?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Human activities have significantly accelerated the translocation of species outside their native range with severe consequences for global biodiversity. Freshwater crayfish are a particularly successful group of invasive species, as exemplified by the increasingly widespread marbled crayfish Procambarus virginalis Lyko, 2017. A significant portion of crayfish populations has regenerating or missing claws. At the same time, claws play important roles in crayfish feeding and intraspecific interactions, altering their ability to invade new areas and impact native communities. Here, using functional response analyses, we combined laboratory experiments and modeling to investigate whether the number of claws on marbled crayfish modulates its ecological impact (focusing on predation rate) as well as its intraspecific interactions via population dynamic modeling. We found that the number of claws did not affect the marbled crayfish’s functional response type (hyperbolic type II), attack rates, or handling times when preying on chironomid larvae. Rates of partial prey consumption were overall low (<2%), and claw number had a significant effect on the partial consumption rate of prey, which tended to increase with the number of claws and prey density. The presence of multiple intraspecific predators influenced marbled crayfish non-trophic behavior, with antagonistic effects prevalent between crayfish pairs regardless of claw status. Moreover, the impact of multiple predators was prey density-dependent, with the highest levels of antagonism shown at the lowest prey density across crayfish groups. Our findings indicate that the ecological influence of invasive crayfish remains unchanged by the number of claws, highlighting the escalating, context-independent threat these species pose to freshwater ecosystems.
Introduction
Accelerating human-mediated species translocations have led to the arrival of thousands of species outside their native ranges (Pyšek et al. Citation2020, Soto et al. Citation2024). Following intentional or accidental introductions, some non-native species establish and spread, often resulting in adverse effects on the environment and/or economy (Lockwood et al. Citation2013, Simberloff et al. Citation2013). These invasive alien species can generate substantial ecological impacts, such as the alteration of food webs (Jeschke et al. Citation2014, David et al. Citation2017) and decline of biodiversity and ecosystem services. In addition, these species frequently pose threats to both socioeconomic stability and human well-being (Pyšek et al. Citation2020); therefore, biological invasions are considered one of the main components of global change (Masters and Norgrove Citation2010). Ongoing trends of environmental degradation from biological invasions are unprecedented and alarming, as exemplified by the situation in freshwater ecosystems, where the risk of species extinction is consistently higher than in the terrestrial and marine environment (Ricciardi and Rasmussen Citation1999, Collen et al. Citation2014).
Freshwater crayfish (Decapoda: Astacidea), especially those of North American origin, are considered a particularly successful group of invasive species, as exemplified by the myriad of widespread invasive crayfish species and populations in Europe (Kouba et al. Citation2014, Weiperth et al. Citation2020). However, their temporal trends vary across species and geographic scales (Soto et al. Citation2023). These introductions were originally motivated to replace declining native crayfish stocks, affected by the so-called “crayfish plague” (caused by the North American oomycete Aphanomyces astaci Schikora). This originally proposed fishery and aquaculture use is largely responsible for the broad distribution of invasive species, such as the spiny-cheek crayfish, Faxonius limosus (Rafinesque, 1817), signal crayfish, Pacifastacus leniusculus (Dana, 1852), and red swamp crayfish, Procambarus clarkii (Girard, 1852) (Holdich et al. Citation2009, Mojžišová et al. Citation2024). In recent decades, crayfish also became popular as ornamental aquatic pets (Chucholl Citation2013, Patoka et al. Citation2014, Weiperth et al. Citation2020), consequently leading to the establishment of several additional crayfish species in Europe (Bláha et al. Citation2022 and references therein). Of these, the parthenogenetic marbled crayfish Procambarus virginalis Lyko, 2017 is a well-recognised invader (European Commission Citation2016, Lipták et al. Citation2017), increasingly present across continental Europe (Grandjean et al. Citation2021, Sanna et al. Citation2021, Scheers et al. Citation2021) as well as overseas (Hossain et al. Citation2018).
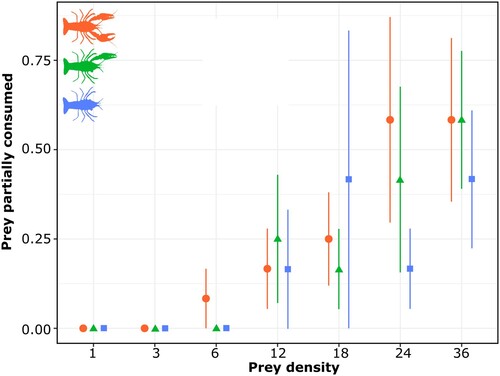
Crayfish are characterized by the presence of 3 pairs of chelate pereiopods (hereafter called claws), the first of which is considerably enlarged, especially in adult and sexually active males (Holdich Citation2002, Buřič et al. Citation2010, Citation2021). Crayfish claws are essentially involved in courtship and mating (Holdich Citation2002) as well as burrowing (Kouba et al. Citation2016) and chemoreception (Moore and Belanger Citation2009). Claws are additionally critical during intrasecific and interspecific interactions (Fořt et al. Citation2019, Roje et al. Citation2021) and food intake, mainly when prey is relatively large, mobile, or hard-shelled (Keller and Hazlett Citation1996, Sanders and Mills Citation2022). Crayfish can lose their claws, usually via autotomy during, for example, aggressive interactions through predator defense or unsuccessful molting. Their partial or complete absence imparts lower performance in the roles noted earlier, leading to potential ecological consequences (Mariappan et al. Citation2000, Kouba et al. Citation2011). The loss of claws can affect the individual’s ability to handle and consume prey, whereas individuals with both claws can manipulate and process food more efficiently (Duermit et al. Citation2015). Thus, crayfish with fully developed claws could plausibly leave more prey partially eaten when new prey items can be more easily acquired, possibly reflecting a selective feeding behavior in crayfish. Crayfish, especially those with more claws, may preferentially consume the most nutritious parts of their prey, which is particularly advantageous in environments where prey availability is abundant. This selective behavior optimizes their energy intake when they can acquire new prey items with ease, thus maximizing their overall energy gain while minimizing the effort involved in foraging. By contrast, injured crayfish will more often eat the prey entirely to cover their regeneration needs. We also expected, in line with optimal foraging theory, that higher densities of prey would stimulate greater rates of partial prey consumption, owing to selective consumption of more nutritious body parts (Jeschke et al. Citation2002, Zimmermann et al. Citation2015). Partial prey consumption can have severe consequences on ecosystems via changes in nutrient cycling, such as by prey remains being seized by scavengers and other secondary consumers (Jochum and Eisenhauer Citation2022). Thus, determining if the number of claws can modulate predation rate and partial consumption could help us better understand the impacts of invasive crayfish on local communities.
