 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Little is known about the effect of strategy use on working memory training in aging. Here, we aim to assess the impact of strategy on trained and transfer tasks for accuracy and P300, while investigating the effect of age, gender and education. We recruited 26 elderly and recorded EEGs from the cognitive training group and active control group. Our results showed changes in P300, but only a positive trend in accuracy for the trained task across groups. We found transfers to untrained tasks, but we did not observe any difference in transfer effects between training groups. However, our results showed significant differences between training groups for a similar version of the trained task, suggesting that strategy use might have prolonged effects. Individual characteristics had a significant impact on accuracy and P300 for trained and transfer tasks. These findings underline the role of strategy use and individual characteristics during N-Back training.
Introduction
Working memory (WM) is one of the most studied but also debated cognitive function considered in cognitive training settings. Most frequently, it refers to a mental construct that stores for a short period of time and manipulates information needed for complex cognitive task performance (Baddeley & Hitch, Citation1974). Despite the variety in hypotheses, there is general agreement about its role as an executive controller with limited capacity to store visual and verbal information (Baddeley, Citation2003; Cantarella et al., Citation2017; Miyake & Shah, Citation1999; Shah & Miyake, Citation1999). Baddeley (Citation2003) adopted a model originally proposed by Norman and Shallice (Citation1986), which suggests that actions are driven by routine behaviour and controlled automatically by well-learned processes or driven by the supervisory attentional system in case something unexpected happens. It has been shown that changes in WM are affected by age (Delaloye et al., Citation2008).
Recent functional imaging studies focused on WM have used an N-Back task to investigate brain structural correlates of working memory and divided attention (Rowe et al., Citation2000), as this task requires a continuous variable load upon WM. During N-Back task performance, an attention-driven comparison process evaluates the representation of the previous event in WM (Heslenfeld, Citation2003). When there is no detection of a change in stimulus attributes, the mental model of the stimulus context is maintained, and only sensory evoked potentials are recorded—N100, P200, N200. Differently, when a new stimulus is detected, attentional processes provide an “updating” of the stimulus representation that is concomitant with P300, a positive Event-related Potential (ERP) deflection that occurs around 250–500 ms after stimulus onset (Donchin, Citation1986). Consequently, the processing system is modulated by arousal level that controls the amount of attention available during task performance (Kahneman & Tversky, Citation1973). Furthermore, when task conditions are very simple and less attentional resources are needed, the P300 exhibits a large amplitude and short peak latency (Watter et al., Citation2001). In contrast, for tasks that require more attentional resources, the P300 amplitude is smaller and peak latency longer as more processing resources are used for task performance (Kok, Citation2001). Additionally, attentional resource and internal distribution of controlled attention affect P300 amplitude: when memory load increases, P300 magnitude decreases (Kok, Citation2001). It has been shown that for N-Back tasks, P300 amplitude decreases for increasing WM load (Gevins et al., Citation1996; Scharinger et al., Citation2017; Watter et al., Citation2001). Pesonen et al. (Citation2007) observed, using spectral power bands, differences in alpha power when participants were performing a higher N-back load level (2 and 3-Back) compared to a lower load level (1-Back). All N-Back levels require that each stimulus is encoded, that a representation of the target stimulus is maintained in memory, and that each item’s representation is matched against the stored target’s representation. However, information maintenance and manipulation load changes with increasing N. In the 0-Back, participants need to maintain only one item in memory, whereas in the 1-Back, participants similarly need to maintain only one item, but in addition, it requires the regular updating of WM. Consequently, WM updating is not a 1-step replacement, but a 2- step shift and replacement operation (Chen et al., Citation2008).
Several studies have investigated the impact of WM training in aging in terms of behavioural outcomes, some showing benefits after intervention and transfers to untrained WM tasks and fluid intelligence (Brehmer et al., Citation2012; Bürki et al., Citation2014; Heinzel et al., Citation2016; Li et al., Citation2008; Loosli et al., Citation2016; Richmond et al., Citation2011; Zinke et al., Citation2014), whereas others failed to report enhancements in the trained task and transfers (Guye & Von Bastian, Citation2017; Hering et al., Citation2017; Sala & Gobet, Citation2019; Teixeira-Santos et al., Citation2019). The review of Simons et al. (Citation2016) reports ample evidence of training enhancements for trained compared to “within-training” tasks, when involving cognitive processes similar to the trained task, and far less evidence for “beyond-training” tasks, when calling upon different mental processes, and everyday cognitive performance, especially in older adults. However, many studies revealed to have major issues with its design or analysis that preclude to strong conclusions about the efficacy of training. In line with the latter, a more recent work published by Pergher et a.l (Citation2019), supported the idea that WM training interventions include a wide variety in training task features and transfer measures, and sparsely addressed effect of individual differences, concluding that all these factors could be the source of the ongoing disagreement about possible transfers to untrained tasks. Besides assessing behavioural training and transfer effects, only few studies with young adults explored the effect of N-Back training on ERPs–characteristic deflections in (usually) EEG amplitude in sync with a specific sensory, cognitive, or motor event (Luck, Citation2005). Oelhafen et a.l (Citation2013) observed that training using an N-Back task showed stronger P300 responses in the parietal cortex, which may be related to improvements in processing speed, attentional control, or both. However, although to a lesser extent, other ERP components might be affected as well such as the early visual ERP components—P1, N1, N2, P2—and late positive components (LPC) or slow wave (Luria et al., Citation2016; Ruchkin et al., Citation1995). Salmi et al. (Citation2019) suggested that early and late ERPs are differentially affected by N-Back training. In particular, pre- and post-tests showed ERP changes indicative of a decreased load-effect for the training group, and slow wave amplitude that decreased in the easy task and increased in the difficult task, reflecting maintenance of to-be-remembered items (Luria et al., Citation2016; Vilà-Balló et al., Citation2018).
More recently, a series of studies shed light on the relation between individual differences and WM training outcomes in older adults, focusing on strategy use (Borella et al., Citation2017; Borella et al., Citation2017; Laine et al., Citation2018; Morrison et al., Citation2016), as well as motivation (Katz et al., Citation2018) and pre-existing abilities (Zinke et al., Citation2014; Borella, Carbone et al., Citation2017). Morrison et al. (Citation2016) examined whether strategy choice could modulate WM training outcomes. When using mental rehearsal as a specific strategy, they observed an increased performance. Benson et a.l (Citation2011) demonstrated that an explicit strategy can provide performance improvements during sensorimotor adaptation. However, this has been rarely investigated in elderly. One such study is that one of Borella et al. (Citation2017) showing improvements for two trained groups (with and without instructed strategy) compared to the control group. Also, they observed transfers to increased processing speed- and short-term memory tasks that persisted for 6 months. However, the training group that adopted the specific strategy showed long-term large effect sizes for all tasks involving memory processes, whereas for the other training group only for the processing speed task. Although this confirms the effect of strategy training in aging, other studies reported that it leads to a very narrow transfer to executive control tasks only (Neely & Bäckman, Citation1995) but it does not enhance performance in attentional blink paradigms (Basak et al., Citation2008).
According to the literature, the impact of a subject’s individual differences has been extensively examined from a behavioural viewpoint after a single session of cognitive performance (Christensen, Citation2001; Drag et al., Citation2010; MacPherson et al., Citation2002). Results showed the impact of age with lower accuracies and increased reaction times for older individuals compared to young ones, gender (Wiederholt et al., Citation1993), and education level (Ruff & Parker, Citation1993). However, the effect of these moderators in terms of ERPs during or pre- and post-training has not yet been reported. Studies using a single session of cognitive performance in older adults, reported a smaller P300 amplitude and a larger P300 latency over the midline central and parietal locations compared to young adults (Ashford et al., Citation2011; Polich, Citation2007), and a more frontal scalp distribution, interpreted as memory decay and reduced working memory capacity (Li et al., Citation2013). Steffensen et al. (Citation2008) showed that females were characterised by a greater P300 response compared to males for a relevant stimulus during an object recognition task, indicating that females process visual information differently compared to males. However, the evidence of gender-related differences might be due to differences in brain size, skull thickness, or other biological gender variation (Conroy & Polich, Citation2007; Pfefferbaum et al., Citation2001). Furthermore, the study of Begum et a.l (Citation2014) and Angel et a.l (Citation2010) indicated that ERP components are modulated by education level. Specifically, Angel et al. (Citation2010) showed that the effect of age on ERP responses was smaller for higher educated people compared to those with a lower level of education, during the performance of a word-stem cued-recall task.
In the present study, we aim to: (1) assess the impact of strategy use during an N-Back training in terms of behavioural accuracy and P300 changes in the trained task and transfers to other cognitive domains, in older individuals. Furthermore, (2) we want to investigate the effect of individual characteristics, such as age, gender and education, on the trained task in terms of behavioural accuracy and P300 responses, and transfer tasks in terms of behavioural accuracy. We consider two training groups, one group was instructed to use mental rehearsal as a strategy (Borella et al., Citation2017; Bailey et al., Citation2014) and the other group not, in which case participants developed their own strategies, and a passive control group that underwent only pre- and post-testing.
