?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ottawa Sands Lake is a retired mine lake located in a protected dune ecosystem adjacent to Lake Michigan in western Michigan, USA. Development activities are planned for the lake and surrounding land, potentially threatening the lake’s ecological health. We conducted a baseline study of water quality and macroinvertebrates, combined with exclusion cage experiments, to examine the role of predation. Our results indicated the lake’s littoral zone had good water quality with low phosphorus (mean soluble reactive phosphorus [SRP] < 5 µg/L; mean total phosphorus [TP] < 10 µg/L) and nitrogen (mean nitrate [NO3-N] = 38–190 µg/L; mean ammonia [NH3-N] = 8–19 µg/L, excluding deep hole) concentrations, high dissolved oxygen (>9.5 mg/L, excluding deep hole), and low chlorophyll a (<12 µg/L, except for 1 sampling date) concentrations. The macroinvertebrate community was dominated by 9 families, with Chironomidae the most abundant. Three environmental factors—specific conductivity, total dissolved solids, and ammonia—were significantly related, albeit weakly, to macroinvertebrate community structure. The exclusion cages did not result in a statistically significant difference in macroinvertebrate community structure, although high variance may have masked differences. Overall, Ottawa Sands Lake is a high-quality system, but development plans, including the installation of a paved trail around the lake and the implementation of a nonmotorized boating program, may result in changes. The baseline conditions outlined in this work will allow lake managers to assess the impact of development activity and add to the limited database regarding the limnology of retired mine lakes around the globe.
KEYWORDS:
Introduction
The ecology of sand mine lakes has received relatively little attention in the limnological literature (Meng et al. Citation2018). Sand mines often are closely linked to aquatic systems because as mine pits deepen, they can drop below the water table and begin to fill with groundwater (Tavernini et al. Citation2009a). Mine pits are most often filled via groundwater influx to form lakes but also may be filled by runoff, precipitation, or flooding (Castendyk and Eary Citation2009, USGS Citation2022). These lakes may be prone to contamination resulting from legacy pollutants associated with sand extraction, washing, and drying, thus presenting both an ecological and societal hazard to surrounding areas (McCullough and Lund Citation2006). Given the high demand for sand for use in the production of concrete, metal castings, and glass (Duan et al. Citation2019), mine lakes are likely to become more prevalent. Indeed, in the United States alone, sand and gravel mining represent 48% of all mining activity occurring annually (Aschenbach and Poling Citation2015).
Although generally understudied, a number of studies, especially in Europe, have focused on mine lakes (cf. Klapper and Geller Citation2001, Mollema and Antonellini Citation2016, Søndergaard et al. Citation2018, Seelen et al. Citation2021). These studies reveal that mine lakes undergoing active dredging have low water quality because of sediment suspension and high levels of turbidity, which negatively impact filter feeders and decrease light penetration, placing stress on photosynthetic organisms (Tavernini et al. Citation2009b). An actively mined lake was shown to have lower phytoplankton biomass compared to a retired counterpart because dredging disrupts water column stability (Tavernini et al. Citation2009b). Additionally, actively mined lakes do not typically stratify because constant mixing can result in an oxygen-rich hypolimnion that alters lake redox state water and water chemistry (Tavernini et al. Citation2009a, Citation2009b). By contrast, retired sand mine lakes have been shown to stratify and exhibit much higher water clarity compared with their actively mined counterparts (Tavernini et al. Citation2009a). The topography of mine lakes depends on prior mining activity and can strongly influence the limnology of the lake ecosystem. For example, mining activity can lead to extensive or limited littoral shelves, which when present can harbor distinct emergent and submergent vegetation (Seelen et al. Citation2021) and provide critical habitat for fish and invertebrates. In addition, deep holes can form as a function of mining, creating either limited or extensive profundal zones in these lakes.
Although retired mine lakes do not face the same threats to water quality and biodiversity as active mine lakes, they can still harbor legacy contaminants and are susceptible to cultural eutrophication (Tavernini et al. Citation2009b, Waajen et al. Citation2016). Because many of these systems lack surface inflows and outflows, nutrients can become concentrated, especially in lakes that form in areas where anthropogenic influence is present (Waajen et al. Citation2016). Regardless of mine lake status, their water quality, similar to non-mine lakes, is heavily influenced by the quality of groundwater and any other water sources feeding the lake, local geology, and land use/land cover of the area drained by the lake (Marszelewski et al. Citation2017).
The ecological status of mine lakes can be assessed not only via water chemistry but also with biotic indicators such as macroinvertebrates, organisms shown to be useful bioindicators of habitat and water quality in lotic and lentic environments (Weatherhead and James Citation2001, White and Irvine Citation2003, Orzechowski and Steinman Citation2022). Macroinvertebrate community assemblages tend to be tightly related to the ecological condition of aquatic environments, with certain taxa found only in high quality habitat while others tolerate degraded conditions (Nelson and Steinman Citation2013, Wiederholm Citation1980). Nonetheless, bioindication has limits; biophysical processes within a system (e.g., predation, wind–wave action) also may influence macroinvertebrate community structure. Hence, water quality may not be the sole driver, or even the most important driver, of macroinvertebrate taxonomic composition (Weatherhead and James Citation2001). Additionally, the assimilative capacity of aquatic systems means that not all negative impacts of degradation or pollution may be immediately visible in macroinvertebrate communities (Cairns Citation1999). Despite these potential limitations, bioindication using macroinvertebrates can provide a robust understanding of system condition over space and time, especially when complemented by water quality data (Wiederholm Citation1980). Macroinvertebrates may be especially useful in unique or atypical lentic systems, such as lakes that have formed in retired sand mines, although their presence can be dependent, at least in part, on the establishment of aquatic vegetation (Gammons et al. 2009).