The dynamics of ecosystems are shaped not only by direct predation but also by non-trophic interactions among multiple predators that influence the behavior and survival of individual species (McCoy et al. Citation2012). Multiple predator effects can manifest antagonistically (negative non-trophic interaction; prey risk reduction) or synergistically (positive non-trophic interactions; prey risk enhancement), whereas additive interactions comprise a lack of multiple predator effects (Sih et al. Citation1998). Specifically, in crayfish, the number of claws could enhance multiple predator effects through, for example, increased fighting behavior and predator–predator interference. Additionally, this effect may be further amplified at low prey densities where competition for resources becomes more intense and predators are more likely to fully consume the prey. Multiple predator effects are thus prey density-dependent and tend to be highest at intermediate prey densities (i.e., unimodal; Sentis et al. Citation2017). The presence of multiple predators has potentially severe effects on both predator and prey populations. In the case of antagonistic effects, prey populations may be able to persist at higher predator densities. Conversely, synergistic effects may result in increased mortality for prey populations, leading to decreased population sizes or even local extinctions (Jackson et al. Citation2014, Carvalho et al. Citation2022). Despite the increasing research on the strength of non-trophic interaction in multi-predator systems, scientific research focusing on the interaction between conspecific crayfish pairs, particularly in relation to claw numbers, has been less explored (Sentis and Boukal Citation2018, but see Cuthbert et al. Citation2020, Grimm et al. Citation2020). Understanding the influence of claw number on these intraspecific interactions is crucial for comprehending the broader ecological impacts of crayfish in freshwater ecosystems.
Considering the numerous roles of claws, we aimed to investigate the influence of claw status and intraspecific interactions on foraging activity in a model invasive crayfish species, including partial prey consumption. We first estimated the single predator functional response, which describes the relationship between the feeding rate and prey density for a single individual of marbled crayfish (Holling Citation1959). We next used these per capita functional responses to predict and quantify non-trophic interactions (e.g., interference, facilitation) between conspecific crayfish groups (Sentis et al. Citation2017). We thereby determined the sign and strength of potential multiple predator effects between intraspecific marbled crayfish pairs (i.e., whether predator–predator interactions are mediated by claw status and prey density; Sentis and Boukal Citation2018).
We thus hypothesized that (1) the loss of claws and lower prey density would reduce prey consumption, as well as (2) lower the rate of partial prey consumption, while (3) altering the severity of antagonistic multiple predator effects.
Methods
Animal collection and maintenance
Experiments were conducted in July 2022 at the Research Institute of Fish Culture and Hydrobiology in Vodňany, Faculty of Fisheries and Protection of Waters, University of South Bohemia in České Budějovice, Czech Republic. The marbled crayfish was chosen as a study species, originating from our own laboratory culture consisting of a closed recirculating aquaculture system with rearing aquaria fitted with numerous shelters for crayfish, such as baked clay bricks with cross holes of different sizes and pieces of plastic pipes (Lunda et al. Citation2020). Animals were fed daily in excess using defrosted chironomid larvae, grated carrots, and commercial fish pellets containing microalgae (Sera Granugreen, Sera GmbH, Germany). The light regime was held at 14 h light:10 h dark, and the water temperature was usually 21–22 °C. We selected a similar-sized group of intermoult, nonreproductive females (i.e., females without pleopodal eggs; males are not present in the species) and developed glair glands, ranging from 16 to 21 mm in their carapace length. These specimens were assigned to 3 groups: individuals having (1) both claws fully developed, (2) 1 claw fully developed and the other missing, and (3) no claws, hereafter indicated as both claws, 1 claw, and clawless groups, respectively. Because females belonging to the latter 2 groups did not occur in sufficient numbers in our stock, an autotomy of preferably regenerating claws was induced in some. The propodus of the given claw (usually its palm) was pinched with a thumb nail of laboratory personnel while keeping the animals underwater to allow the caridoid escape reaction. Thereby, the process was rapid and effective, adequately simulating autotomy under natural conditions. Before their use, these animals were allowed to recover for at least 3 days in the communal stock with similarly treated individuals. No special permission is needed for such a manipulation.
We employed Chironomus plumosus (Linnaeus, 1758) larvae as prey because of its high abundance and availability, harvested from a local pond every 2–3 days and maintained in plastic boxes (inner dimensions 26 cm × 18 cm × 7.5 cm) filled with 2 L of aged tap water and continuous aeration. The water was exchanged daily and the dead larvae and debris were removed. The individual weight of the prey was 29.5 (3.83) mg (mean [standard deviation]).