Transfer tasks can be arranged along a continuum spanning within (short-term memory) to beyond WM (fluid intelligence, attention, inhibitory control, etc.) tasks (Pergher, Shalchy et al., Citation2019). The “within WM” tasks involve cognitive sub-processes similar to the ones practiced during the training task, whereas the “beyond WM” include different mental processes, although their mechanisms were shown to correlate with WM (De Ribaupierre & Lecerf, Citation2006). Generally, we expected to observe larger improvements for the N-Back training task in the CTG (instructed strategy) compared to the ACG (self-developed strategy), and in line with the behavioural responses, to detect higher P300 amplitudes, especially for the more difficult N-Back levels (2 and 3-Back) for the CTG compared to the ACG (Pesonen et al., Citation2007), suggesting that the task became easier for the participants in the strategy training group compared to the control group, although literature on this is scarce (Borella et al., Citation2017; Bailey et al., Citation2014). In addition, we hypothesise to find larger transfers to both within and beyond WM tasks for the CTG (instructed strategy) compared to the ACG (self-developed strategy). Specifically, we expect to find larger transfers to within WM tasks compared to beyond WM tasks, as the former benefit from a better encoding of the stimuli due to repeated practice, whereas the latter might be affected negatively due to the fact that the strategy used affects only memory but not other cognitive functions. Finally, differences in individual characteristics can lead to differences in baseline outcomes, but we expect that their impact will even be larger for the last training session and post-tests of both training groups.
Methods
Participants
A group of 26 healthy adults, between 55 and 70 years old (M = 61.86, SD = 5.37, 12 women, 14 men) were recruited via flyers and advertisements posted at Senior Citizens Centers in Leuven (). The selection criteria included: a MMSE score above 27, no history of neurological or psychiatric diseases, no experience with WM training, normal vision, and not taking any medication that could interfere with cognitive functioning. Participants were randomly assigned to three different experimental groups: a cognitive training group (CTG) (N = 7) that was instructed to use a specific strategy, called mental rehearsal, where participants had to repeat mentally each stimulus on the screen, an active control group (ACG) (N = 7) that underwent the same N-Back training and could self-develop any type of strategy during each session, and a passive control group (PCG) that did not undergo any training (N = 12). The PCG was invited to the laboratory only two times, to complete two sessions of pre- and post- cognitive tests to account for possible variations in tests re-tests outcomes, while the CTG and ACG completed 10 N-Back training sessions in approximately 4 weeks, with a frequency of 2–3 times per week, and performed one session of cognitive tests before (pre) and one after (post) training. All participants were asked which strategy they used during the training. Specifically, at the end of each session ACG participants were asked to report which strategy they adopted. This way done to ensure, when processing their data, that the strategy used was different from that of CTG participants (instructed to use mental rehearsal). Our ACG participants reported to have used counting on fingers, making short words of the pictures in English, etc. Our CTG participants were instructed to use mental rehearsal before starting a training session, and we asked them to confirm they used it at the end of that training session. The choice of mental rehearsal was motivated by evidence of its effectivity in young adults (Morrison et al., Citation2016) and, in general, the beneficial impact the strategy use can have in older adults (Borella et al., Citation2017; Basak et al., Citation2008). All the recruited participants attended the 10 training sessions and two cognitive testing sessions (one before and one after training). However, we discarded two participants after the first training session due to bad recording and/or too many artifacts in their EEG data. Prior to start the experiment, participants were informed about the experimental procedure and signed an informed consent form. The study was approved by our university hospital’s ethical committee (UZ KU Leuven, S59475).
Materials and procedure
Training participants were invited to our laboratory for a total of 12 visits, during which each participant underwent individual sessions. Participants were blinds for the condition they were in, as they ignored the existence of the three experimental groups. We randomly assigned participants to one of the three groups: CTG, ACG and PCG, and as we aimed to investigate whether individual differences may have played an important role in the training outcomes, we did not control, at this stage (baseline), for age, gender, education and performance level. The two trained groups (CTG, ACG) underwent a 10 sessions-N-Back training during which EEG was recorded, the purpose of which is to verify whether the P300-ERP amplitude changes as a function of training, and cognitive tests administered before and after training (pre/post-testing). The choice of the intervention length was taken from studies that reported positive outcomes on older adults (Bürki et al., Citation2014—10 sessions; Heinzel et al., Citation2016—12 sessions; Loosli et al., Citation2016—10 sessions). Differently, PCG underwent only pre- and post-tests (A). Both training groups received a monetary reward of 10 euros and an additional compensation (maximum 10 euros) based on achieved accuracy.
Figure 1. Experimental procedure and paradigm. (A) 20 participants included in the two training groups underwent 10 sessions of N-Back training, and were administered pre- and post-tests to assess transfer effects. (B) Example of stimulus sequence during the N-Back task (2-Back), and its timeline.
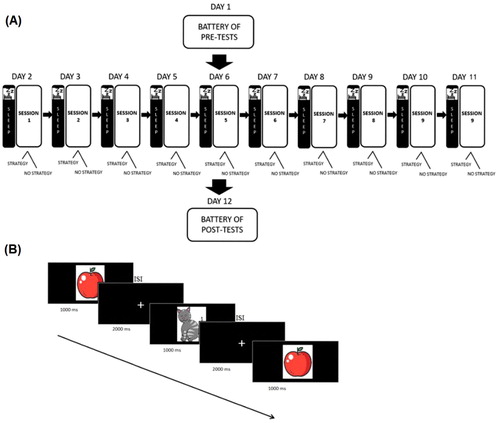
Table 1. Participants characteristics.
Training task
N-Back
We used the same WM task adopted in our previous study (Pergher et al., Citation2019). The goal of the task is to decide if the presented stimulus is the same as the one presented N-times before (B). We divided the N-Back task into three difficulty levels, named 1-Back, 2-Back and 3-Back. For each N-Back level, a minimum of 100 stimuli (i.e. meaningful drawings) were presented in pseudorandom order. The task was adaptive as participants were required to correctly answer 70% of the current trials before passing on to the next level. In total, each training session was divided in 6 blocks, each one consisting of 100 stimuli. A given N-Back level consisted of a minimum of 100 stimuli (1 block) with a maximum of 600 stimuli (6 blocks). When the participant reached the 70% accuracy threshold in the last block, training moved onto the next N-Back level. For each block of 100 stimuli, 33% of them were a target. The stimuli were presented for 1 s followed by a 1.5 s inter-stimulus interval (ISI) and 0.5 s of feedback presentation (red frowny/green smiley). Between stimuli, participants were presented with a crosshair centred on the screen and were instructed to respond during this interval. In total, there were 10 N-Back training sessions (1 h/session) performed in approximately 4 weeks (2–3 time/week).
Pre- and post-tests
N-Back task
The task was a similar version of the trained N-Back task (see above “Training task”) except that it was not adaptive, as N-Back level was increasing stepwise, using the same number of stimuli, and no feedback to the user was provided. For each N-Back level, 100 stimuli were presented in pseudorandom order. In total, there were 6 blocks, 2 for each N-Back level.
Transfer tasks
Pre- and post-cognitive tests were used to investigate behavioural differences before and after training, and detect age, gender and education level influence, in both training and passive control groups. The selected cognitive tests were those used in the Memory Clinic of Gasthuisberg University Hospital, Leuven, with which we collaborate. In addition, based on literature, we considered those tests for which correlations were demonstrated with the cognitive processes involved in working memory (here N-Back) (Jaeggi et al., Citation2008; Lilienthal et al., Citation2013; Meier & Kane, Citation2013; Ross et al., Citation2007; Sánchez-Cubillo et al., Citation2009). With the selected tests near and far transfer tasks are probed, whereby near transfer refers to “within WM-modality”, thus involving cognitive sub-processes similar to the one practiced during training, whereas far transfer is refers to “beyond WM-modality”, such as attention and fluid intelligence, thus calling upon other mental processes (albeit that the underlying mechanisms have been shown to correlate with WM, see De Ribaupierre & Lecerf, Citation2006). Concretely, selected “within WM-modality” tasks included Digit Span Forward and Backward (short-term memory), CORSI (short-term WM) and VAT (short-term memory), whereas selected “beyond WM-modality” tasks included TMT-A and B (attention and task switching), COWAT (verbal fluency; COWAT 1 for professions, 2 for animals, 3 for letter “A”, 4 for letter “K”, and 5 for letter “N”), Stroop (inhibitory control and attention), Visual Search (attention) and RAVEN (fluid intelligence). The computerised tasks were Digit Span, Visual Search and CORSI block tapping test, whereas Trail-Making, Controlled Oral Word Association, Visual Association, Stroop and Raven Progressive Matrices were paper and pencil tests.
“Within WM-modality” tasks
Digit Span
This test is a subtest of the Wechsler Adult Intelligence Scale (WAIS) (Wechsler, Citation1955). It is commonly used to determine short-term memory capacities. Participants are orally presented with a sequence of digits ranging from easy to more difficult. The test is divided into two sections, a forward and a backward. During the forward section, participants are required to repeat a sequence of digits in the same order as orally presented. In the backward version, participants are required to repeat a sequence of digits in the opposite order. It takes approximately 10 min (including instruction).
CORSI block tapping test
The CORSI block test is used to assess visuo-spatial short-term WM (Kessels et al., Citation2000). During the task, participants are presented with a screen consisting of identical spatially separated squares. As the task commences, participants are presented with a sequence of blue squares turning yellow, while all the other blue squares remain blue. The goal of the task is to mimic the sequence of yellow squares as the task ranges from easy to more difficult. It takes approximately 10 min (including instruction and practice phase).
