ABSTRACT
The purpose of this study was to investigate whether symptoms of attention deficit hyperactivity disorder (ADHD) impact distraction by unexpected deviant sounds and vibrations. The hypothesis was a difference between individuals with low and high ADHD symptom severity in deviance distraction. In a cross-modal oddball task, we measured the impact of to-be-ignored deviating auditory and vibrotactile stimuli in 45 adults. No difference was observed between groups with low and high symptoms of ADHD in their propensity for distraction between modalities using both frequentist and Bayesian methods. The impact of the deviating sounds and vibrations on performance was similar between groups. However, the amount of missed trials, which possibly reflects mind wandering or attention away from the focal task, was higher in the high-symptom group. The findings indicate some differences in habituation across the duration of the task. The complexity of adult ADHD symptomatology, especially differences in attentional control is discussed.
ADHD symptoms and auditory and tactile distraction in adults
Attention Deficit Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder that affects approximately 5-10% of the adult population worldwide (Adler et al., Citation2017; Faraone et al., Citation2021). Individuals with ADHD often experience difficulties in inhibiting irrelevant stimuli and maintaining attention to the task at hand (McLoughlin et al., Citation2011; Pedersen & Ohrmann, Citation2018). Previous studies have suggested that sensory processing differences may contribute to these attentional difficulties in individuals with ADHD (Dellapiazza et al., Citation2021). However, the extent to which different sensory modalities, such as auditory and tactile, may impact attention differentially in adults with different levels of ADHD symptoms remains unclear.
Studies on younger and older adults without an ADHD diagnosis have shown that attention can differ across modalities, with some individuals exhibiting better performance in auditory tasks and others in tactile tasks (e.g. Rienäcker et al., Citation2018; Santangelo et al., Citation2010). The oddball paradigm has been frequently used to measure attention in different modalities (e.g. Senderecka et al., Citation2012; Sokhadze et al., Citation2018) and across modalities using the cross-modal oddball task (Escera et al., Citation1998). In auditory-visual oddball tasks, while focussing on a visual categorisation task, participants are required to inhibit distraction from infrequent, rare and unexpected deviant sounds that are presented in a sequence of repeated standard sounds. Similarly, in tactile-visual oddball tasks, participants are required to inhibit distraction from infrequent, rare and unexpected deviant vibrations that are presented in a sequence of repeated standard vibrations (Ljungberg et al., Citation2012). Typically, the standard stimuli, in the oddball task, are presented in 80% of the trials, whereas the deviant stimuli are presented in 20% of the trials (see Parmentier, Citation2014 for a review). Deviant stimuli have been observed to elicit notable brain responses, including mismatch negativity, ERP component P3a, and re-orienting negativity, indicating their impact on cognitive processes (for a review see Wetzel & Schröger, Citation2014). Furthermore, these deviant stimuli in this paradigm can lead to behavioural consequences such as prolonged reaction times, a phenomenon known as deviance distraction (e.g. Parmentier, Citation2014). Interestingly, the influence of deviance can extend beyond a single trial, as post-deviance distraction is also observed in standard trials following a deviant trial, also resulting in extended reaction times (Wetzel, Citation2015). Therefore, the oddball paradigm is utilised in research on ADHD cognitive symptoms due to its ability to directly represent the aforementioned behavioural consequences of deviance distraction.
These cross-modal oddball tasks, named such for including sensory processing in more than one modality (e.g. a visual task with auditory distractors), have been used to study attentional control in children and adolescents with ADHD. Individuals with ADHD exhibit deficits in both sensory processing and orienting attention, indicated by neural differences in specific brain regions using fMRI (Orinstein & Stevens, Citation2014; Stevens et al., Citation2007) and ERP (Alexander et al., Citation2008; Janssen et al., Citation2016). Another study on adolescents using an auditory oddball paradigm found response speed was improved in trials with to-be-ignored deviant stimuli in 25 boys diagnosed with ADHD. The authors argued that stimulation of the orienting network by novel sounds facilitates subsequent focal task performance (Tegelbeckers et al., Citation2022).