Experiment 1: functional responses
Prior to the experiment, each crayfish was independently acclimated to the experimental arenas (plastic box with opaque walls, inner dimensions 26 cm × 18 cm × 7.5 cm) without shelter and supplied with 1 L of aged tap water. The acclimation lasted for 24 h to attain identical levels of hunger among animals. The light regime (14 h light:10 h dark) and a temperature of 21.3 (0.5) °C was maintained in the experimental incubators (Lovibond TC 445 S/445 L, Liebherr, Germany). To evaluate the effect of claws on foraging activity, each individual crayfish from 1 of the 3 groups (i.e., both claws, 1 claw, and clawless) was exposed to 7 prey densities (larvae of C. plumosus; densities: 1, 3, 6, 12, 18, 24, 36) for 4 h (during the light period), resulting in 21 treatment combinations, each replicated 12 times (i.e., 252 independent observations). Each animal was used only once in Experiment 1 (n = 252 individuals). In addition, to account for the potential natural mortality of the prey, a predator-free control was established in each trial with a density of 24 prey, resulting in 7 controls (i.e., 1 per day) over a 1-week experimentation period. Each day, 42 replicates were run at the same time (7 prey densities × 3 crayfish group × 2) with a random selection of the crayfish within each group, plus 1 day extra for those failing trials because of, for example, molting. At the end of the trial, we counted how many larvae were Alive, Eaten (entirely or partially: the latter categorized as 25%, 50%, or 75% of the body eaten to assess partly eaten prey), and Dead (no apparent signs of consumption). To address the ecological impacts associated with the crayfish claw number, we focused on the number of prey killed, disregarding the extent of their consumption.
Experiment 2: non-trophic interactions
We conducted a second experiment to quantify the strength of non-trophic interactions between multiple predators (i.e., crayfish groups), quantified by their per capita resource utilization (through parameters derived from Experiment 1). Following Experiment 1, the animals were allowed to recover in a communal stock fed ad libitum for at least 3 days before their involvement in Experiment 2, where they were not used more than twice (i.e., always allowed to recover and randomly assigned to the respective tested groups; discussed later). The experimental design comprised the simultaneous release of 2 crayfish (using all 6 claw combinations possible: clawless vs. clawless, clawless vs. 1 claw, clawless vs. both claws, 1 claw vs. 1 claw, 1 claw vs. both claws, and both claws vs. both claws) exposed to 3 initial prey densities (12, 24, and 36) representing low, medium, and high levels for the duration of the experiment, resulting in 18 combinations, each replicated 12 times (i.e., 216 independent observations with 2 crayfish each). The experimental conditions such as light regime, temperature, acclimation time, and exposure were identical to the previous experiment. Crayfish pairs were allowed to forage and interact freely. In addition, 1 predator-free control was established in each trial to account for the potential mortality of prey under the 24 density. Prey consumption was categorized as in Experiment 1.
Statistical analyses
All statistical analyses were conducted in R 4.1.3 (R Core Team Citation2022). We used the fair_test function of the frair R package (Pritchard et al. Citation2017) to identify the shape of the functional responses via logistic regression per group. All responses exhibited evidence for type-II form (i.e., significant negative first-order term) and, because we did not replace the consumed prey, we fitted the Rogers’ random predator equation to each dataset using the frair_fit function of the frair R package (Rogers Citation1972, Pritchard et al. Citation2017) as follows:
(1)
(1) where Ne refers to the number of prey killed, N0 is the initial prey density, a is the attack rate (i.e., rate at which a predator attacks the prey), h is the handling time (i.e., the time needed to consume a prey) and T is the total experimental period (i.e., 4 h). We compared the shape of the functional response for the 3 crayfish groups using 95% confidence intervals. The confidence intervals of each functional response were calculated using the frair_boot function of the frair R package, with 999 nonparametric bootstraps for a and h parameters per functional response treatment (i.e., for each of the 3 groups; Pritchard et al. Citation2017). Therefore, significant differences among groups were noted when their confidence intervals diverged, or a lack of significance was inferred when the confidence intervals overlapped (Barrios-O’Neill et al. Citation2015). We also calculated the functional response ratio (FRR) by integrating data from both the attack rate (a) and handling time (h), following the method described by Cuthbert et al. (Citation2019), where we divided the attack rate by the handling time as follows:
(2)
(2)
We fit a zero-inflated generalised linear model (GLM) with binomial family and logit link function using the function glmmTMB of the R package glmmTMB (Magnusson et al. Citation2017) to investigate any differences in the numbers of partial larvae consumed by the 3 crayfish groups (i.e., clawless, 1 claw, and both claws). These models are commonly used to assess count data with an excess of 0 observations (in our case, 1025 of 1060 values were 0: 96.69%), using a general maximum likelihood (ML) estimation algorithm. The logit link function was used to model the partial consumption (i.e., partial consumption rate out of total larvae consumed) as a function of the predictor variables (i.e., prey density, group of crayfish, and their interaction). Additionally, we performed a post hoc test using Tukey correction to compare all crayfish groups pairwise using the lsmeans function of the lsmeans R package (Lenth Citation2016).
In Experiment 2, we aimed to quantify the strength of non-trophic interactions. We first calculated the interaction strength (IS; i.e., the magnitude of the observed interactions between predator and prey) as follows:
The IS includes both trophic and non-trophic interaction (Sentis and Boukal Citation2018). To partition the empirical measurement of IS into its trophic component (i.e., trophic interaction strength, IST) and non-trophic interaction (ISNT), we first predicted IST using our estimate of functional response parameters in a population-dynamic model (McCoy et al. Citation2012). We next quantified ISNT as the difference between the observed IS and the predicted IST (Sentis et al. 2017, Cuthbert et al. Citation2021).