Visual association test
The Visual Association Test (VAT) is a test relying on short-term memory (Lindeboom et al., Citation2002). During the test, participants are presented with 12 pictures in total. During the first part of the test, participants are instructed to look at the first 6 pictures. After a certain amount of time, the pictures are removed, and the participants are presented with the other 6 pictures which look the same as the first pictures but have one object added. Again, the pictures are removed, and the participants have to determine what object was added to the last 6 pictures comparing them with the first 6 presented pictures. It takes approximately 10 min (including instruction).
“Beyond WM-modality” tasks
Trail-making-test
The Trail-Making-Test (TMT), a subtest of the Army Individual Test Battery is designed to assess visual attention and task switching capacity (Army, Citation1944). The TMT test is divided in two parts, A and B, in which the first part includes only letters, while the second letters and numbers. Participants are required to list in alphabetic order letters (TMT-A) and alternate letters and numbers in their logical order (TMT-B). It takes approximately 8 min (including instruction). Stop timing when the Trail is completed, or when maximum time is reached (for part A: 2.5 min; for part B: 5 min).
Controlled oral word association test
The Controlled Oral Word Association Test (COWAT) gauges verbal fluency and is part of the Halstead-Reitan Neuropsychological Test Battery (Reitan & Wolfson, Citation1986). During the test, participants are required to write down as many words that fit into a specific category for one minute. The categories used were professions (1), animals (2), letters “A” (3), “K” (4) and “N” (5). It takes approximately 10 min (including instruction).
Stroop
The Stroop task depends on the principle of inhibitory cognitive interference and attention (Stroop, Citation1935). The task consists of 3 variations of the same test. First, participants are presented with a sheet consisting of names of colours printed in black ink. During this part of the task, participants are required to simply read all the names of the colours. During the second part of the task, participants are presented with a sheet consisting of squares printed in different colours. The goal of the task is to verbalise which colours are printed. During the third and final part of the task, participants are presented with a sheet consisting of names of colours printed in a different colour than the name, called conflict words or interference (for example, the word “blue” printed in green). The goal of this last task is to read the written colour names which interfere with colour in which they are printed. In our study, we considered only the third and last part of the task for the pre- and post-tests assessment. It takes approximately 5 min.
Visual search
The Visual Search task is a cognitive test that gauges the efficiency of search, reflecting the distribution of attention from item to item (Wolfe, Citation1998). During some trials, a letter “T” can be seen between the letters “L”. The goal of the task is to determine if a letter “T” is presented in between all the letters “L”. It takes approximately 15 min.
Raven progressive matrices test
The Raven test is a test for measuring abstract reasoning and fluid intelligence consisting of 60 multiple choice questions (Raven, Citation1938). All of the questions consist of a geometrical design with one missing piece. The goal of the task is to determine, out of 6 multiple choices, which the missing piece is. The maximum time is 30 min.
EEG recordings
EEG was recorded continuously during the N-Back task with a SynAmpsRT device (Compumedics, Australia) at a sampling rate of 2 kHz and using 32 Ag/AgCl electrodes. The electrodes were placed at O1, Oz, O2, PO3, P8, P4, Pz, P3, P7, TP9, CP5, CP1, CP2, CP6, TP10, T7, C3, Cz, C4, T8, FC6, FC2, FC1, FC5, F3, Fz, F4, AF3, AF4, Fp1, Fp2, with the reference placed at AFz and the ground at CPz. We placed four electrodes around the eyes for electro-oculogram recording (EOG) following the instructions of Croft and Barry (Citation2000). The recorded EEG signal was re-referenced offline to the average of the two mastoid signals (average mastoid reference, TP9 & TP10), the EOGs used for removing eye movement and blinking artifacts, using Croft and Barry’s aligned-artifact average (AAA) procedure (Croft & Barry, Citation1998), band-pass filtered in the 0.1–30 Hz range, and cut into epochs starting from 100 ms pre- till 1500 ms post-stimulus onset. Baseline correction was performed by subtracting the average of the 100 ms pre-stimulus onset activity from the 1500 ms post-stimulus onset activity. Finally, the epochs were downsampled to 1000 Hz and stored for ERP component detection.
Statistical analyses
For the training groups, we considered the mean accuracy of the trials for each N-Back level and session. Differently from Jaeggi et al. (Citation2011), we assessed each training session separately, without averaging across training sessions, and considered for analyses the first, the middle and the last training sessions, in order to detect the potential effect of the intervention length. A mixed ANOVA with two within-subject factors (load x session) and one between-subject factor (group) was performed to assess any differences in N-Back accuracy over training sessions for both training groups. To investigate transfers, a mixed ANOVA with one within subject factor (pre–post) and one between subject factor (group) was performed. Furthermore, for the two training groups, repeated mixed-ANOVAs for the ERP analyses including three within-subject factors: level (1-back, 2-back, 3-back), session (t1, t2, t3), and channel (frontal (FZ), central (CZ), posterior (PZ)); and one between-subject factor (group: cognitive group, active group, control group) were applied to the P300 amplitudes, calculated as the average amplitude over a time window between 250–500 ms, and P300 latencies, calculated as the highest positive peak over a time window between 250 and 500 ms, for each channel Fz, Cz and Pz separately. The Holm–Bonferroni correction was applied to account for multiple comparisons and the Greenhouse-Geisser epsilon correction when the assumption of sphericity was violated (Mauchly’s Test of Sphericity). The choice to focus on P300 was based on the fact that this ERP component is the most prominent and affected one during N-Back task performance (Watter et al., Citation2001). P300 amplitude depends on task complexity, stimulus complexity, stimulus probability and sequential expectation of stimuli. P300 amplitude increases with allocated processing capacity (Kramer & Strayer, Citation1988). Moreover, we used a two-way ANOVA to investigate individual differences in the two training groups (CTG, ACG). For the training task, each N-Back level had a minimum of 100 stimuli and a maximum of 600 stimuli, depending on whether the participant reached the 70% accuracy threshold, hence, each training session had a total of 600 stimuli (trials). For the pre- and post-N-Back task, which was not adaptive, we had 200 stimuli per N-Back level, thus, 600 stimuli in total. Hence, for both training- and pre- and post-N-Back tasks, even when participants responded incorrectly to several trials, their average minimal number of correctly responded trials per N-Back level always exceeded 70, and therefore also the average minimal number of corresponding EEG response epochs, a number that is in line with the recommendations for conducting P300 data analysis (Duncan et al., Citation2009), and per session above 450 epochs. Epochs with incorrect behavioural responses were excluded from further analysis. In addition, epochs with EEG signals greater than 100 µV were also excluded as they could be motion artifacts.
Finally, (Cohen, Citation1973) were reported [η2 = SSeffect/SSeffect + SSerror, where SS = sum of squares] to indicate the magnitude of the significant differences for behavioural outcomes, and for significant differences in ERP outcomes.
Results
Training data
Behaviour
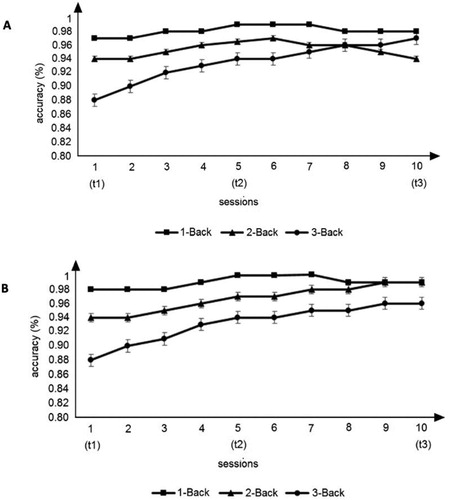
We assessed differences in accuracy over first (t1), middle (t2) and last (t3) training sessions, using a mixed ANOVA with two within-subject factors (N-Back level × session) and one between-subject factor (group). Furthermore, Greenhouse-Geisser epsilon correction was applied as the assumption of sphericity was violated for all factors (sphericity was assessed using Mauchly’s test), except for N-Back level, and Holm–Bonferroni correction was used to account for multiple comparisons. Our results showed a significant main effect for N- Back level (F(2, 24) = 16.339, p < 0.001, = 0.577). A post-hoc analysis showed a significant difference comparing 1-Back and 3-Back (p = 0.001) and comparing 2-Back and 3-Back (p < 0.001), suggesting that participants achieved a higher level of accuracy for 1-Back (M = 98.67) and 2-Back (M = 98.19) compared to 3-Back (M = 94.98). We did not find any significant differences when comparing 1- and 2-Back (p = 0.546), indicating that there was no significant difference in accuracy comparing the first two N-Back levels. Moreover, no main effect for Session was found, suggesting that overall there was no significant difference in accuracy between sessions. However, when considering the descriptive data, we observed an increase in accuracy level over sessions for 1-Back (Mt1 = 96.36, Mt2 = 99.71, Mt3 = 99.23), 2-Back (Mt1 = 96.57, Mt2 = 98.43, Mt3 = 99.57) and 3-Back (Mt1 = 92.36, Mt2 = 95.57, Mt3 = 97) (see Supplemental Material, Table S1). Additionally, our results did not show evidence of a main effect for Group (F(1,12) = 0.541, p = 0.476), suggesting that there were no significant differences in accuracy level between CTG and ACG. Furthermore, our results did not show any significant interaction effects, indicating that the effect of N-Back level and session for accuracy was not significantly different between both training groups and that the effect of N-Back level and session in terms of accuracy was not significantly different over training sessions between both training groups. All together, these results indicate that there were no significant differences in accuracy over training sessions when comparing the two training groups.