Ottawa Sands Lake (OSL) is a retired sand mine lake located in Ottawa County, Michigan, USA (). In 2020, Ottawa County Parks completed a master plan for Ottawa Sands County Park that includes many elements that will directly or indirectly involve and impact OSL. Direct impacts include plans to construct a swimming area, kayak launch, fishing piers, and boardwalks in OSL (Viridis Design Group Citation2020); indirect impacts include plans to construct large parking areas, paved trails, restrooms, camping structures (i.e., yurts, cabins, tent pads), and artificial wetlands near the lake (Viridis Design Group Citation2020). Because many aspects of the Ottawa Sands master plan involve human interaction with the mine lake and its ecological communities, this study was conducted to assess its condition prior to the start of construction. This study had 2 goals: (1) identify baseline conditions in OSL to inform effective management, reclamation, and conservation practices; and (2) assess the role of trophic linkages in this constructed lake by deploying cages to exclude predators from macroinvertebrate communities in OSL’s littoral zone.
Materials and methods
Study area
OSL is a 32.4 ha sand mine lake located in Ferrysburg, Michigan, USA (), lying adjacent to the Grand River and separated from Lake Michigan by a 0.8 km-wide stretch of critical dune ecosystem. A sand mining operation began in the 1930s and ceased activity in 2017 (Lakeshore Environmental Citation2017). In July 1985, mining below the water table began to form what is now OSL as the mine pit filled with water. Although the mining permit for the site remains open, mining has ended, and the site was purchased in 2017 by Ottawa County Parks and the Land Conservancy of West Michigan. The site was opened to the public as a park in fall 2018 (Viridis Design Group Citation2020).
OSL is “perched” above Lake Michigan’s water table as a result of its encasement by a clay formation (Viridis Design Group Citation2020), and because it has no connected surface inflows or outflows, it is susceptible to changes in water level as a result of fluctuating annual precipitation (Applied Ecological Services Citation2020). Groundwater connections exist among OSL, the Grand River, and Lake Michigan, and therefore OSL water levels rise and drop in concert with Grand River and Lake Michigan, but at present little is known concerning their interactions, including flow paths and hydraulic conductivities. OSL exhibits a highly irregular bathymetry as a result of mining activity. The north side of the lake has relatively steep drop-offs while other areas have a shallow littoral shelf (Applied Ecological Services Citation2020). The deepest point of the lake is ∼20 m in the southwest quadrant of the lake (Viridis Design Group Citation2020). A natural feature survey conducted in 2020 classified the natural vegetative communities of the mine lake as emergent marsh in the littoral zone and submergent marsh in the shallow pelagic zone (Martinus Citation2020). The land immediately surrounding the lake was classified as disturbed dune field, most of which has been reclaimed or is in the process of reclamation via native planting efforts (Applied Ecological Services Citation2020, Martinus Citation2020).
Although OSL has been referred to as “pristine” because of its water clarity, environmental concerns persist resulting from its former use as a sand mine (Lakeshore Environmental Citation2017, Viridis Design Group Citation2020). One such concern is high levels of iron (Fe) and manganese (Mn) in groundwater, thought to be a legacy effect of wash plant fuels, lubricants, and other chemicals used on site beginning in the 1960s (Lakeshore Environmental Citation2017). However, current environmental reports show no concern of contamination by these or other contaminants in the surface water of OSL (Lakeshore Environmental Citation2017, Viridis Design Group Citation2020). Another concern is the potential degradation of OSL by invasive species because of its proximity to other waterbodies and its use by local waterfowl. A few invasive macrophytes have been documented to date (Myriophyllum spicatum, Nitellopsis obtusa, and Phragmites australis), although their proliferation may be limited by low nutrient levels in OSL (Lakeshore Environmental Citation2017, Elgin Citation2020).
Other concerns regarding the ecological integrity of OSL relate to a fragile critical dune ecosystem surrounding the lake (Lakeshore Environmental Citation2017). A recent study that ranked areas in the Great Lakes basin based on conservation importance for rare birds highlighted OSL and its surrounding area as a hotspot of critical habitat for imperiled migratory fowl (National Audubon Society Citation2021). Because of its unique position near the juncture of the Grand River and Lake Michigan, and its connectivity with other public lands, OSL is considered a high priority for conservation, not only for waterfowl but for other local flora and fauna (Herpetological Resource and Management Citation2020, Martinus Citation2020, National Audubon Society Citation2021).
Field sampling and lab analysis
Water quality
Water chemistry was monitored monthly from July 2020 to June 2021 at 3 sites in the OSL littoral zone (), selected based on ease of access and the presence of littoral vegetation, which was not continuous around the lake. A YSI 6600 V2 multi-parameter sonde (YSI, Yellow Springs, OH, USA) was used to assess water temperature, dissolved oxygen (DO) concentration, pH, specific conductance (SpCond), total dissolved solids (TDS), and turbidity. We logged 33 YSI samples (3 sites × 11 months); January 2021 YSI data were lost because of a logging instrument malfunction. Water samples were collected at the water surface of each site and assessed for ammonia (NH3), nitrate (NO3−), soluble reactive phosphorus (SRP), and total phosphorus (TP), resulting in 36 samples in the lake (3 sites × 12 months).