The estimated functional response parameters from the single predator’s experiment were used to predict multiple predator feeding rates using the following population dynamic model:
where N refers to prey density, P is predator density (i.e., P2: both claws; P1: one claw; P0: clawless), a is attack rate (i.e., a2: both claws; a1: 1 claw; a0: clawless), and h is handling time (i.e., h2: both claws; h1: 1 claw; h0: clawless) (estimated in Experiment 1). To predict the prey killed in a multi-predator experiment (i.e., Experiment 2), the initial values of N and P2, P1, P0 matched the prey and predator densities of the specific experimental treatment. Additionally, to estimate the variance for each predicted value, we used a sensitivity analysis using the sensRange function in the R package FME (Soetaert and Petzoldt Citation2010). We generated 100 sets of randomized parameters using a Latin hypercube sampling algorithm (Soetaert and Petzoldt Citation2010). The population dynamic model was applied to each of the 100 parameter sets generated randomly (Sentis et al. 2017, Cuthbert et al. Citation2021), yielding the predicted number of prey killed in each treatment in the absence of non-trophic interactions (Sentis et al. Citation2017, Veselý et al. Citation2019).
For each treatment in Experiment 2, we next calculated IST as follows:
Last, we estimated the ISNT, which refers to the magnitude of the interaction between multiple predators aside from direct predation and can thus mediate prey risk. Positive values refer to synergistic effects (i.e., combined predators kill more prey than predicted) while negative values refer to antagonism effects (i.e., fewer prey are killed than predicted). For each treatment, non-trophic interaction strength was calculated as follows:
To investigate differences in IS and/or ISNT among crayfish groups, we fit 2 linear models with IS and ISNT as the response variable for each model, respectively, and crayfish group, prey density, and their interaction as predictors. The assumptions of the model were verified using the gvlma function from the gvlma R package (Pena and Slate Citation2019).
In addition, we also conducted a sensitivity analysis for all analyses presented in the main text except the GLM of partially consumed prey to determine the impact of non-consumed larvae on our results. For this, we excluded larvae that were killed but not eaten (i.e., 0% eaten) from our models, following the same analytical approach described earlier (sensitivity analysis in Supplementary Material).
Results
Experiment 1
Natural prey mortality was 2.08% and thus was not needed to correct for background prey mortality. All 3 functional responses among marbled crayfish were classified as hyperbolic type II, owing to a significantly negative first-order term for each claw group (). Therefore, the predation rate decreased with increasing prey density, showing high feeding rates at low densities and reaching an asymptote at higher densities. We found that, based on the convergence of their 95% confidence intervals across all prey densities, there were no statistical differences among functional responses of the 3 crayfish groups (). Likewise, attack rates and handling times did not differ significantly among groups (). Crayfish maximum feeding rates ranged from 7.87 to 9.09 prey per hour, while the FRR ranged from 21.02 to 26.35 ().
Figure 1. Functional responses from the Rogers’ random predator equation with 95% non-parametric bootstrapped (n = 999) confidence intervals for each tested group of crayfish: (a) both claws, (b) 1 claw, (c) clawless. Points represent raw data.
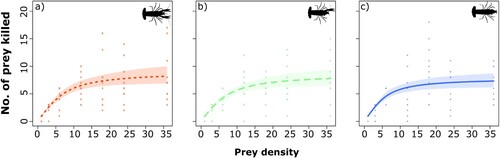
Figure 2. Non-parametric bootstrapped parameters (n = 999) for (a) attack rate and (b) handling time estimates from Rogers’ random predator equation with 95% confidence intervals considering each tested group of crayfish. Points represent the initial estimate from the random predator equation.
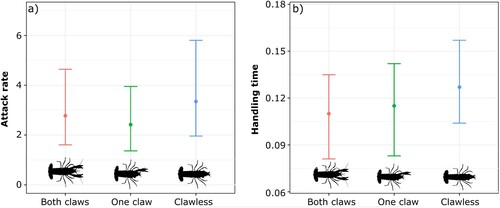
Table 1. First-order terms and p-values from logistic regression of prey killed as a function of prey density for each tested group and functional response parameters from the Rogers’ random predator equation per hour. Bold numbers indicate statistically significant values.
Rates of partial prey consumption differed significantly among tested crayfish groups (likelihood ratio = 2.442, df = 2, p < 0.01) and increased with prey density (likelihood ratio = 11.74, df = 1, p < 0.001), but not their interaction (likelihood ratio = 0.271, df = 2, p = 0.32). Partial prey consumption rates tended to increase with claw numbers but were generally low compared to total consumption (Supplemental Table S1). In the clawless group, 14 of 1200 (1.16%) prey items were partially consumed across all prey densities, whereas the 1 claw group partially consumed 17 of 1200 (1.41%) prey items, and lastly, both claws partially consumed 20 of 1200 (1.66%) prey items (). Crayfish with both claws had significantly greater rates of partial prey consumption than clawless or 1 claw crayfish (both p < 0.05), which were in turn statistically similar (p > 0.05). Overall, no partially consumed prey were found at the 1 or 3 prey density, and only 1 partially consumed prey was found at the 6 prey density. On average 0.38 of 14 (2.71%) and 0.52 of 19 (2.73%) partially consumed prey were found at the 24 and 36 prey densities, respectively, across all crayfish groups ().
Experiment 2
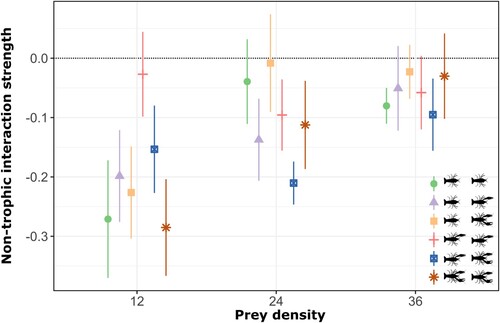
As in experiment 1, natural prey mortality rate was low (1.05%), and thus we did not correct for background prey mortality. Interaction strength (IS) decreased significantly with increasing prey density and did not differ significantly with crayfish groups considering the number of claws (Supplemental Table S2). Non-trophic interaction strengths were generally negative, suggesting that most of the interactions between crayfish groups were predominantly antagonistic (Supplemental Table S3). This finding reflects a general decrease in prey risk when crayfish were in pairs. Non-trophic interactions did not significantly differ among crayfish combinations (likelihood ratio = 0.233, df = 5, p = 0.548) but were significantly affected by prey density (likelihood ratio = 3.680, df = 1, p = 0.001) and not significantly effected by the interaction of both crayfish group and prey density (likelihood ratio = 0.334, df = 5, p = 0.332). Antagonisms were therefore prey density-dependent, with highest levels at the lowest prey density for most crayfish groups ().