Overall, participants were more accurate in the 1-Back compared to the 2- and 3-Back (). Moreover, after correcting for multiple comparisons, we did not find a significant main effect of Session, indicating that participants did not improve significantly over training sessions, although we found a trend of improvement for all participants over sessions (Mt1 = 95.095, Mt2 = 97.905, Mt3 = 98.833). Specifically, higher accuracy level was achieved in the first five training sessions for both training groups, showing that mental rehearsal did not have a beneficial effect on training outcomes, although the group that was instructed to use a specific strategy (CTG) showed a trend of additional accuracy gain in the last five training sessions compared to the self-developed strategy group (ACG). The lack of enhancements across sessions may be due to the small sample size.
Figure 2. Mean ± SD of accuracy during 10 sessions of N-Back training. (A) Cognitive training performance of the cognitive training group (CTG) in the first (t1), middle (t2) and last (t3) session. (B) Cognitive training performance of the active control group (ACG) in t1, t2 and t3.
Interestingly, when we investigated the difference between the same N-Back training task administered a few days before and after the training, our results showed a significant pre- and post-test difference (F(1,23) = 91.92, p < 0.001, = 0.8). This effect was significantly different between the three experimental groups—CTG vs. ACG vs. PCG (F(2.23) = 14.66, p < 0.001,
= 0.56), and indicated that ACG (M = 5.01) performed overall significantly better than CTG (M = 3.45, p = 0.046), CTG (M = 3.47) performed significantly better than PCG (M = 1.40, p = 0.005) and that ACG (M = 5.28) performed significantly better than PCG (M = 1.46, p < 0.001). As our results showed a significant pre- and post-test x group interaction, we performed a post-hoc one-way ANOVA to assess baseline differences between groups. Our results did not show any significant difference at baseline between groups. However, when investigating differences between groups during post-tests, we found significant results (F(2,25) = 31.18, p < 0.001, η2 = 0.73) indicating that ACG (M = 7.87) performed significantly better than CTG (M = 5.71, p = 0.022), ACG (M = 7.87) performed significantly better than PCG (M = 1.94, p < 0.001) and that CTG (M = 5.71) performed significantly better than PCG (M = 1.94, p < 0.001). These results suggest that participants who underwent 10 session-training intervention achieved a higher N-Back accuracy level at post-test compared to the PCG that did not undergo any training. Specifically, participants included in the ACG showed a higher accuracy level compared to participants instructed to use a specific strategy (CTG).
P300-ERPs component
An additional way to assess the effect of strategy choice is by investigating the P300 amplitude and latency at channels Fz, Cz and Pz of the CTG and ACG at t1, t2 and t3 ( and ) by using a mixed ANOVA including three within-subject factors (load, session and channel) and one between-subject factor (group). Furthermore, the Greenhouse-Geisser epsilon correction was applied when the assumption of sphericity was violated for all factors, except for N-Back level.
Figure 3. ERPs responses comparing the first session of CTG and ACG. P300 responses (target minus non-target) for the first sessions of the 1-Back, 2-Back and 3-Back task (Supplementary data are shown in Table S2).
Notes: CTG = cognitive training group (mental rehearsal); ACG = active control group (self-developed strategy).
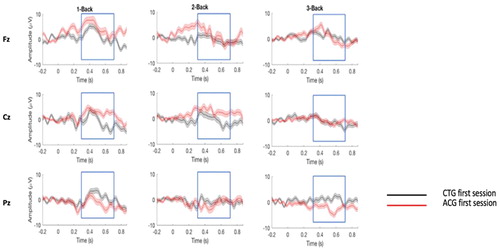
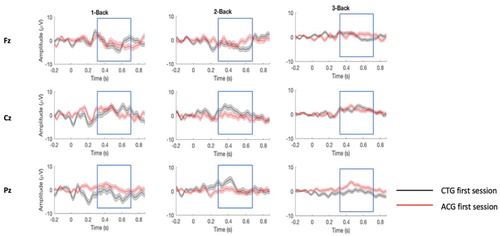
Figure 4. ERPs responses comparing the last session for CTG and ACG. P300 responses (target minus non-target) for the last sessions of the 1-Back, 2-Back and 3-Back (Supplementary data are shown in Table S2).
Notes: CTG = cognitive training group (mental rehearsal); ACG = active control group (self-developed strategy).
When performing the within-subjects analysis, our results showed significant three-way interactions for N-Back level × Session × Channel (F(6.59,1206.19) = 4.53, p < 0.01, =0.02), and Session x Channel x Group (F(3.01,551.09) = 7.72, p < 0.001,
= 0.04) (see Supplemental Material, Table S2 for further details). These results suggest that the interaction between session and channel differed between N-Back difficulty levels, and that the interaction between the latter and training group changed over sessions. Moreover, after correcting for multiple comparisons, our results did not show any significant two-way interactions nor any main effects. However, when considering the descriptive data, we observed a higher overall P300 amplitude for 1-Back (M = 2.53, SD = 0.38) compared to 2-Back (M = 1.58, SD = 0.47) and 3-Back (M = 0.90, SD = 0.46).
Furthermore, when we performed the between-subjects analysis, our results did not show a main effect for Group (CTG vs. ACG), indicating that overall the P300 amplitudes did not differ significantly between the two training groups (ACG (M = 1.73) and CTG (M = 1.61)).
We performed the same analyses for P300 latency. When performing the within-subjects analysis, our results showed a significant interaction effect for N-Back level × Session × Channel × Group (F(7.7,4216.60) = 4.64, p < 0.001, = 0.08), for N-Back level × Group (F(1.96,1071.25) = 24.95, p < 0.001,
= 0.044) and for Session × Channel (F(3.84,2103.58) = 4.89, p = 0.01,
= 0.009) (see Supplemental Material, Table S3 and Table S4 for further details). We also found a main effect for Channel (F(1.98,1084.01) = 46.34, p < 0.001,
= 0.078). A post-hoc pairwise-comparison analysis showed that channel Fz differed significantly from channel Pz in terms of P300 latency (p < 0.001) and that channel Cz differed significantly from channel Pz (p < 0.001) in terms of P300 latency. These results indicate that P300 latencies for Fz were overall shorter (M = 0.320) compared to those for Pz (M = 0.338) and that similarly also P300 latencies for Cz (M = 0.324) were shorter compared to those for Pz (M = 0.338).
Furthermore, when performing the between-subjects analysis, we found a main effect for Group (F(1,548) = 74.26, p < 0.001, = 0.119) suggesting that overall shorter P300 latencies for ACG (M = 0.339) compared to CTG (M = 0.316).
Transfer effects
To investigate whether transfers to other cognitive domains occurred after the N-Back training, we first compared the three groups at pre-tests using a one-way ANOVA, to determine whether differences would emerge at baseline. No significant differences were found. Next, we compared pre- and post-test differences of the three groups to see whether the strategy use might have played an important role in affecting transfers using mixed-ANOVA’s with one within-subject factor (pre-post) and one between subject factor (group). Our results showed significant transfer effects when comparing pre- and post-tests for VAT (F(1,12) = 12.79, p = 0.004, = 0.516), Raven (F(1,12) = 6.76, p = 0.023,
= 0.36) and Visual Search (F(1,12) = 27.19, p < 0.001,
= 0.694). However, these transfer effects were not significantly different when comparing the two training groups, suggesting that strategy did not affect transfer effects. Similarly, when we investigated pre- and post-test differences considering CTG and PCG as the factor group, we found a significant difference for Visual Search (F(1,17) = 9.55, p = 0.007,
= 0.36). However, we did not find any significant difference between groups, indicating that both training and PCG groups showed significant transfer effects. Last, when we explored pre- and post-test differences considering ACG and PCG as factor group, we found a significant for Raven (F(1,17) = 7.06,
= 0.293), but not between groups ().
Figure 5. Mean ± SD of accuracy for pre- and post-tests of CTG, ACG and PCG. All participants underwent pre- and post-tests before and after the N-Back training to assess transfers to other cognitive domains.
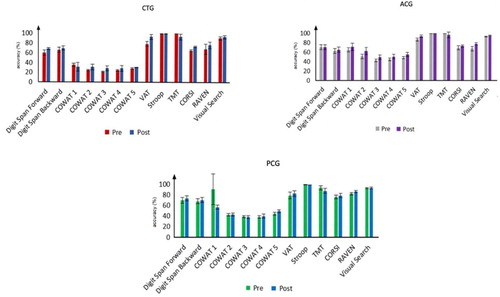
Moreover, when we investigated pre- and post-test differences for each group separately, using a repeated ANOVA, our results showed a significant effect for the ACG for CORSI (F(1,6) = 9.75, p = 0.021, = 0.619), a significant difference for the CTG for VAT (F(1,6) = 10.5, p = 0.018,
= 0.636), Raven (F(1,6) = 6.502, p = 0.044,
= 0.52) and Visual Search (F(1,6) = 17.754, p = 0.006,
= 0.747), whereas no significant pre- and post-test differences were found for the PCG.