Water chemistry was also assessed on a seasonal basis at the surface, mid-depth (∼7.7 m), and near-bottom (∼14.2 m) of a central part of OSL using the same methods described earlier. Water samples were taken in July 2020, September 2020, April 2021, and July 2021 via kayak. A winter sample was not possible because of unstable ice cover. All nutrient analyses were conducted using Standard Methods protocols (APHA Citation2005). After returning to the lab, water from each site was gently inverted and subsampled for analysis of (1) phosphorus (P) as both SRP and TP and (2) nitrogen (N) as both NO3−-N and NH3-N species. Duplicate water quality samples were collected once a month for quality control. Water for SRP and NO3− analyses was syringe-filtered through acid-washed 0.45 μm membrane filters into scintillation vials; SRP was refrigerated at 4 °C, and NO3− samples were frozen until analysis. NH3 was preserved using sulfuric acid and refrigerated at 4 °C until analysis. Nutrients were analyzed on a SEAL AQ2 (SEAL Analytical, Mequon, WI, USA) discrete automated analyzer (US EPA Citation1993). Chlorophyll a (Chl-a) samples were filtered on glass-fiber filters (GF/F, Whatman, Buckinghamshire, UK), frozen for 24 h, and concentrations determined spectrophotometrically after extraction in 90% (v/v) acetone/water solution on a Shimadzu UV-1601 (Shimadzu Scientific Instruments, Columbia, MD, USA) spectrophotometer (Steinman et al. Citation2017).
Exclusion experiment
Two cages (1 m × 1 m × 1 m) were deployed at each of the 3 littoral sampling sites, ∼1–2 m from each other at the same approximate depth. The cages (mesh size: 3.175 mm) were constructed of PVC frames; the control cage had no wire mesh and the exclusion cage had wire mesh attached to all sides except the bottom. One Hester-Dendy sampler (Hester and Dendy Citation1962) was placed in the middle of each cage to serve as a substrate for macroinvertebrate colonization. The 9-plate Hester-Dendy samplers were constructed from high-density fiberboard (Masonite) and exchanged approximately monthly to ensure adequate colonization of invertebrates (Mamola Citation2005, Bacey and Barry Citation2008) over the 12-month sampling period. The Hester-Dendy samplers from each site were returned to the lab, and invertebrates were scraped into a 500 µm mesh sieve using a soft-bristle toothbrush. The contents were transferred to labeled jars with 90% ethanol until sorting and identification. Macroinvertebrates were identified to the family level using protocols outlined in the State of Michigan surface water assessment guidance (Procedure 51) using the keys developed by Merritt et al. (Citation2019) and Peckarsky et al. (Citation1990).
Data analysis
Macroinvertebrate community structure was assessed for overall trends using non-metric multidimensional scaling (NMDS) to compare spatial and temporal patterns. A Bray-Curtis dissimilarity coefficient was used as the distance metric for NMDS analysis (Bray and Curtis Citation1957). Stress values were <0.20. A permutational multivariate analysis of variance using distance matrices (adonis) was used as a post hoc test to determine the presence of significant differences in macroinvertebrate community composition among sites using a 95% confidence interval. A similarity to percentages (SIMPER) analysis was conducted to determine which macroinvertebrate families contributed to the highest level of dissimilarity among paired sites and paired seasons. NMDS, adonis, and SIMPER analyses were performed using R 4.2.2 and the vegan package (Venables and Ripley Citation2002, R Core Team Citation2019, Oksanen et al. Citation2020, Wickham et al. Citation2021).
Macroinvertebrate diversity was calculated using the Shannon-Wiener index (), which was then converted into the effective number of species (
) for easier comparison between sites (n = 3), seasons (n = 4), and treatments (n = 2) (Jost Citation2010). Macroinvertebrate density for each site was calculated by totaling the counts for each family by treatment type and dividing the sum by the average surface area for the respective Hester-Dendy sampler. Submerged surface area of the substrates decreased as water depth declined because of the fixed distance at which the samplers were originally placed. A paired sample Wilcoxon signed-rank test was performed to determine whether site or season had a significant influence on median macroinvertebrate density based on treatment type (Wilcoxon Citation1945). Normality was tested using Shapiro-Wilk and homogeneity was tested using Bartlett’s test of homogeneity of variance (Shapiro and Wilk Citation1965). All analyses were conducted using the stats and rstatix packages (R 4.2.2).
Results
Water quality
Overall, water quality in OSL is good. The lake is alkaline (pH ∼8.3), with relatively low SpCond (∼275 µS/cm), near or entirely saturated DO at all surface and middle depths, and Chl-a concentrations ∼10 µg/L in the littoral region and <2 µg/L in the pelagic region (). DO near the deepest part of the lake remained >2 mg/L, but we did not sample in midwinter when concentrations may become hypoxic or anoxic. The relatively high mean Chl-a concentration at the deep site is driven by a single anomalously high reading (16.8 µg/L) and should be viewed with caution (). The mean water quality values were similar among the 3 littoral sites, with the exception of lower Chl-a at site 3, suggesting the lake was well-mixed, at least horizontally.
Table 1. Water quality data (mean and range values) collected from the 3 littoral sites and 1 pelagic site in Ottawa Sands Lake from June 2020 to July 2021. Total samples: littoral (n = 13); pelagic (n = 4).
Vertical profiles of temperature and DO at the deep hole in July and September 2020 and July 2021 provide evidence of stratification in OSL (). Near-bottom temperatures dropped ∼50–67% compared to the surface, while near-bottom DO concentrations declined ∼2 mg/L from the surface and 6–7 mg/L from mid-depth in both July 2020 and 2021, and declined ∼3-fold from the surface and mid-depth in September 2020. Near-bottom DO concentration did not drop below the hypoxic threshold of 2 mg/L on the dates sampled (). Chl-a concentrations were ≤2 µg/L at all depths during the first 3 sampling events; in July 2021, the final sampling period, concentrations were barely above 2 µg/L at the surface and mid-depth but spiked to 16.8 µg/L at the near-bottom (), assuming this data point is accurate.