Figure 4. Non-trophic interaction strengths across each tested group of crayfish (i.e., both claws, 1 claw, and clawless) and prey densities. The error bars represent standard error. The dotted line indicates expected values in the absence of non-trophic effects. Each color and shape refer to each treatment evaluated in this study.
Discussion
Overview
The spread of invasive crayfish in Europe is a growing threat to aquatic ecosystems because of their profound ecological and economic impacts, such as competition for resources, shelter, or refuge, as well as predation pressure (Souty-Grosset et al. Citation2016, Bucciarelli et al. Citation2019, Kouba et al. Citation2021). A substantial portion of the crayfish population may be represented by individuals with missing or regenerating claws, especially when occurring at high population densities (Kouba et al. Citation2011, Graham et al. Citation2021). Our results suggest that the number of claws does not significantly influence chironomid prey consumption, therefore failing to support our first hypothesis (i.e., the loss of claws reduces prey consumption). The partial consumption rate of prey, however, tended to increase with the number of claws and prey density, thereby supporting our second hypothesis (i.e., claw presence and higher prey density will increase the rate of partial prey consumption). Lastly, our results did not fully support our third hypothesis (i.e., the loss of claws, and lower prey density, will alter the severity of antagonistic multiple predator effects) because most interactions among claw groups were antagonistic and enhanced at low prey densities.
Functional responses
Type II functional responses manifest in higher rates of consumption at low prey densities, creating a greater risk of extirpation of natural resources (Jeschke et al. Citation2004). All crayfish groups followed a hyperbolic type II functional response and exhibited statistically different attack rates (a) and handling times (h). These results indicate that the loss of crayfish claws did not quantitatively change their predation rates relating to their ability to capture and handle the prey we provided. The situation may change when crayfish are exposed to larger, faster, or hard-bodied prey where the contribution of claws could be greater. Additionally, crayfish might display differential resource preferences based on claw presence, but this hypothesis requires further examination.
Recent studies have shown that the occurrence of missing claws in crayfish populations is a common phenomenon (Wood et al. Citation2020) dependant on population density (Powell et al. Citation1998, Savolainen et al. Citation2003, González et al. Citation2010). For instance, 75% of virile crayfish, Faxonius virilis (Hagen, 1870), individuals were found with missing or regenerating claws (Graham et al. Citation2021). So far, little is known about marbled crayfish wild populations, but the share of injured individuals might be relatively low (e.g., 8.7% reported by Maciaszek et al. Citation2022). However, about half of all individuals can be injured when co-occurring with larger and more aggressive crayfish counterparts, such as red swamp crayfish and common yabby, Cherax destructor (Clark, 1936) (Kouba et al. Citation2021). Thereby, affected individuals could have learned to adapt their foraging behavior, utilizing other pairs of their pereiopods (especially the second- and third-clawed pairs) to handle prey, therefore retaining their invasion risk and potential impacts (McCall and Mead Citation2008, Buřič et al. Citation2010). In addition, claw regeneration requires substantial energy that would otherwise be used in other vital processes, such as growth and reproduction (Aiken and Waddy Citation1992, Juanes and Smith Citation1995). In our study, we found that the rate of prey consumption was consistent across different crayfish claw groups, regardless of the number of claws or the presence of other food competitors. This observation suggests that the energy expended in claw regeneration does not directly influence, or is not compensated by, variations in prey consumption rates among these groups.
Partial larvae consumption
We suspected that the loss of claws could affect the ability of crayfish to handle and consume prey effectively, signaled through differences in rates of partial prey consumption (Sih et al. Citation1998). A higher number of claws could provide increased capacity and manipulation abilities, promoting potential selection of more nutritious parts of prey. The lack of claws may conversely make it difficult to capture, process, and select prey parts, resulting in more frequent full prey consumption. Our results support these relationships between partial prey consumption and claw numbers with prey density, suggesting that partial prey consumption is affected positively by claw number and prey density, as stated in optimal foraging theory.
The higher frequency of partially consumed larvae can have severe consequences for ecosystems. However, the remaining matter and energy from partially consumed larvae does not ascend into higher trophic levels, but instead is consumed by scavengers and secondary consumers. This alteration in the resource provisioning for these groups can potentially modify ecological interactions (Odum, Citation1968, Jochum and Eisenhauer Citation2022). Nevertheless, note that the total number of partially consumed prey in this experiment was negligible (51) compared with the number of totally consumed prey (1209), and thus partial consumption is expected to have a weaker ecological impact than the full consumption of prey.
Non-trophic interaction strength
The non-trophic interaction strength did not differ among crayfish groups, although it differed across prey densities. Our results suggest that the interaction among crayfish groups was mostly antagonistic (i.e., predator interference) as prey mortality was significantly reduced for most multiple predator assemblages. These results support previous evidence surrounding the interaction of multiple predators and their prey density (Sentis et al. Citation2017, Cuthbert et al. Citation2021). Antagonistic interactions suggest that predators interfere with each other or that prey escape predators better when they face multiple predators. When predators interfere and thus compete for resources, we may expect stronger interference at lower prey densities and weaker interference at high prey density where resource competition is also weaker. In line with our expectations, we found that increasing prey availability decreased the frequency and magnitude of antagonistic interactions by reducing the competition between predators for food resources. Therefore, our study suggests that the number of claws does not necessarily determine the ecological impact of crayfish populations. Additionally, the ecological impact of crayfish populations may be influenced by other factors such as population density, size distribution, and prey density. In areas with high prey density, crayfish may have a strong impact on their ecosystem because of their ability to consume large amounts of prey. By contrast, in areas where prey populations are low, crayfish may have a weaker impact because of competition among individuals for limited resources. Therefore, even in populations where the frequency of clawless individuals is higher, we can expect ecological impacts similar to populations with more claws. Nevertheless, like many laboratory feeding experiments, we caution that our confined aquaria may have influenced the intensity of interaction strengths as well as non-trophic interaction strengths. Other environmental contexts such as alternative prey or interspecific multiple predators as well as biotic conditions could have further influenced our results (Faria et al. Citation2023). While future studies could include field-based observations, the present study provides a carefully controlled analysis of density-dependence in trophic interactions according to crayfish conditions.