Individual differences
N-Back task
In order to assess whether individual differences could affect N-Back training outcomes, we investigated P300 component and accuracy level for gender (males vs females), age (55–63 vs 64–70 years old) and education (<9 years vs >8 years) comparing CTG and ACG, by using mixed ANOVA analyses and Holm–Bonferroni correction for multiple comparisons. We first evaluated the role of individual differences in the first (t1) training session, and then in the first and last (t1–t3) training session. In the first training session, we found significant differences for age between young (M = 0.78) and older individuals (M = 1.899) in terms of P300 amplitude (F(1,890) = 6.16, p = 0.026, = 0.007), indicating lower amplitudes for young adults compared to older adults. However, this effect was not significant between the two training groups. Furthermore, our results showed significant differences for age between channels (Fz, Cz and Pz) (F(1.86,1657.48) = 18.75, p < 0.001,
= 0.021), suggesting that overall young adults exhibited lower P300 amplitudes for central and parietal areas (Cz: M = 1.53; Pz: M = −0.50) compared to older adults (Cz: M = 1.67; Pz: M = 2.74). However, differently from the previous findings, young adults showed larger P300 amplitudes (Fz: M = 1.31) compared to old participants (Fz: M = 1.22) in frontal areas. Again, this effect was not significant between the two training groups. No significant differences for gender nor education were found for the first session.
Of interest, when comparing t1 and t3, we found a significant age × session interaction (t1–t3) (F(1,819) = 14.18, p < 0.01, = 0.017), suggesting that P300 amplitude differed significantly between young (Mt1 = 0.789, Mt3 = 1.923) and older (Mt1 = 2.082, Mt3 = 0.971) adults. Specifically, young adults exhibited lower P300 amplitudes in t1 compared to older adults, whereas higher P300 amplitudes in t2 compared to older adults. However, we did not find any significant difference between the two training groups (CTG vs. ACG). Additionally a significant channel × education interaction was found (F(1.91,469.60) = 8.17, p < 0.01,
= 0.032), indicating that the effect of education differed significantly between channels. However, we did not find any significant difference between the two training groups. Moreover, when we considered the last training session, we did not find any significant main effect for gender, age or education.
We performed the same analyses for P300 latency in t1, and we found a significant difference for age between young (M = 0.350) and older (M = 0.323) adults (F(1,804) = 47.64, p < 0.001, = 0.056), showing longer latencies for young adults compared to older adults. However, we did not find any significant difference between the two training groups. Additionally, we found a significant difference for gender between male (M = 0.326) and female (M = 0.348) in terms of P300 latency (F(1,825) = 42.381, p < 0.001,
= 0.049). However, we did not find any significant difference between the two training groups as well as significant difference in P300 latency for education level. Moreover, our results showed a significant group x education level interaction in t1 (F(1,817) = 34.609, p < 0.001,
= 0.041), suggesting the different impact of education level between CTG (high education: M = 0.348; low education: M = 0.320) and ACG (high education: M = 0.338; low education: M = 0.353).
Of interest, when comparing t1 and t3, we did not find any significant difference considering all individual characteristics and session (t1–t3) interaction, suggesting that P300 latency did not significantly differ when comparing individual characteristics between the first and last session. As expected, our results showed a significant group x education level interaction comparing t1 and t3 (F(1,748) = 72.494, p < 0.001, = 0.089), indicating that high educated individuals that used the instructed strategy (mental rehearsal) (M = 0.340) showed overall longer P300 latencies compared to high educated individuals that were not instructed to use a specific strategy (M = 0.337). Furthermore, that low educated individuals that used the instructed strategy (M = 0.315) showed shorter P300 latencies compared to low educated individuals that were not instructed to use a specific strategy (M = 0.358).
Furthermore, when considering pre- and post-test differences of the similar version of the N-Back training task administered a few days before and after the training, our results showed no significant main effect for age, gender and education, suggesting that individual characteristics did not affect pre- and post-test outcomes.
Transfer tasks
Since individual differences modulated P300 responses recorded during N-Back training in the first and last training session, we investigated the impact of individual differences in comparing pre- and post-tests by using mixed-ANOVAs and Holm–Bonferroni correction for multiple comparisons. Our results showed a significant effect for gender for Visual Search (F(1,20) = 6.75, p = 0.017, = 0.252), indicating that female (M = 115.08) performed better than male (M = 111.82). However, this effect was not significantly different between groups. Also, we did not find any significant pre- and post-test main effects for age and education.
When considering baseline differences, we found that gender significantly affected Digit Span (F(1,25) = 5.18, p = 0.032, η2 = 0.18). Furthermore, when performing a two-way ANOVA with gender × group (CTG, ACG and PCG) interaction, our results indicated no significant differences between groups. Additionally, we found that education significantly affected CORSI (F(1,25) = 5.44, p = 0.028, η2 = 0.18). However, this effect was not significantly different between groups. Furthermore, we did not find any significant baseline differences for age. (see Table S5, Table S6 and Table S7 in Supplemental Material for further details).
Discussion
In the current study, we demonstrated that for healthy older adults, strategy use does not affect N-Back training outcome and does not lead to “within- and beyond-WM modality” transfers. We also verified the impact of three individual variables, age, gender and education, on training outcome as well as transfer effects. In addition, besides behavioural responses, we also recorded our participants’ P300 responses and verified the effect of these three individual factors on P300 amplitude and latency.
Despite the lack of improvement in the trained task in both training groups, our results revealed a difference between the two training groups for the non-adaptive version of the trained N-Back task, administered after intervention completion, with a higher accuracy for the ACG compared to CTG. This seems to support the “consolidation theory” which suggests that plasticity processes can occur during and immediately after training, but also days up to months after the intervention ends (Scullin et al., Citation2012). With the term “consolidation”, we imply the process of transforming a temporary perceptual representation into a durable WM representation that can survive the presentation of new sensory inputs (Jolicœur & Dell’Acqua, Citation1998). The enhancements observed in the trained task the next day might be related to a period of sleep after training that has been shown to enhance WM performance (Kuriyama et al., Citation2008). Indeed, both sleep and dopamine level has shown to regulate the degree of plasticity observed after WM training, with and without transcranial electrical stimulation (Walker & Stickgold, Citation2004). Consequently, this theory led us to believe that the use of a task-specific strategy might have a later impact on the trained task performance.
As to the P300, its amplitude and latencies showed no significant differences between the two groups across training sessions, channels and N-Back levels. The P300 amplitude revealed significant interactions between session and channels which in addition differed between difficulty levels, an interaction between group and difficulty level which differed significantly over sessions with overall a higher P300 amplitude for 1-Back compared to the more difficult levels. Moreover, we found a shorter overall P300 latency for ACG compared to CTG. However, we did not find any differences in P300 amplitude between training groups.
Previous studies on N-Back training (Guye & Von Bastian, Citation2017; Hering et al., Citation2017; Sala & Gobet, Citation2019; Teixeira-Santos et al., Citation2019) reported a minimal or even absent effectivity of N-back training in terms of transference to other cognitive domains. We investigated transfer effects both in terms of baseline (pre-tests) differences between groups and pre- and post-tests performance. After verifying pre-tests differences at baseline, for which we observed no differences between the three groups, we compared pre- and post-test performance. We found transfers in both training groups, and specifically transfers to “within-WM modality” (VAT), and “beyond-WM modality” (Raven and Visual Search) tasks. However, when comparing training groups, we did not observe any difference. These results suggest that the use of a specific strategy did not play an important role in affecting N-Back training outcomes for both “within-WM modality” and “beyond-WM modality” tasks. This is in line with Hertzog et al. (Citation1998) who also showed that mental rehearsal negatively affected performance in a free recall memory task, especially in older adults, and similarly in Sanders et al. (Citation1980) when using as strategy single mentions of each item in a list. Differently, the studies of Laine et al. (Citation2018), Morrison et al. (Citation2016) and Borella et al. (Citation2017) reported larger gains for participants that used a specific strategy, reason why we instructed one participant group in this way, although generally N-Back training did not enhance other cognitive domains in older adults. However, the results could not be comparable as these studies did not use mental rehearsal or an N-Back task to train their subjects. Furthermore, when comparing training groups, we did not observe any difference. Accordingly, the meta-analysis by Sala and Gobet (Citation2019) concluded that WM training interventions do not improve older adults’ cognitive functions, especially for transfers to “beyond-WM modality” tasks. Moreover, our results partially confirmed the findings observed in the studies by Colom et al. (Citation2013) and Harrison et al. (Citation2013) that reported enhancements in WM. Moreover, other recent meta-analyses (Lampit et al.’s, Citation2014; Melby-Lervåg et al., Citation2016; Melby-Lervåg & Hulme, Citation2013) supported the conclusions of Sala and Gobet (Citation2019) by highlighting the limited impact of WM training on transfer to other cognitive domains in older adults, especially to “beyond-WM modality” tasks.
Although our results partially differed with the limitations reported in those meta-analyses, we can agree that N-Back training can resort a controversial efficacy in both trained and untrained tasks, and that it is more difficult to observe transfers to “beyond-WM modality” tasks after WM training performance (Sala & Gobet, Citation2019; Soveri et al., Citation2017).
Besides assessing the effect of strategy use and transfer to “within-WM modality” and “beyond-WM modality” tasks after N-Back training, we also evaluated the impact of individual differences from a neurophysiological point of view. Age was observed to affect P300 amplitude for both training groups at baseline (first N-Back session), whereas age, education and gender for P300 latency. However, no differences between groups at baseline (t1) were observed, except for education. Moreover, age affected P300 amplitude when comparing the first and last training sessions, but it was not significant between the two training groups, whereas education, in line with the outcomes in t1, had an impact on P300 latency between groups. P300 latency outcomes at baseline were in line with the results observed by Polich (Citation2007) and partially in agreement with those of Steffensen et al. (Citation2008) which indicated larger P300 amplitudes to target stimuli for females, although gender did not affect P300 latency. Furthermore, the impact of education on P300 latency we observed supports the evidence presented by Patel and Azzam (Citation2005) who showed that individuals with a lower educational level might have more difficulties in the stimulus identification process, causing their P300 latency to become shorter. Also Angel et al. (Citation2010), using a word-stem cued-recall task, observed the effect of educational level on ERP responses when comparing young and older individuals, with a smaller effect of age for higher educated people compared to those with a lower level of education.