Table 2. Nutrient data (mean and range values) collected from the 3 littoral sites and 1 pelagic site in Ottawa Sands Lake and nutrient data from other nearby lakes in west Michigan for comparison. Total samples: littoral (n = 13); pelagic (n = 4). BD = below detection (<5 µg/L); ND = not determined. Sampling dates: Ottawa Sands (July 2020, September 2020, April 2021, and July 2021); Muskegon Lake (May, July, September 2003–2005); Mona Lake (May–October 2002, February 2003, April–October 2003), Little Black Lake (August 2007), Spring Lake (July 2003).
P and N concentrations at the littoral sites were consistent with OSL’s oligotrophic state (). SRP concentrations were below detection at all sites while TP remained ≤10 µg/L. Mean NO3-N concentrations were <60 µg/L at all sites except site 3, and NH3-N concentrations were low at all sites; NH3-N was highest at the near-bottom depth at the deep hole, suggesting possible sediment release during low DO periods, but still reached a mean of only 136 µg/L (, ). Occasional spikes in nutrients occurred, but these were episodic and relatively modest in magnitude; the only nutrient spike observed at all 3 littoral sites was SRP in March 2021, but the absolute increase was small, increasing from below detection to 5–6 µg/L (). Chl-a concentrations remained ≤20 µg/L at all littoral sites and on all dates except for the first sampling date (June 2020), when they were much higher, ranging from ∼30 (site 3) to 100 µg/L (site 1; ).
Figure 2. (a) Soluble reactive phosphorus (SRP), (b) total phosphorus (TP), (c) nitrate (NO3-N), (d) ammonia (NH3-N), and (e) chlorophyll a (Chl-a) concentrations at the 3 sampling sites in Ottawa Sands Lake.
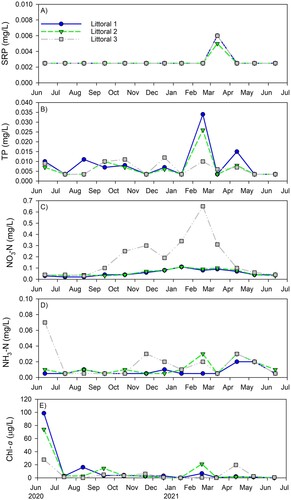
Mean nutrient concentrations in OSL were compared with nearby natural lakes (within a 15 km distance) we previously sampled and measured. OSL contains far less N and P than the 3 drowned river mouth lakes in the immediate region and has nutrient concentrations comparable to an impoundment lake ().
Exclusion experiment
Because of non-normal data distribution, we performed a Wilcoxon signed-rank test comparison of the median macroinvertebrate density values between caged and uncaged treatments; both the mean and median values for the 9 most abundant families (composing ∼98% of the total macroinvertebrate abundance in OSL) are reported (). No statistically significant differences (p > 0.05) in the median values for the macroinvertebrate community structure were found between the control and exclusion treatments at any site for any family. Overall, Chironomidae was the most abundant macroinvertebrate family present on our substrates. The density of chironomids collected on the caged (experiment) treatment substrates during the fall season was >100 times higher than in the uncaged (control) substrates. Although the mean chironomid density was higher in the caged than open treatments at each site (), the median values were not statistically different, likely because of the high variation in collected organisms. In addition, no statistically significant differences (p > 0.05) in macroinvertebrate diversity were observed between caged and uncaged treatments, regardless of site and season ().
Table 3. Contrast of total macroinvertebrate individuals per square meter (density) in the caged (excluded) and uncaged (control) treatments for each site and season. Calculations include mean (SE, 1 standard error) and median (25th–75th quartile) for each of the 9 families that comprised 98% of the total abundance. No significant differences were detected among either sites or seasons (separately) based on the paired samples Wilcoxon test. Winter data were not available. N/A indicates an absence of taxa.
Table 4. Shannon-Wiener scores (exp(H’); diversity) for each site in Ottawa Sands Lake, Michigan across 3 seasons (9 months) and treatment type (caged vs. uncaged). Values are mean (SE, 1 standard error) for each site and n is the total number of sampling events per group. Based on a pairwise comparison of the estimated marginal means with a Bonferroni adjustment, no statistically significant differences were observed among caged vs. uncaged treatments. * denotes 1 winter sample, which brings the total to 46.
Macroinvertebrate community structure
The macroinvertebrate community analysis (NMDS) was based on pooling the uncaged and caged samples, given the lack of significant differences in their densities. Our analysis revealed no obvious clustering by treatment type (, ), but significant clustering was present with site and season (, ). Fall had the highest variation in macroinvertebrate community distribution in ordination space while the spring and summer community structures were clustered more tightly (). The envfit analysis revealed 3 statistically significant vectors, although each with relatively low R2 values: NH3-N = 0.15, TDS = 0.31, and SpCond = 0.33. The macroinvertebrate community structure in the spring experienced higher levels of specific conductivity and TDS while NH3-N levels affected macroinvertebrate community structure in all seasons, but primarily in spring and summer.
Figure 3. Non-metric multidimentional scaling (NMDS) ordination of macroinvertebrate community structure from the 3 sampling sites based on caged vs. uncaged treatment. OS = Ottawa Sands; SpCond = specific conductance; TDS = total dissolved solids; TP = total phosphorus; NO3 = nitrate; NH4 = ammonia; ODO = optical dissolved oxygen.