Conclusions
Our results suggest that the number of claws does not influence chironomid prey consumption when crayfish are kept individually or in groups. The different crayfish groups exhibited similar functional responses (i.e., their feeding strength remained unchanged despite the loss of claws). Additionally, crayfish with higher numbers of claws tended to increase the number of partially consumed prey. Finally, the non-trophic interaction strength among crayfish groups was always antagonistic, greater at low prey densities, suggesting more intense competition or interference at lower prey densities. Overall, our results provide compelling evidence that even clawless crayfish possess effective mechanisms for capturing and consuming prey, significantly influencing freshwater ecosystems. This finding highlights the potential risk posed by invasive crayfish to global biodiversity because they are capable of preying on a variety of aquatic organisms, including threatened and endangered species. Our findings contribute to identifying the mechanisms underlying the ecological role and efficiency of foraging behavior of crayfish with different numbers of claws, both in response to prey and predator densities. Based on these results, further research is needed to understand the role that claws play in the foraging behavior of crayfish. Future experiments could involve other factors such as prey preferences (e.g., hard-/soft-bodied), interspecific predatory interactions, and the role of crayfish sex. This comprehensive knowledge would help to fully unravel the functional effects of claw status in the foraging behavior and potential ecological impact of crayfish.
Supplementary Material.docx
Download MS Word (172.5 KB)Acknowledgements
This study was supported by the Ministry of Education, Youth, and Sports of the Czech Republic – project “CENAKVA” no. LM2018099. RNC is funded by the Leverhulme Trust (ECF-2021-001). This research was conducted in accordance with the objectives of the European consortium DANUBIUS RI.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aiken DE, Waddy SL. 1992. The growth process in crayfish. Rev Aquatic Sci. 6(3):4.
- Barrios-O’Neill D, Dick JT, Emmerson MC, Ricciardi A, MacIsaac HJ. 2015. Predator-free space, functional responses, and biological invasions. Funct Ecol. 29(3):377–384.
- Bláha M, Weiperth A, Patoka J, Szajbert B, Balogh ER, Staszny Á, Kouba A. 2022. The pet trade as a source of non-native decapods: the case of crayfish and shrimps in a thermal waterbody in Hungary. Environ Monit Assess. 194(11):795.
- Bucciarelli GM, Suh D, Lamb AD, Roberts D, Sharpton D, Shaffer HB, Kats LB. 2019. Assessing effects of non-native crayfish on mosquito survival. Conserv Biol. 33(1):122–131.
- Buřič M, Haubrock, PJ, Veselý L, Kozák P, Kouba A. 2021. Effective investments due to seasonal morphological changes? Possible reasons and consequences of allometric growth and reproduction in adult signal crayfish (Pacifastacus leniusculus). Can J Zool. 99(2):85–96.
- Buřič M, Kouba A, Kozák P. 2010. Intra-sex dimorphism in crayfish females. Zoology. 113(5):301–307.
- Carvalho F, Sousa R, Cássio F, Pascoal C. 2022. Temperature and interspecific competition alter the impacts of two invasive crayfish species on a key ecosystem process. Biol Invasions. 24(12):3757–3768.
- Chucholl C. 2013. Invaders for sale: trade and determinants of introduction of ornamental freshwater crayfish. Biol Invasions. 15:125–141.
- Collen B, Whitton F, Dyer EE, Baillie JE, Cumberlidge N, Darwall WR, Böhm M. 2014. Global patterns of freshwater species diversity, threat, and endemism. Glob Ecol Biogeogr. 23(1):40–51.
- Cuthbert RN, Dalu T, Wasserman RJ, Weyl OL, Froneman PW, Callaghan A, Dick JT. 2020. Examining intraspecific multiple predator effects across shifting predator sex ratios. Basic Appl Ecol. 45:12–21.
- Cuthbert RN, Dickey JW, Coughlan NE, Joyce PW, Dick JT. 2019. The functional response ratio (FRR): advancing comparative metrics for predicting the ecological impacts of invasive alien species. Biol Invasions. 21:2543–2547.
- Cuthbert RN, Wasserman RJ, Dalu T, Briski E. 2021. Warming mediates intraspecific multiple predator effects from an invasive crustacean. Mar Biol. 168:1–7.
- David P, Thebault E, Anneville O, Duyck PF, Chapuis E, Loeuille N. 2017. Impacts of invasive species on food webs: a review of empirical data. Adv Ecol Res. 56:1–60.
- Duermit E, Kingsley-Smith PR, Wilber DH. 2015. The consequences of claw removal on stone crabs Menippe spp. and the ecological and fishery implications. N Am J Fish Manag. 35(5):895–905.
- EU. 2016. Commission implementing regulation (EU) 2016/1141 of 13 July 2016 adopting a list of invasive alien species of Union concern pursuant to regulation (EU) No 1143/2014 of the European Parliament and of the Council. Off J European Union. 189(4):4–8.
- Faria L, Cuthbert RN, Dickey JW, Jeschke JM, Ricciardi A, Dick JT, Vitule JR. 2023. The rise of the functional response in invasion science: a systematic review. NeoBiota. 85:43–79.