In addition, we investigated the effect of individual differences from a behavioural perspective using a similar version of the trained task and any transfer effects at baseline and for pre- and post-tests. Interestingly, individual differences for the two training groups at baseline did not impact on N-Back task performance. Moreover, when we investigated transfer effects, we found that baseline performance was significantly affected by gender for Digit Span and by education for CORSI, and that pre- and post-test performance was affected by gender for Visual Search. In contrast, age and education did not result to affect any pre- and post-tests outcomes.
The present study suggests that N-Back training in older adults does not significantly enhances trained task performance, as well as leads to transfers to other cognitive domains (“within-WM modality” and “beyond-WM modality” tasks), in line with previous WM training studies that highlight the difficulty of enhancing “beyond-WM modality” tasks such as fluid intelligence. Moreover, our findings add novelties in measuring the impact of strategy use and individual differences on accuracy and in showing the effect on P300 amplitudes and latencies in the trained as well as transfer tasks, across training sessions. Except for two previous studies (Hertzog et al., Citation1998; Sanders et al., Citation1980), albeit using different memory tasks, we demonstrated in the case of the N-Back task mental rehearsal is not effective in improving trained WM task performance and in increasing transfers to other untrained cognitive domains. We also revealed the impact of differences in age, gender and education on WM training outcomes. This study has potential limitations that need to be considered. The first one is the small sample size, suggesting to carefully interpret our findings when generalising to other cognitive training domains. However, to ensure that our populations were large enough for our case, we performed a power analysis using G*power (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower.html) for accuracy showing that ≥7 subjects for the N-Back training task for the transfer tasks are sufficient to support a power of 90%. We admit that the picture is more complex from P300 perspective since individual differences can have an impact on P300 signature, especially age, which we hypothesise to have an effect on training outcome. Apart from that, our EEG recordings were made with the same device and electrode set-up (in our case, the 10–20 EEG SynAmpsRT device system with Ag/AgCl electrodes), electrode referencing system (here, average mastoid reference), environmental conditions (between 22°C and 25°C), experimental setup (the averaged minimal trial number per condition was above 70 epochs, in line with the recommendations for conducting P300 data analysis, see Cohen & Polich, Citation1997; Pontifex et al., Citation2010), preprocessing pipeline and P300 signature extraction (see Methods section). But, overall, quantitative sample size calculations are still scarce in ERP literature although it would add scientific rigour (Larson & Carbine, Citation2017). The second limitation concerns the fact that we investigated only a few individual differences, albeit other factors such as leisure activities, life style, language, etc., might play an important role in WM training outcome. The third limitation concerns the need for additional investigations in order to explore whether other ERP components, such as early and late ERPs, are modulated across N-Back training sessions. The fourth limitation regards the lack of EEG recordings for the pre- and post-N-Back task. For instance, it might have been interesting to see whether there was already a discernable signature of a consolidation process. Additionally, these results should be interpreted with caution due to the lack of follow-ups for testing whether transfers to other cognitive domains are preserved in older individuals. Moreover, although we were aware that other ERP components might be affected by WM training, such as early visual and late positive components (LPC) (Luria et al., Citation2016; Salmi et al., Citation2019) reflecting load effect and maintenance of to-be-remembered materials (Vilà-Balló et al., Citation2018), it was impossible to investigate these positive and negative slow waves as they occur in a time window that overlaps our feedback stimuli and collected our button press responses. Consequently, it would have been very difficult to extract this component from the feedback stimulus and button press responses. Last, the transfer tasks we selected and administered in this study involved several cognitive domains, “within-” and “beyond-WM modality”, however we did not include tests for detecting changes in daily-life activities. In view of these observations, we suggest future studies to consider the role of strategy use and individual differences on daily-life performance and to further investigate the factors that might modulate the impact of the N-Back training on transfers to other cognitive domains.
Author contributions
VP and NV collected the data and conducted the experiments; VP designed the experiments, wrote the manuscript and performed the analyses; NV contributed to the performance of the analyses. All authors discussed the results and contributed to the final manuscript.
Data sharing
The data of this study are available via this link: “https://drive.google.com/drive/folders/1kZwTZl5foxbMQQJ3SanMt-zuKtEFOmp4?usp=sharing”.
supplementary material
Download MS Word (396.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Angel, L., Fay, S., Bouazzaoui, B., Baudouin, A., & Isingrini, M. (2010). Protective role of educational level on episodic memory aging: An event-related potential study. Brain and Cognition, 74(3), 312–323. https://doi.org/10.1016/j.bandc.2010.08.012
- Army, U. S. (1944). Army individual test battery. Manual of Directions and Scoring.
- Ashford, J. W., Coburn, K. L., Rose, T. L., & Bayley, P. J. (2011). P300 energy loss in aging and Alzheimer’s disease. Journal of Alzheimer’s Disease, 26(s3), 229–238. https://doi.org/10.3233/JAD-2011-0061
- Baddeley, A. (2003). Working memory: Looking back and looking forward. Nature Reviews Neuroscience, 4(10), 829. https://doi.org/10.1038/nrn1201
- Baddeley, A. D., & Hitch, G. J. (1974). Working memory. In G. A. Bower (Ed.), The psychology of learning and motivation (pp. 47–89). Academic Press.
- Bailey, H. R., Dunlosky, J., & Hertzog, C. (2014). Does strategy training reduce age-related deficits in working memory? Gerontology, 60(4), 346–356. https://doi.org/10.1159/000356699
- Basak, C., Boot, W. R., Voss, M. W., & Kramer, A. F. (2008). Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychology and Aging, 23(4), 765. https://doi.org/10.1037/a0013494
- Begum, T., Reza, F., Ahmed, I., & Abdullah, J. M. (2014). Influence of education level on design-induced N170 and P300 components of event related potentials in the human brain. Journal of Integrative Neuroscience, 13(1), 71–88. https://doi.org/10.1142/S0219635214500058
- Benson, B. L., Anguera, J. A., & Seidler, R. D. (2011). A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. Journal of Neurophysiology, 105(6), 2843–2851. https://doi.org/10.1152/jn.00002.2011
- Borella, E., Carbone, E., Pastore, M., De Beni, R., & Carretti, B. (2017). Working memory training for healthy older adults: The role of individual characteristics in explaining short-and long-term gains. Frontiers in Human Neuroscience, 11, 99. https://doi.org/10.3389/fnhum.2017.00099
- Borella, E., Carretti, B., Sciore, R., Capotosto, E., Taconnat, L., Cornoldi, C., & De Beni, R. (2017). Training working memory in older adults: Is there an advantage of using strategies? Psychology and Aging, 32(2), 178–191. https://doi.org/10.1037/pag0000155
- Brehmer, Y., Westerberg, H., & Bäckman, L. (2012). Working-memory training in younger and older adults: Training gains, transfer, and maintenance. Frontiers in Human Neuroscience, 6, 63. https://doi.org/10.3389/fnhum.2012.00063
- Bürki, C. N., Ludwig, C., Chicherio, C., & De Ribaupierre, A. (2014). Individual differences in cognitive plasticity: An investigation of training curves in younger and older adults. Psychological Research, 78(6), 821–835. https://doi.org/10.1007/s00426-014-0559-3
- Cantarella, A., Borella, E., Carretti, B., Kliegel, M., & de Beni, R. (2017). Benefits in tasks related to everyday life competences after a working memory training in older adults. International Journal of Geriatric Psychiatry, 32(1), 86–93. https://doi.org/10.1002/gps.4448
- Chen, Y. N., Mitra, S., & Schlaghecken, F. (2008). Sub-processes of working memory in the N-back task: An investigation using ERPs. Clinical Neurophysiology, 119(7), 1546–1559. https://doi.org/10.1016/j.clinph.2008.03.003
- Christensen, H. (2001). What cognitive changes can be expected with normal ageing? Australian and New Zealand Journal of Psychiatry, 35(6), 768–775. https://doi.org/10.1046/j.1440-1614.2001.00966.x
- Cohen, J. (1973). Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and Psychological Measurement, 33(1), 107–112. https://doi.org/10.1177/001316447303300111
- Cohen, J., & Polich, J. (1997). On the number of trials needed for P300. International Journal of Psychophysiology, 25(3), 249–255. https://doi.org/10.1016/S0167-8760(96)00743-X
- Colom, R., Román, F. J., Abad, F., Shih, P. C., Privado, J., Froufe, M., Escorial, S., Martínez, K., Burgaleta, M., Quiroga, MÁ, Karama, S., Haier, R., Thompson, P., & Jaeggi, S. (2013). Adaptive n-back training does not improve fluid intelligence at the construct level: Gains on individual tests suggest that training may enhance visuospatial processing. Intelligence, 41(5), 712–727. https://doi.org/10.1016/j.intell.2013.09.002
- Conroy, M. A., & Polich, J. (2007). Normative variation of P3a and P3b from a large sample: Gender, topography, and response time. Journal of Psychophysiology, 21(1), 22–32. https://doi.org/10.1027/0269-8803.21.1.22
- Croft, R. J., & Barry, R. J. (1998). EOG correction: A new perspective. Electroencephalography and Clinical Neurophysiology, 107(6), 387–394. https://doi.org/10.1016/S0013-4694(98)00086-8
- Croft, R. J., & Barry, R. J. (2000). Removal of ocular artifact from the EEG: A review. Neurophysiologie Clinique/Clinical Neurophysiology, 30(1), 5–19. https://doi.org/10.1016/S0987-7053(00)00055-1
- Delaloye, C., Ludwig, C., Borella, E., Chicherio, C., & De Ribaupierre, A. (2008). L’Empan de lecture comme épreuve mesurant la capacité de mémoire de travail: normes basées sur une population francophone de 775 adultes jeunes et âgés. European Review of Applied Psychology, 58(2), 89–103. https://doi.org/10.1016/j.erap.2006.12.004
- De Ribaupierre, A., & Lecerf, T. (2006). Relationships between working memory and intelligence from a developmental perspective: Convergent evidence from a neo-Piagetian and a psychometric approach. European Journal of Cognitive Psychology, 18(1), 109–137. https://doi.org/10.1080/09541440500216127
- Donchin, E. (1986). Cognitive psychophysiology and human information processing. Psychophysiology: Systems, Processes and Applications, 244–267.