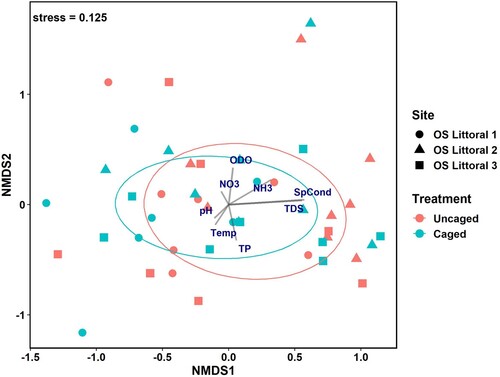
Figure 4. Non-metric multidimentional scaling (NMDS) ordination of macroinvertebrate community structure from the 3 sampling sites based on season. OS = Ottawa Sands; SpCond = specific conductance; TDS = total dissolved solids; TP = total phosphorus; NO3 = nitrate; NH4 = ammonia; ODO = optical dissolved oxygen.
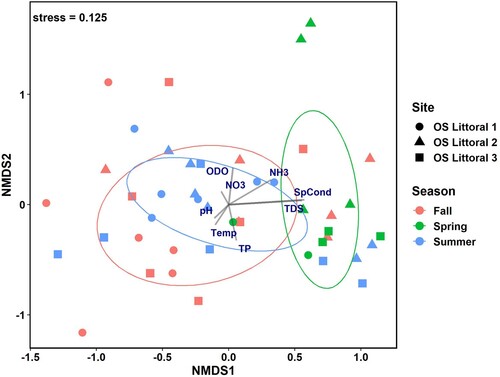
Table 5. Results from permutational multivariate analysis of variance (PERMANOVA) test using adonis for site (Littoral 1, 2, and 3), season (spring, summer, and fall), treatment (caged vs. uncaged), and interactions between both site and season as well as site and treatment. Values in bold indicate significant differences among groups.
The permutational analysis of variance (adonis) results further revealed which taxa most influenced the macroinvertebrate community structure for both between-site and between-season dissimilarities. Sites 1 and 3 had the highest percent dissimilarity (78.2%; ), with 3 taxa significantly contributing to their relative percent dissimilarity. Site 3 had significantly higher average abundances of Coenagrionidae, Amphipoda, and Asellidae than site 1. Similarly, sites 1 and 2 showed high between-site percent dissimilarity (75.6%), driven largely by significantly higher average abundances of both Caenidae and Ceratopogonidae at site 2 than site 1; sites 2 and 3 had the lowest percent dissimilarity (74.3%), but no taxa significantly contributed to the difference. Seasonal comparisons of the macroinvertebrate community structure based on adonis results revealed that the greatest dissimilarity was between fall and spring (80.4%; ). Three macroinvertebrate groups were found in greatest average abundance during spring compared to both fall and summer: Ephemerellidae, Ceratopogonidae, and Collembola. The percent dissimilarity between summer and fall was 75.1%, with Caenidae and Coenagrionidae found in greater average abundances during fall than in summer ().
Table 6. Average macroinvertebrate family abundances that account for at least 80% of the variance among sites and seasons based on the similarity to percentages (SIMPER) calculations from the non-metric multidimensional scaling (NMDS) analysis. Values in bold with an asterisk indicate significant (p < 0.05) differences in macroinvertebrate family between groups.
Discussion
Sand mining activity represents a significant economic global market (US$17.4 billion as of 2020) but can result in major alterations of terrestrial and aquatic ecosystems (IMARC Citation2020). Most sand mining in the United States occurs in marine coastal areas or in freshwater dune systems, especially in the Great Lakes Region (Schrotenboer and Arbogast Citation2010, Aschenbach and Poling Citation2015). Much of the need for sand in Michigan comes from foundries, which use sand to produce metal castings, primarily for the automotive industry (Schrotenboer and Arbogast Citation2010). Additionally, hydraulic fracturing (where sand is used as an additive to fracking fluids) has increased in recent years, although the increasing influence of antifracking campaigns may prevent it from becoming a significant draw on sand resources in the future (Pearson Citation2017). Mining Great Lakes sand dunes is highly controversial because these dunes represent a rare and fragile ecosystem supporting a range of flora and fauna that survive only in these systems (Richardson and Nicholls Citation2021). These unique dunes also represent important cultural ecosystem services for residents of the Great Lakes basin and beyond, although to date few studies have been published concerning these values (Richardson and Nicholls Citation2021).
The nutrient concentrations in OSL were low, consistent with prior studies (Obot et al. Citation2019, Seelen et al. Citation2021) and the literature survey conducted by Gammons et al. (2009) of pit mine lakes; they found that most pit lakes are oligotrophic, which they attributed to their relatively young age, high volume to surface area ratio, and close association with low organic carbon lithology (cf. Blanchette and Lund Citation2016). We suspect the lack of surface inflows, combined with groundwater influx from the pristine dune ecosystem, also contributes to the generally oligotrophic nature of OSL. Although sand mining can release nutrients from the sediment into the water column (as shown for shallow Lake Hongze, China) because of physical disturbance of the lake bottom (Zou et al. Citation2019), this release is less likely to occur in OSL because of its greater depth. However, nutrient release may occur from sediments on the shallower ledges in the littoral zone where macrophytes appear (Seelen et al. Citation2021) and, after senescence, can lead to accumulation of organic matter; bioturbation or diffusion of nutrients from sediment may account for the anomalously high chlorophyll values measured on our first sampling event but never replicated. In addition, lake water clarity rebounds quickly after the cessation of dredging (Meng et al. Citation2021), which also would apply to nutrient flux.