- Fořt M, Hossain MS, Kouba A, Buřič M, Kozák P. 2019. Agonistic interactions and dominance establishment in three crayfish species non-native to Europe. Limnologica. 74:73–79.
- González R, Celada JD, González A, García V, Carral JM, Sáez-Royuela M. 2010. Stocking density for the intensive rearing of juvenile crayfish, Pacifastacus leniusculus (Astacidae), using Artemia nauplii to supplement a dry diet from the onset of exogenous feeding. Aquac Int. 18:371–378.
- Graham ZA, Vargas C, Angilletta MJ Jr, Palaoro AV. 2021. Regenerated claws of the virile crayfish Faxonius virilis (Hagen, 1870) (Decapoda: Astacidea: Cambaridae) generate weaker pinching forces compared to original claws. J Crustac Biol. 41(3):ruab036.
- Grandjean F, Collas M, Uriarte M, Rousset M. 2021. First record of a marbled crayfish Procambarus virginalis (Lyko, 2017) population in France. BioInvasions Rec. 10(2):341–347.
- Grimm J, Dick JT, Verreycken H, Jeschke JM, Linzmaier S, Ricciardi A. 2020. Context-dependent differences in the functional responses of conspecific native and non-native crayfishes. NeoBiota. 54:71–88.
- Holdich DM. 2002. Distribution of crayfish in Europe and some adjoining countries. Bull Fr Pêche Pisc. 367:611–650
- Holdich DM, Reynolds JD, Souty-Grosset C, Sibley PJ. 2009. A review of the ever-increasing threat to European crayfish from non-indigenous crayfish species. Knowl Manag Aquat Ecosyst. 11:394–395.
- Holling CS. 1959. Some characteristics of simple types of predation and parasitism. Can Entomol. 91(7):385–398.
- Hossain MS, Patoka J, Kouba A, Buřič M. 2018. Clonal crayfish as biological model: a review on marbled crayfish. Biologia. 73:841–855.
- Jackson MC, Jones T, Milligan M, Sheath D, Taylor J, Ellis A, Grey J. 2014. Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshw Biol. 59(6):1123–1135.
- Jeschke JM, Bacher S, Blackburn TM, Dick JT, Essl F, Evans T, Kumschick S. 2014. Defining the impact of non-native species. Conserv Biol. 28(5):1188–1194.
- Jeschke JM, Kopp M, Tollrian R. 2002. Predator functional responses: discriminating between handling and digesting prey. Ecol Monogr. 72(1):95–112.
- Jeschke JM, Kopp M, Tollrian R. 2004. Consumer-food systems: Why type I functional responses are exclusive to filter feeders. Biol Rev. 79(2):337–349.
- Jochum M, Eisenhauer N. 2022. Out of the dark: using energy flux to connect above- and belowground communities and ecosystem functioning. Eur J Soil Sci. 73(1):e13154.
- Juanes F, Smith LD. 1995. The ecological consequences of limb damage and loss in decapod crustaceans: a review and prospectus. J Exp Mar Biol Ecol. 193(1–2):197–223.
- Keller TA, Hazlett BA. 1996. Mechanical use of crayfish chelae. Mar Freshw Behav Physiol. 28(3):149–162.
- Kouba A, Buřič M, Policar T, Kozák P. 2011. Evaluation of body appendage injuries to juvenile signal crayfish (Pacifastacus leniusculus): relationships and consequences. Know Manag Aquatic Ecos. 401:4.
- Kouba A, Lipták B, Kubec J, Bláha M, Veselý L, Haubrock PJ, Buřič M. 2021. Survival, growth, and reproduction: comparison of marbled crayfish with four prominent crayfish invaders. Biology. 10(5):422.
- Kouba A, Petrusek A, Kozák P. 2014. Continental-wide distribution of crayfish species in Europe: update and maps. Knowl Manag Aquat Ecosyst. 413:5.
- Kouba A, Tíkal J, Císař P, Veselý L, Fořt M, Příborský J, Buřič M. 2016. The significance of droughts for hyporheic dwellers: evidence from freshwater crayfish. Sci Rep. 6(1):26569.
- Lenth RV. 2016. Least-squares means: the R package lsmeans. J Stat Softw. 69:1–33.
- Lipták B, Mojžišová M, Gruľa D, Christophoryová J, Jablonski D, Bláha M, Kouba A. 2017. Slovak section of the Danube has its well-established breeding ground of marbled crayfish Procambarus fallax f. virginalis. Knowl Manag Aquat Ecosyst. 418:40.
- Lockwood JL, Hoopes MF, Marchetti MP. 2013. Invasion ecology. Hoboken (NJ): John Wiley & Sons.
- Lunda R, Roy K, Dvorak P, Kouba A, Mraz J. 2020. Recycling biofloc waste as novel protein source for crayfish with special reference to crayfish nutritional standards and growth trajectory. Sci Rep. 10(1):19607.
- Maciaszek R, Jabłońska A, Prati S, Wróblewski P, Gruszczyńska J, Świderek W. 2022. Marbled crayfish Procambarus virginalis invades a nature reserve: How to stop further introductions? Europ Zool J. 89(1):888–901.
- Magnusson A, Skaug H, Nielsen A, Berg C, Kristensen K, Maechler M, Brooks MM. 2017. Package ‘glmmtmb’. R Package Version 0.2.0.
- Mariappan P, Balasundaram C, Schmitz B. 2000. Decapod crustacean chelipeds: an overview. J Biosci. 25:301–313.
- Masters G, Norgrove L. 2010. Climate change and invasive alien species. CABI Working Paper 1, 30.
- McCall JR, Mead KS. 2008. Structural and functional changes in regenerating antennules in the crayfish Orconectes sanborni. Biol Bull. 214(2):99–110.