- Drag, L. L., Bieliauskas, L. A., Langenecker, S. A., & Greenfield, L. J. (2010). Cognitive functioning, retirement status, and age: Results from the cognitive changes and retirement among senior surgeons study. Journal of the American College of Surgeons, 211(3), 303–307. https://doi.org/10.1016/j.jamcollsurg.2010.05.022
- Duncan, C. C., Barry, R. J., Connolly, J. F., Fischer, C., Michie, P. T., Naatanen, R., Polich, J., Reinvang, I., & Van Petten, C. (2009). Event-related potentials in clinical research: Guidelines for 116 eliciting, recording, and quantifying mismatch negativity, P300, and N400. 117. Clinical Neurophysiology, 120(11), 1883–1908. https://doi.org/10.1016/j.clinph.2009.07.045
- Gevins, A., Smith, M. E., Le, J., Leong, H., Bennett, J., Martin, N., & Whitfield, S. (1996). High resolution evoked potential imaging of the cortical dynamics of human working memory. Electroencephalography and Clinical Neurophysiology, 98(4), 327–348. https://doi.org/10.1016/0013-4694(96)00288-X
- Guye, S., & Von Bastian, C. C. (2017). Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychology and Aging, 32(8), 732. https://doi.org/10.1037/pag0000206
- Harrison, T. L., Shipstead, Z., Hicks, K. L., Hambrick, D. Z., Redick, T. S., & Engle, R. W. (2013). Working memory training may increase working memory capacity but not fluid intelligence. Psychological Science, 24(12), 2409–2419. https://doi.org/10.1177/0956797613492984
- Heinzel, S., Lorenz, R. C., Pelz, P., Heinz, A., Walter, H., Kathmann, N., & Stelzel, C. (2016). Neural correlates of training and transfer effects in working memory in older adults. Neuroimage, 134, 236–249. https://doi.org/10.1016/j.neuroimage.2016.03.068
- Hering, A., Meuleman, B., Bürki, C., Borella, E., & Kliegel, M. (2017). Improving older adults’ working memory: The influence of age and crystallized intelligence on training outcomes. Journal of Cognitive Enhancement, 1(4), 358–373. https://doi.org/10.1007/s41465-017-0041-4
- Hertzog, C., McGuire, C. L., & Lineweaver, T. T. (1998). Aging, attributions, perceived control, and strategy use in a free recall task. Aging. Neuropsychology, and Cognition, 5(2), 85–106. https://doi.org/10.1076/anec.5.2.85.601
- Heslenfeld, D. J.. (2003). Visual mismatch negativity. In J. Polich (Ed.), Detection of change (pp. 41–59). Boston, MA: Springer.
- Jaeggi, S. M., Buschkuehl, M., Jonides, J., & Perrig, W. J. (2008). Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences, 105(19), 6829–6833. https://doi.org/10.1073/pnas.0801268105
- Jaeggi, S. M., Buschkuehl, M., Jonides, J., & Shah, P. (2011). Short-and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences, 108(25), 10081–10086. https://doi.org/10.1073/pnas.1103228108
- Jolicœur, P., & Dell’Acqua, R. (1998). The demonstration of short-term consolidation. Cognitive Psychology, 36(2), 138–202. https://doi.org/10.1006/cogp.1998.0684
- Kahneman, D., & Tversky, A. (1973). On the psychology of prediction. Psychological Review, 80(4), 237. https://doi.org/10.1037/h0034747
- Katz, B., Jaeggi, S. M., Buschkuehl, M., Shah, P., & Jonides, J. (2018). The effect of monetary compensation on cognitive training outcomes. Learning and Motivation, 63, 77–90. https://doi.org/10.1016/j.lmot.2017.12.002
- Kessels, R. P., Van Zandvoort, M. J., Postma, A., Kappelle, L. J., & De Haan, E. H. (2000). The corsi block-tapping task: Standardization and normative data. Applied Neuropsychology, 7(4), 252–258. https://doi.org/10.1207/S15324826AN0704_8
- Kok, A. (2001). On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology, 38(3), 557–577. https://doi.org/10.1017/S0048577201990559
- Kramer, A. F., & Strayer, D. L. (1988). Assessing the development of automatic processing: An application of dual-task and event-related brain potential methodologies. Biological Psychology, 26(1-3), 231–267. https://doi.org/10.1016/0301-0511(88)90022-1
- Kuriyama, K., Mishima, K., Suzuki, H., Aritake, S., & Uchiyama, M. (2008). Sleep accelerates the improvement in working memory performance. Journal of Neuroscience, 28(40), 10145–10150. https://doi.org/10.1523/JNEUROSCI.2039-08.2008
- Laine, M., Fellman, D., Waris, O., & Nyman, T. J. (2018). The early effects of external and internal strategies on working memory updating training. Scientific Reports, 8(1), 4045. https://doi.org/10.1038/s41598-018-22396-5
- Lampit, A., Hallock, H., & Valenzuela, M. (2014). Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Medicine, 11(11). https://doi.org/10.1371/journal.pmed.1001756
- Larson, M. J., & Carbine, K. A. (2017). Sample size calculations in human electrophysiology (EEG and ERP) studies: A systematic review and recommendations for increased rigor. International Journal of Psychophysiology, 111, 33–41.
- Li, L., Gratton, C., Fabiani, M., & Knight, R. T. (2013). Age-related frontoparietal changes during the control of bottom-up and top-down attention: An ERP study. Neurobiology of Aging, 34(2), 477–488. https://doi.org/10.1016/j.neurobiolaging.2012.02.025
- Li, S. C., Schmiedek, F., Huxhold, O., Röcke, C., Smith, J., & Lindenberger, U. (2008). Working memory plasticity in old age: Practice gain, transfer, and maintenance. Psychology and Aging, 23(4), 731. https://doi.org/10.1037/a0014343
- Lilienthal, L., Tamez, E., Shelton, J. T., Myerson, J., & Hale, S. (2013). Dual N-back training increases the capacity of the focus of attention. Psychonomic Bulletin & Review, 20(1), 135–141. https://doi.org/10.3758/s13423-012-0335-6
- Lindeboom, J., Schmand, B., Tulner, L., Walstra, G., & Jonker, C. (2002). Visual association test to detect early dementia of the Alzheimer type. Journal of Neurology. Neurosurgery & Psychiatry, 73(2), 126–133. https://doi.org/10.1136/jnnp.73.2.126
- Loosli, S. V., Falquez, R., Unterrainer, J. M., Weiller, C., Rahm, B., & Kaller, C. P. (2016). Training of resistance to proactive interference and working memory in older adults: A randomized double-blind study. International Psychogeriatrics, 28(3), 453–467. https://doi.org/10.1017/S1041610215001519
- Luck, S. J. (2005). Ten simple rules for designing ERP experiments. Event-related potentials: A methods handbook, 262083337.
- Luria, R., Balaban, H., Awh, E., & Vogel, E. K. (2016). The contralateral delay activity as a neural measure of visual working memory. Neuroscience & Biobehavioral Reviews, 62, 100–108. https://doi.org/10.1016/j.neubiorev.2016.01.003
- MacPherson, S. E., Phillips, L. H., & Della Sala, S. (2002). Age, executive function and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychology and Aging, 17(4), 598. https://doi.org/10.1037/0882-7974.17.4.598
- Meier, M. E., & Kane, M. J. (2013). Working memory capacity and Stroop interference: Global versus local indices of executive control. Journal of Experimental Psychology: Learning, Memory, and Cognition, 39(3), 748. https://doi.org/10.1037/a0029200
- Melby-Lervåg, M., & Hulme, C. (2013). Is working memory training effective? A meta-analytic review. Developmental Psychology, 49(2), 270. https://doi.org/10.1037/a0028228
- Melby-Lervåg, M., Redick, T. S., & Hulme, C. (2016). Working memory training does not improve performance on measures of intelligence or other measures of “far transfer” evidence from a meta-analytic review. Perspectives on Psychological Science, 11(4), 512–534. https://doi.org/10.1177/1745691616635612
- Miyake, A., & Shah, P. (1999). Models of working memory: Mechanisms of active maintenance and executive control. Cambridge University Press.
