin T. 1994. Aquatic insects of China useful for monitoring water quality. Nanjing, People’s Republic of China: Hohai University Press.
- Mysterud A. 2006. The concept of overgrazing and its role in management of large herbivores. Wildl Biol. 12(2):129–141.
- Naeem S, Chair FS, Chapin IIIFS. 1999. Biodiversity and ecosystem functioning: maintaining natural life support processes. Issues Ecol. 4:2–11.
- Nandintsetseg B, Greene JS, Goulden CE. 2007. Trends in extreme daily precipitation and temperature near Lake Hövsgöl, Mongolia. Int J Climatol. 27(3):341–347.
- O’Callaghan P, Kelly-Quinn M, Jennings E, Antunes P, O’Sullivan M, Fenton O, Huallachain DO. 2019. The environmental impact of cattle access to watercourses: a review. J Environ Qual. 48(2):340–351.
- Oksanen J, Blanchet JG, Kindt R. 2007. The vegan package. Community Ecol Package. 10:631–637.
- Orr HG, Carling PA. 2006. Hydro-climatic and land use changes in the River Lune catchment, North West England, implications for catchment management. River Res Appl. 22(2):239–255.
- Otgonsuren A, Goulden CE, Burke IC, Bulgan B. 2008. Soil CO2 flux in Hövsgöl National Park, Northern Mongolia. Mong J Biol Sci. 6(1–2):31–38.
- Oyunbaatar D, Hiller B. 2005. Hydrological studies. In: Goulden CE, Tsogtbaatar J, Mendsaikhan B, editors. The dynamics of biodiversity loss and permafrost melt in Lake Hövsgöl National Park, Mongolia. Ulaanbaatar (Mongolia): Mongolian Academy of Sciences, Institute of Geoecology; p. 174–240.
- Pattison I, Lane SN. 2012. The link between land-use management and fluvial flood risk: a chaotic conception? Prog Phys Geogr Earth Environ. 36(1):72–92.
- Petchey OL, Gaston KJ. 2002. Functional diversity (FD), species richness and community composition. Ecol Lett. 5(3):402–411.
- Petchey OL, Gaston KJ. 2006. Functional diversity: back to basics and looking forward. Ecol Lett. 9(6):741–758.
- Pla L, Casanoves F, Di Rienzo JA. 2008. Confidence intervals for functional diversity indices considering species abundance. Dublin (Ireland): XXIV International Biometric Conference.
- Pla L, Casanoves F, Di Rienzo J. 2012. Quantifying functional biodiversity. New York (NY): Springer.
- Poff NL. 1997. Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J North Am Benthol Soc. 16(2):391–409.
- Puntsag T, Mitchell MJ, Goulden CE, Owen JS, Hale PM. 2006. Relationships between the surface water chemical and soil chemical characteristics in Lake Hövsgöl watershed, Mongolia. Mong Geosci. 29:98–103.
- Puntsag T, Owen JS, Mitchell MJ, Goulden CE, McHale PJ. 2010. Patterns in solute chemistry of six inlet streams to Lake Hövsgöl, Mongolia. J Ecol Environ. 33(4):289–298.
- Quinn J, Davies-Colley R, Hickey C, Vickers ML, Ryan PA. 1992. Effects of clay discharges on streams. 2. Benthic invertebrates. Hydrobiologia. 248(3):235–247.
- Quinn JM, Stroud MJ. 2002. Water quality and sediment and nutrient export from New Zealand hill-land catchments of contrasting land use. N Zeal J Mar Freshw Res. 36(2):409–429.
- R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Reeves PN, Champion PD. 2004. Effects of livestock grazing on wetlands: literature review. Environment Waikato Regional Council.
- SAS Institute Inc. 2010. JMP, version 9.0. Cary (NC): SAS Institute Inc.
- Schmidt-Kloiber A, Hering D. 2015. www.freshwaterecology.info – An online tool that unifies, standardizes and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol Indic. 53:271–282.
- Scrimgeour GJ, Kendall S. 2003. Effects of livestock grazing on benthic invertebrates from a native grassland ecosystem. Freshw Biol. 48(2):347–362.
- Sharkhuu A, Sharkhuu N, Etzelmuller B. 2007. Permafrost monitoring in the Hövsgöl Mountain Region, Mongolia. J Geophys Res Earth Surf. 112(2):11.
- Southwood TR. 1977. Habitat, the templet for ecological strategies? J Anim Ecol. 46(2):336–365.
- Strand M, Merritt RW. 1999. Impacts of livestock grazing activities on stream insect communities and the riverine environment. Am Entomol. 45(1):13–29.
- Townsend CR, Hildrew AG. 1994. Species traits in relation to a habitat templet for river systems. Freshw Biol. 31(3):265–275.
- Tsalolkhin SJ. 1997. Key to freshwater invertebrates of Russia and adjacent lands. Saint Petersburg (Russia): Nauka, Zoological Institute of the Russian Academy of sciences. 3:439.
- Tsalolkhin SJ. 2001. Key to freshwater invertebrates of Russia and adjacent lands. Saint Petersburg (Russia): Nauka, Zoological Institute of the Russian Academy of sciences. 5:825.
- Tsalolkhin SJ. 2004. Key to freshwater invertebrates of Russia and adjacent lands. Saint Petersburg (Russia): Nauka, Zoological Institute of the Russian Academy of sciences. 6:516.
- Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. 1980. The River Continuum Concept. Can J Fish Aquat Sci. 37(1):130–137.
- Vaughn CC. 2010. Biodiversity losses and ecosystem function in freshwaters: emerging conclusions and research directions. BioScience. 60(1):25–35.
- Villéger S, Mason NW, Mouillot D. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology. 89(8):2290–2301.
- Wiederholm T, editor. 1983. Chironomidae of the Holarctic region. Keys and diagnoses. Part 1. Keys and diagnoses – Larvae. Entomologica Scandinavica Supplement 19: 1–457.
- Yadamsuren O, Hayford B, Gelhaus J, Ariuntsetseg L, Goulden C, Podenas S, Podeniene V. 2015. Declines in diversity of crane flies (Diptera: Tipuloidea) indicate impact from grazing by livestock in the Hövsgöl region of Mongolia. J Insect Conserv. 19(3):465–477.
- Zaika VV. 2000. An atlas guide to the aquatic invertebrates of Tuva and West Mongolia, Part 1, 2: Stonefly Insecta, Ectognatha, Plecoptera. Kyzyl: Tuvinian Institute for the Exploration of Natural Resources SB. Russian Academy of Sciences.
- Zhang L, Liu D, Liu S. 2013. Responses of functional diversity of aquatic insect community to land use change in middle reach of Qianntang River, East China. Chin J Appl Ecol. 24(10):2947–2954.