- McCoy E, Burce R, David D, Aca EQ, Hardy J, Labaja J, Araujo G. 2018. Long-term photo-identification reveals the population dynamics and strong site fidelity of adult whale sharks to the coastal waters of Donsol, Philippines. Front Mar Sci. 5:271.
- McCoy MW, Stier AC, Osenberg CW. 2012. Emergent effects of multiple predators on prey survival: the importance of depletion and the functional response. Ecol Lett. 15(12):1449–1456.
- Mojžišová M, Weiperth A, Gebauer R, Laffitte M, Patoka J, Grandjean F, Kouba A, Petrusek A. 2024. Diversity and distribution of Aphanomyces astaci in a European hotspot of ornamental crayfish introductions. J Invertebr Pathol. 202:108040.
- Moore P, Belanger R. 2009. The role of the major chelae in the localization and sampling of female odours by male crayfish, Orconectes rusticus (Girard, 1852). Crustaceana. 82(6):653–668.
- Odum EP. 1968. Energy flow in ecosystems: a historical review. Am Zool. 8(1):11–18.
- Patoka J, Petrtýl M, Kalous L. 2014. Garden ponds as potential introduction pathway of ornamental crayfish. Knowl Manag Aquat Ecosyst. 414:13.
- Pena EA, Slate ME. 2019. Package ‘gvlma’. Global Validation of Linear Model Assumptions. Version 1.0.0.3.
- Pritchard DW, Paterson RA, Bovy HC, Barrios-O’Neill D. 2017. frair: an R package for fitting and comparing consumer functional responses. Meth Ecol Evol. 8(11):1528–1534.
- Powell ML, Hammer HS, Watts SA. 1998. Observations on the frequency of claw loss in the crayfish Procambarus charkii. J World Aquacult Soc. 29(4):485–490.
- Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Richardson DM. 2020. Scientists’ warning on invasive alien species. Biol Rev. 95(6):1511–1534.
- R Core Team. 2022. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/
- Ricciardi A, Rasmussen JB. 1999. Extinction rates of North American freshwater fauna. Conserv Biol. 13(5):1220–1222.
- Rogers D. 1972. Random search and insect population models. J Anim Ecol. 41:369–383.
- Roje S, Richter L, Worischka S, Let M, Veselý L, Buřič M. 2021. Round goby versus marbled crayfish: alien invasive predators and competitors. Knowl Manag Aquat Ecosyst. 422:18.
- Sanders H, Mills DN. 2022. Predation preference of signal crayfish (Pacifastacus leniusculus) on native and invasive bivalve species. River Res Appl. 38(8):1469–1480.
- Sanna D, Azzena I, Scarpa F, Cossu P, Pira A, Gagliardi F, Casu M. 2021. First record of the alien species Procambarus virginalis Lyko, 2017 in fresh waters of Sardinia and insight into its genetic variability. Life. 11(7):606.
- Savolainen R, Ruohonen K, Tulonen J. 2003. Effects of bottom substrate and presence of shelter in experimental tanks on growth and survival of signal crayfish, Pacifastacus leniusculus (Dana) juveniles. Aquac Res. 34(4):289–297.
- Scheers K, Brys R, Abeel T, Halfmaerten D, Neyrinck S, Adriaens T. 2021. The invasive parthenogenetic marbled crayfish Procambarus virginalis Lyko, 2017 gets foothold in Belgium. BioInvasions Rec. 10(2):326–340.
- Sentis A, Boukal DS. 2018. On the use of functional responses to quantify emergent multiple predator effects. Sci Rep. 8(1):1–12.
- Sentis A, Gémard C, Jaugeon B, Boukal DS. 2017. Predator diversity and environmental change modify the strengths of trophic and nontrophic interactions. Global Change Biol. 23(7):2629–2640.
- Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol Evol. 13(9):350–355.
- Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Vilà M. 2013. Impacts of biological invasions: What’s what and the way forward. Trends Ecol Evol. 28(1):58–66.
- Soetaert K, Petzoldt T. 2010. Inverse modelling, sensitivity and Monte Carlo analysis in R using package FME. J Stat Softw. 33:1–28.
- Soto I, Ahmed DA, Beidas A, Oficialdegui FJ, Tricarico E, Angeler DG, Haubrock PJ. 2023. Long-term trends in crayfish invasions across European rivers. Sci Total Environ. 867:161537.
- Soto I, Balzani P, Carneiro L, Cuthbert RN, Macêdo R, Serhan Tarkan A, Ahmed DA, Bang A, Bacela-Spychalska K, Bailey SA, et al. 2024. Taming the terminological tempest in invasion science. Biol Rev. doi:10.1111/brv.13071
- Souty-Grosset C, Anastacio PM, Aquiloni L, Banha F, Choquer J, Chucholl C, Tricarico E. 2016. The red swamp crayfish Procambarus clarkii in Europe: impacts on aquatic ecosystems and human well-being. Limnologica. 58:78–93.
- Veselý L, Boukal DS, Buřič M, Kuklina I, Fořt M, Yazicioglu B, Sentis A. 2019. Temperature and prey density jointly influence trophic and non-trophic interactions in multiple predator communities. Freshw Biol. 64(11):1984–1993.
- Weiperth A, Bláha M, Szajbert B, Seprős R, Bányai Z, Patoka J, Kouba A. 2020. Hungary: a European hotspot of non-native crayfish biodiversity. Knowl Manag Aquat Ecosyst. 421:43.
- Wood TC, Smiley PC, Gillespie RB, Gonzalez JM, King KW. 2020. Injury frequency and severity in crayfish communities as indicators of physical habitat quality and water quality within agricultural headwater streams. Environ Monit Assess. 192(4):1–17.
- Zimmermann B, Sand H, Wabakken P, Liberg O, Andreassen HP. 2015. Predator-dependent functional response in wolves: from food limitation to surplus killing. J Anim Ecol. 84(1):102–112.