Gammons et al. (2009) also noted that DO is often high in shallow regions of pit lakes because of atmospheric gas exchange but can become depleted in deeper zones because of reduced atmospheric exchange and high chemical oxygen demand associated with oxidation of metal sulfides. This scenario is unlikely to be a major issue in OSL because the mining was for sand, which is reflected in the lake’s DO concentrations exceeding the hypoxic threshold even at the deep site.
Sand mining clearly impacts lake benthos. Macroinvertebrate populations in actively dredged areas face constant disturbance and the removal and alteration of habitat. Although dredged areas are recolonized relatively quickly once dredging ends, sensitive species may not be able to inhabit degraded areas with low habitat heterogeneity or altered habitat quality, so these areas will likely be dominated by tolerant, generalist taxa (Meng et al. Citation2018, 2021, Zou et al. Citation2019). Sand mining activities ended in OSL in 2017, yet the most abundant taxa, including those that contributed the most to the dissimilarity between sites and seasons, were a mix of tolerant (Coenagrionidae, Ceratopogonidae, Asellidae, and Amphipoda) to fairly sensitive (Caenidae and Ephemerellidae) families, suggesting the habitat and water quality differences among sites in OSL can support a range of macroinvertebrates in its current condition. We also recognize that local climatological conditions can influence our results. As a consequence, we compared the mean 2 maximum temperature and total precipitation records from 2020–2021 (sampling year) to the prior 20 years with 2-sample t-tests and found no significant (α = 0.05) differences, both when looking at the entire year and also specifically June–July of each year.
Despite the range of tolerant to fairly sensitive macroinvertebrate species identified during this study, 98% of all taxa collected at OSL belonged to only 9 macroinvertebrate families, but this lack of familial diversity could be due to the inherent sampling bias from the use of artificial substrates (Canton and Chadwick Citation1983, Rinella and Feminella Citation2005). The inclusion of active macroinvertebrate sampling approaches, such as D-net sweeps and Ponar samples, may result in a more diverse community.
Overall, macroinvertebrate mean densities were greater in caged than uncaged samples, but because of the extremely high variance and non-normal data, the pairwise comparison of macroinvertebrate median densities were not statistically different. For example, fall densities of Chironomidae at sites 1 and 3 were 100 times higher in the caged versus the uncaged treatment, but the median density between treatments was not significantly different. In addition, fluctuating water levels due to seasonal changes in OSL as well as Lake Michigan caused large variations in the surface area available for macroinvertebrates to colonize the substrates, therefore contributing to the nonsignificant difference in median density between control and experimental treatments. Our data suggest, but not conclusively, that predation of the macroinvertebrate community is occurring in OSL, likely by larger vertebrate species such as fish, amphibians, or reptiles.
A herpetofaunal survey resulted in the documentation of 4 species of amphibians and 2 species of reptiles, including Eastern American toad (Anaxyrus americanus), Fowler’s toad (Bufo [Anaxyrus] fowleri), green frog (Rana clamitans), northern leopard frog (Rana pipiens), eastern snapping turtle (Chelydra serpentine), and northern brown snake (Storeria dekayi), which may account for reduced macroinvertebrate taxa (Herpetological Resource and Management Citation2020). We suspect that over time, a more extensive macrophyte bed will develop in OSL and, with greater habitat and cover, trophic level interactions will increase, possibly resulting in greater predation pressures on the macroinvertebrate community. Alternatively, increased structure and more habitat also imply more refuges, which may promote higher diversity. Hence, when assessing the ecological status of retired mine lakes, it is important to consider time since the cessation of mining (cf. Marszelewski et al. Citation2017) and the lake’s development potential.
Although OSL seems to be in relatively good condition at present, it faces several threats in the near future. First, Ottawa County is moving forward with development plans, including camping, a paved trail around the lake, and nonmotorized boating, which will add pressures on the lake associated with swimming, possible shoreline erosion, and fishing. Second, the submerged macrophyte community is already sparse, and additional loss may impact both macroinvertebrate and fish habitat and community structure (Shupryt and Stelzer Citation2009, Jeppesen et al. Citation2012, Lusardi et al. Citation2018, Son et al. Citation2021); increasing habitat heterogeneity is important to maintain and/or increase a diverse and resilient ecosystem (Thomaz Citation2023). Third, environmental concerns still remain in OSL resulting from its former use as a sand mine (Lakeshore Environmental Citation2017, Viridis Design Group Citation2020), including high levels of Fe and Mn in the groundwater (Lakeshore Environmental, Inc. Citation2017), which may pose a risk to human health. Fourth, invasive species are a potential concern given the presence of waterfowl in OSL; M. spicatum, N. obtusa, and P. australis are of particular concern (Lakeshore Environmental Citation2017, Elgin Citation2020). Finally, while not a threat to OSL itself, increased use of the area risks the ecological integrity of the fragile critical dune ecosystem surrounding OSL, an area highly ranked for conservation importance and considered a hotspot of critical habitat for imperiled migratory fowl (National Audubon Society Citation2021). Hence, establishing baseline conditions in OSL is critical to effective management, reclamation, and conservation to promote both its ecological integrity and value for stakeholders (cf. Zedler Citation2007).
Acknowledgements
Maggie Oudsema and Brian Scull assisted with field and laboratory analyses, respectively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- [APHA] American Public Health Association. 2005. Standard methods for the examination of water and wastewater. Washington (DC): American Public Health Association.
- Applied Ecological Services. 2020. Ottawa sands wetland assessment and restoration concept plan. Ottawa County Parks and Recreation. https://miottawa.org/Parks/pdf/WetlandAssessment.pdf
- Aschenbach TA, Poling M. 2015. Initial plant growth in sand mine spoil amended with organic material. Ecol Restor. 33:197–206.