- Morrison, A. B., Rosenbaum, G. M., Fair, D., & Chein, J. M. (2016). Variation in strategy use across measures of verbal working memory. Memory & Cognition, 44(6), 922–936. https://doi.org/10.3758/s13421-016-0608-9
- Neely, A. S., & Bäckman, L. (1995). Effects of multifactorial memory training in old age: Generalizability across tasks and individuals. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 50(3), 134–P140. https://doi.org/10.1093/geronb/50B.3.P134
- Norman, D. A., & Shallice, T.. (1986). Attention to action. In R. J. Davidson, G. E. Schwartz, & D. Shapiro (Eds.), Consciousness and self-regulation (pp. 1–18). Boston, MA: Springer.
- Oelhafen, S., Nikolaidis, A., Padovani, T., Blaser, D., Koenig, T., & Perrig, W. J. (2013). Increased parietal activity after training of interference control. Neuropsychologia, 51(13), 2781–2790. https://doi.org/10.1016/j.neuropsychologia.2013.08.012
- Patel, S. H., & Azzam, P. N. (2005). Characterization of N200 and P300: Selected studies of the event-related potential. International Journal of Medical Sciences, 2(4), 147. https://doi.org/10.7150/ijms.2.147
- Pergher, V., Shalchy, M. A., Pahor, A., Van Hulle, M. M., Jaeggi, S. M., & Seitz, A. R. (2019). Divergent research methods limit understanding of working memory training. Journal of Cognitive Enhancement, 1–21. https://doi.org/10.1007/s41465-019-00134-7
- Pergher, V., Wittevrongel, B., Tournoy, J., Schoenmakers, B., & Van Hulle, M. M. (2019). Mental workload of young and older adults gauged with ERPs and spectral power during N-Back task performance. Biological Psychology, 107726. https://doi.org/10.1016/j.biopsycho.2019.107726
- Pesonen, M., Hämäläinen, H., & Krause, C. M. (2007). Brain oscillatory 4–30 Hz responses during a visual n-back memory task with varying memory load. Brain Research, 1138, 171–177. https://doi.org/10.1016/j.brainres.2006.12.076
- Pfefferbaum, A., Rosenbloom, M., Deshmukh, A., & Sullivan, E. V. (2001). Sex differences in the effects of alcohol on brain structure. American Journal of Psychiatry, 158(2), 188–197. https://doi.org/10.1176/appi.ajp.158.2.188
- Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. https://doi.org/10.1016/j.clinph.2007.04.019
- Pontifex, M. B., Scudder, M. R., Brown, M. L., O’Leary, K. C., Wu, C. T., Themanson, J. R., & Hillman, C. H. (2010). On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology, 47(4), 767–773. https://doi.org/10.1111/j.1469-8986.2010.00974.x
- Raven, J. C. (1938). Raven’s progressive matrices. Western Psychological Services.
- Reitan, R. M., & Wolfson, D. (1986). The halstead-reitan neuropsychological test battery. In D. Wedding, A. M. Horton, Jr., & J. S. Webster (Eds.), The neuropsychology handbook: Behavioral and clinical perspectives (p. 134–160). Springer Publishing Co.
- Richmond, L. L., Morrison, A. B., Chein, J. M., & Olson, I. R. (2011). Working memory training and transfer in older adults. Psychology and Aging, 26(4), 813. https://doi.org/10.1037/a0023631
- Ross, T. P., Calhoun, E., Cox, T., Wenner, C., Kono, W., & Pleasant, M. (2007). The reliability and validity of qualitative scores for the controlled oral word association test. Archives of Clinical Neuropsychology, 22(4), 475–488. https://doi.org/10.1016/j.acn.2007.01.026
- Rowe, J. B., Toni, I., Josephs, O., Frackowiak, R. S., & Passingham, R. E. (2000). The prefrontal cortex: Response selection or maintenance within working memory? Science, 288(5471), 1656–1660. https://doi.org/10.1126/science.288.5471.1656
- Ruchkin, D. S., Canoune, H. L., Johnson Jr, R., & Ritter, W. (1995). Working memory and preparation elicit different patterns of slow wave event-related brain potentials. Psychophysiology, 32(4), 399–410. https://doi.org/10.1111/j.1469-8986.1995.tb01223.x
- Ruff, R. M., & Parker, S. B. (1993). Gender-and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the finger tapping and grooved pegboard tests. Perceptual and Motor Skills, 76(3_suppl), 1219–1230. https://doi.org/10.2466/pms.1993.76.3c.1219
- Sala, G., & Gobet, F. (2019). Cognitive training does not enhance general cognition. Trends in Cognitive Sciences, 23(1), 9–20. https://doi.org/10.1016/j.tics.2018.10.004
- Salmi, J., Vilà-Balló, A., Soveri, A., Rostan, C., Rodríguez-Fornells, A., Lehtonen, M., & Laine, M. (2019). Working memory updating training modulates a cascade of event-related potentials depending on task load. Neurobiology of Learning and Memory, 166, 107085. https://doi.org/10.1016/j.nlm.2019.107085
- Sánchez-Cubillo, I., Perianez, J. A., Adrover-Roig, D., Rodriguez-Sanchez, J. M., Rios-Lago, M., Tirapu, J. E. E. A., & Barcelo, F. (2009). Construct validity of the trail making test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society, 15(3), 438–450. https://doi.org/10.1017/S1355617709090626
- Sanders, R. E., Murphy, M. D., Schmitt, F. A., & Walsh, K. K. (1980). Age differences in free recall rehearsal strategies. Journal of Gerontology, 35(4), 550–558. https://doi.org/10.1093/geronj/35.4.550
- Scharinger, C., Soutschek, A., Schubert, T., & Gerjets, P. (2017). Comparison of the working memory load in n-back and working memory span tasks by means of EEG frequency band power and P300 amplitude. Frontiers in Human Neuroscience, 11, 6. https://doi.org/10.3389/fnhum.2017.00006
- Scullin, M. K., Trotti, L. M., Wilson, A. G., Greer, S. A., & Bliwise, D. L. (2012). Nocturnal sleep enhances working memory training in Parkinson’s disease but not Lewy body dementia. Brain, 135(9), 2789–2797. https://doi.org/10.1093/brain/aws192
- Shah, P., & Miyake, A. (1999). Models of working memory–an introduction.
- Simons, D. J., Boot, W. R., Charness, N., Gathercole, S. E., Chabris, C. F., Hambrick, D. Z., & Stine-Morrow, E. A. (2016). Do “brain-training” programs work? Psychological Science in the Public Interest, 17(3), 103–186. https://doi.org/10.1177/1529100616661983
- Soveri, A., Antfolk, J., Karlsson, L., Salo, B., & Laine, M. (2017). Working memory training revisited: A multi-level meta-analysis of n-back training studies. Psychonomic Bulletin & Review, 24(4), 1077–1096. https://doi.org/10.3758/s13423-016-1217-0
- Steffensen, S. C., Ohran, A. J., Shipp, D. N., Hales, K., Stobbs, S. H., & Fleming, D. E. (2008). Gender-selective effects of the P300 and N400 components of the visual evoked potential. Vision Research, 48(7), 917–925. https://doi.org/10.1016/j.visres.2008.01.005
- Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643. https://doi.org/10.1037/h0054651
- Teixeira-Santos, A. C., Moreira, C. S., Magalhães, R., Magalhães, C., Pereira, D. R., Leite, J., Carvalho, S., & Sampaio, A. (2019). Reviewing working memory training gains in healthy older adults: A meta-analytic review of transfer for cognitive outcomes. Neuroscience & Biobehavioral Reviews, 103, 163–177. https://doi.org/10.1016/j.neubiorev.2019.05.009
- Vilà-Balló, A., Salmi, J., Soveri, A., Rodríguez-Fornells, A., Lehtonen, M., & Laine, M. (2018). Neural signatures for active maintenance and interference during working memory updating. Biological Psychology, 132, 233–243. https://doi.org/10.1016/j.biopsycho.2018.01.007
- Walker, M. P., & Stickgold, R. (2004). Sleep-dependent learning and memory consolidation. Neuron, 44(1), 121–133. https://doi.org/10.1016/j.neuron.2004.08.031
- Watter, S., Geffen, G. M., & Geffen, L. B. (2001). The n-back as a dual-task: P300 morphology under divided attention. Psychophysiology, 38(6), 998–1003. https://doi.org/10.1111/1469-8986.3860998
- Wechsler, D. (1955). Manual for the Wechsler adult intelligence scale.
- Wiederholt, W. C., Cahn, D., Butters, N. M., Salmon, D. P., Kritz-Silverstein, D., & Barrett-Connor, E. (1993). Effects of age, gender and education on selected neuropsychological tests in an elderly community cohort. Journal of the American Geriatrics Society, 41(6), 639–647. https://doi.org/10.1111/j.1532-5415.1993.tb06738.x
- Wolfe, J. M. (1998). Visual search. Attention, 1, 13–73.
- Zinke, K., Zeintl, M., Rose, N. S., Putzmann, J., Pydde, A., & Kliegel, M. (2014). Working memory training and transfer in older adults: Effects of age, baseline performance, and training gains. Developmental Psychology, 50(1), 304. https://doi.org/10.1037/a0032982