- Bacey J, Barry T. 2008. A comparison study of the proper use of Hester-Dendy® samplers to achieve maximum diversity and population size of benthic macroinvertebrates Sacramento Valley, California. Sacramento (CA): California Environmental Protection Agency. Report No. EH08-2.
- Blanchette ML, Lund MA. 2016. Pit lakes are a global legacy of mining: an integrated approach to achieving sustainable ecosystems and value for communities. Curr Opin Environ Sustain. 23:28–34.
- Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 27:325–349.
- Cairns J. 1999. Assimilative capacity – the key to sustainable use of the planet. J Aquat Ecosyst Stress Recovery. 6:259–263.
- Canton SP, Chadwick JW. 1983. Aquatic insect communities of natural and artificial substrates in a montane stream. J Freshwater Ecol. 2:153–158.
- Castendyk DN, Eary LE III, editors. 2009. Mine pit lakes: characteristics, predictive modeling, and sustainability. Littleton (CO): Society for Mining, Metallurgy, and Exploration (SME).
- Duan H, Cao Z, Shen M, Liu D, Xiao Q. 2019. Detection of illicit sand mining and the associated environmental effects in China’s fourth largest freshwater lake using daytime and nighttime satellite images. Sci Total Environ. 647:606–618.
- Elgin E. 2020. Aquatic plant invaders in Ottawa County. Presented at the Ottawa County Water Quality Forum, West Olive, MI, USA, November 2, 2020. Available online: https://www.miottawa.org/departments/boc/waterquality/
- Gammons CH, Harris LN, Castro JM, Cott PA, Hanna BW. 2009. Creating lakes from open pit mines: processes and considerations, emphasis on northern environments. Can Technol Rep Fish Aquat Sci. 2826:ix–106.
- Herpetological Resource and Management. 2020. Ottawa Sands herpetological assessment. Chelsea (MI): Herpetological Resource and Management technical report 48118.
- Hester FE, Dendy JS. 1962. A multiple-plate sampler for aquatic macroinvertebrates. Trans Am Fish Soc. 91:420–421.
- IMARC. 2020. Silica sand market: global industry trends, share, size, growth, opportunity, and forecast 2021–2026. https://www.imarcgroup.com/silica-sand-manufacturing-plant
- Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K, editors. 2012. The structuring role of submerged macrophytes in lakes (Vol. 131). New York (NY): Springer Science & Business Media.
- Jost L. 2010. The relation between evenness and diversity. Diversity. 2(2):207–232.
- Klapper H, Geller W. 2001. Water quality management of mining lakes—a new field of applied hydrobiology. Acta Hydrochim Hydrobiol. 29(6–7):363–374.
- Lakeshore Environmental I. 2017. Environmental report for Ottawa Sand Company, LLC, Ferrysburg, Michigan, November 2017. Prepared for: Grand Rapids (MI): Ottawa County Parks Trust Fund Application. Project no. 17-860.
- Lusardi RA, Jeffres CA, Moyle PB. 2018. Stream macrophytes increase invertebrate production and fish habitat utilization in a California stream. River Res Appl. 34:1003–1012.
- Mamola M. 2005. Procedure for collecting benthic macroinvertebrates using a Hester-Dendy sampler. Sacramento (CA): Department of Pesticide Regulation. Environmental Monitoring Program. SOP #EQWA. 006.
- Marszelewski W, Dembowska EA, Napiórkowski P, Solarczyk A. 2017. Understanding abiotic and biotic conditions in post-mining pit lakes for efficient management: a case study (Poland). Mine Water Environ. 36:418–428.
- Martinus W. 2020. A vascular flora inventory: Ottawa Sands, Ottawa County Parks, Michigan. Ottawa County Parks and Recreation. https://miottawa.org/Parks/pdf/OttawaSandsNaturalFeaturesInventory.pdf
- McCullough CD, Lund MA. 2006. Opportunities for sustainable mining pit lakes in Australia. Mine Water Environ. 25:220–226.
- Meng X, Cooper KM, Liu Z, Li Z, Chen J, Jiang X, Ge Y, Xie Z. 2021. Integration of α, β and γ components of macroinvertebrate taxonomic and functional diversity to measure of impacts of commercial sand dredging. Environ Pollut. 269:116059.
- Meng X, Jiang X, Li Z, Wang J, Cooper KM, Xie Z. 2018. Response of macroinvertebrates and local environment to short-term commercial sand dredging practices in a floodplain lake. Sci Total Environ. 631-632:1350–1359.
- Merritt R, Cummins K, Berg MB. 2019. An introduction to the aquatic insects of North America, 5th ed. Dubuque (IA): Kendall Hunt Publishing Company.
- Mollema PN, Antonellini M. 2016. Water and (bio)chemical cycling in gravel pit lakes: a review and outlook. Earth Sci Rev. 159:247–270.
- National Audubon Society. 2021. Audubon’s vision: restoring the Great Lakes for birds and people. Great Lakes – National Audubon. https://nas-national-prod.s3.amazonaws.com/restoring_the_great_lakes_web_60mb.pdf
- Nelson WA, Steinman AD. 2013. Changes in the benthic communities of Muskegon Lake, a great lakes area of concern. J Great Lakes Res. 39:7–18.
- Obot OI, Ekpo IF, David GS. 2019. The effect of sand mining on the physico-chemical parameters of Ikot Ekpan River, Akwa Ibom State, Nigeria. J Aquat Sci Mar Biol. 2:21–24.
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, et al. 2020. vegan: Community Ecology Package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan
- Orzechowski RM, Steinman AD. 2022. Assessment of shoreline restoration using macroinvertebrates in a Great Lakes Area of Concern. Environ Monit Assess. 194:260.
- Pearson TW. 2017. When the hills are gone. St. Paul (MN): University of Minnesota Press.
- Peckarsky BL, Fraissinet PR, Penton MA, Conklin DJ. 1990. Freshwater macroinvertebrates of northeastern North America. Ithaca (NY): Cornell University Press.
- R Core Team. 2019. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/
- Richardson RB, Nicholls S. 2021. Characterizing the cultural ecosystem services of coastal sand dunes. J Great Lakes Res. 47:546–551.
- Rinella DJ, Feminella JW. 2005. Comparison of benthic macroinvertebrates colonizing sand, wood, and artificial substrates in a low-gradient stream. J Freshwat Ecol. 20:209–220.
- Schrotenboer BR, Arbogast AF. 2010. Locating alternative sand sources for Michigan’s foundry industry: a geographical approach. Appl Geogr. 30:697–719.
- Seelen LMS, Teurlincx S, Bruinsma J, Huijsmans TMF, van Donk E, Lürling M, de Senerpont Domis LN. 2021. The value of novel ecosystems: disclosing the ecological quality of quarry lakes. Sci Total Environ. 769:144294.
- Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality (complete samples). Biometrika. 52(3/4):591–611.
- Shupryt MP, Stelzer RS. 2009. Macrophyte beds contribute disproportionately to benthic invertebrate abundance and biomass in a sand plains stream. Hydrobiologia. 632:329–339.
- Son S-H, Kwon S-J, Im J-H, Kim S-K, Kong D, Choi J-Y. 2021. Aquatic macrophytes determine the spatial distribution of invertebrates in a shallow reservoir. Water. 13:1455.
- Søndergaard M, Lauridsen TL, Johansson LS, Jeppesen E. 2018. Gravel pit lakes in Denmark: chemical and biological state. Sci Total Environ. 612:9–17.
- Steinman AD, Lamberti GA, Leavitt PR, Uzarski DG. 2017. Biomass and pigments of benthic algae. In: Lamberti GA, Hauer FR, editors. Methods in stream ecology Vol. 1. San Diego (CA): Academic Press; p. 223–241.
- Steinman AD, Ogdahl M, Rediske R, Ruetz CR III, Biddanda BA, Nemeth L. 2008. Current status and trends in Muskegon Lake, Michigan. J Great Lakes Res. 34:169–188.
- Steinman AD, Ogdahl ME, Ruetz CR III. 2011. An environmental assessment of a small shallow lake (Little Black Lake, MI) threatened by urbanization. Environ Monit Assess. 177:193–209.
- Steinman A, Rediske R, Reddy KR. 2004. The reduction of internal phosphorus loading using alum in Spring Lake, Michigan. J Environ Qual. 33:2040–2048.
- Steinman A, Rediske R, Denning R, Nemeth L, Chu X, Uzarski D, Biddanda B, Luttenton M. 2006. An environmental assessment of an impacted, urbanized watershed: the Mona Lake Watershed, Michigan. Arch Hydrobiol. 166(1):117–144.
- Tavernini S, Nizzoli D, Rossetti G, Viaroli P. 2009a. Trophic state and seasonal dynamics of phytoplankton communities in two sand-pit lakes at different successional stages. J Limnol. 68:217–228.
- Tavernini S, Viaroli P, Rossetti G. 2009b. Zooplankton community structure and inter-annual dynamics in two sand-pit lakes with different dredging impact. Int Rev Hydrobiol. 94:290–307.
- Thomaz SM. 2023. Ecosystem services provided by freshwater macrophytes. Hydrobiologia. 850(12):2757–2777.
- [US EPA] United States Environmental Protection Agency. 1993. Methods for chemical analysis of inorganic substances in environmental samples. Cincinnati (OH):USEPA. EPA-600/4-79R-93-020/100.
- [USGS] United States Geographical Survey. 2022. Construction sand and gravel statistics and information [accessed 24 September 2022]. https://www.usgs.gov/centers/national-minerals-information-center/construction-sand-and-gravel-statistics-and
- Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th ed. New York (NY): Springer.
- Viridis Design Group. 2020. Ottawa Sands Park: a collective community visioning plan. Spring Lake (MI): Ottawa County Parks and Recreation. https://miottawa.org/Parks/pdf/plan/OS-Master-Plan-Report_FINAL_LowRes.pdf
- Waajen G, Van Oosterhout F, Douglas G, Lürling M. 2016. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant: lanthanum modified bentonite treatment. Water Res. 97:83–95.
- Weatherhead MA, James MR. 2001. Distribution of macroinvertebrates in relation to physical and biological variables in the littoral zone of nine New Zealand lakes. Hydrobiologia. 462:115–129.
- White J, Irvine K. 2003. The use of littoral mesohabitats and their macroinvertebrate assemblages in the ecological assessment of lakes. Aquat Conserv Mar Freshwater Ecosyst. 13:331–351.
- Wickham H, François R, Henry L, Müller K. 2021. dplyr: a grammar of data manipulation. R package version 1.0.6. https://CRAN.Rproject.org/package=dplyr
- Wiederholm T. 1980. Use of benthos in lake monitoring. J Water Pollut Res Fed. 52:537–547.
- Wilcoxon F. 1945. Individual comparisons by ranking methods. Biometr Bull. 1:80–83.
- Zedler JB. 2007. Success: an unclear, subjective descriptor of restoration outcomes. Ecol Restor. 25:162–168.
- Zou W, Tolonen KT, Zhu G, Qin B, Zhang Y, Cao Z, Peng K, Cai Y, Gong Z. 2019. Catastrophic effects of sand mining on macroinvertebrates in a large, shallow lake with implications for management. Sci Total Environ. 695:133706.