Re-orienting attention is an important cognitive process that allows individuals to redirect their attention from a distracting stimulus back to a task at hand (Corbetta et al., Citation2008). Adults with ADHD may have difficulties specifically with re-orienting attention, particularly in response to unexpected or salient stimuli (Gumenyuk et al., Citation2023). These difficulties may manifest as longer reaction times on post-deviant standard trials, indicating a delay in the re-engagement of attention to the task. Other studies support this idea that individuals with ADHD have sensory processing differences and difficulties with re-orienting attention, particularly in the visual modality (Mueller et al., Citation2017; Roberts et al., Citation2018). However, relatively little research has examined the impact of unexpected task-irrelevant changes in other sensory modalities, such as auditory and tactile, on attentional performance in adult individuals with attention deficits (Ben-Sasson et al., Citation2017). Furthermore, although the oddball task has been widely used to examine the impact of unexpected changes in attentional performance, few studies have directly compared the effects of auditory and vibrotactile deviants. Therefore, a knowledge gap exists regarding the possible impact of sensory deviance on attentional performance in adults with ADHD and the possible sensory processing differences associated with ADHD symptom severity.
Cross-modal processing has been studied in individuals with ADHD. For example, research has shown that individuals with ADHD have difficulties in integrating information from multiple sensory modalities, leading to impaired cross-modal processing (e.g. visual-auditory integration) and a reduced ability to use multisensory information to guide attention and behaviour (Lin et al., Citation2017; Schmidt et al., Citation2019). Studying cross-modal sensory processing can not only add to our understanding of how sensory differences may contribute to ADHD symptoms but also shed light on the extent to which cross-modal processing deficits may underlie ADHD-related attentional impairments. For instance, affective startle paradigms and inhibition paradigms have been found to be impacted by symptoms of ADHD, and some theories suggest that sensory gating is implicated in the development of symptoms (Schulz et al., Citation2023). A potential difference in how those with ADHD process tactile information compared to same-aged peers exists in the literature (Keating et al., Citation2022). Children with ADHD show slower reaction times to tactile stimuli on certain tasks of attentional control (Puts et al., Citation2017). This knowledge can potentially inform the development of more effective interventions aimed at improving cross-modal processing and attention in individuals with ADHD.
The current study aimed to compare the effects of to-be-ignored unexpected vibrotactile and auditory unexpected task-irrelevant changes on performance in a visual focal task in adult participants with low or high scores on the adult ADHD self-reported scale (ASRS) screening tool measuring self-reported symptoms of ADHD. The ASRS is a commonly used and validated screening tool within the sample population to measure ADHD symptom severity (Anbarasan et al., Citation2020; Brevik et al., Citation2020). We used a cross-modal oddball paradigm to measure the impact of deviance distraction in a visual selective attention task. Specifically, we investigated whether healthy adults with high or low ADHD symptom severity show differential effects of vibrotactile and auditory unexpected task-irrelevant changes in a visual task and whether their ability to re-orient attention in post-deviant trials is affected by these sensory modalities. This study's contribution is its focus on cross-modal processing in adults with ADHD. While previous studies have examined auditory or visual processing in ADHD, none of the authors’ knowledge have investigated tactile processing. We hypothesised that if ADHD symptoms are associated with sensory processing differences, having high ADHD symptoms (falling within the scoring thresholds for clinical diagnosis on the ASRS screening tool) may be related to differences in reaction time to auditory and tactile deviant trials compared to standard trials.
Methods
Participants
In this study, we included a sample of 51 Swedish adults. We used the R packages “mixedpower” (Kumle et al., Citation2020) and “simr” (Green & MacLeod, Citation2016) to perform a power analysis by simulation using previously collected data from a pilot study of healthy adults (n = 20). Our goal was to obtain .95 power to detect an effect size of .25 between low and high levels of ADHD groups at a .05 alpha level. According to this power analysis by simulation using previously collected data, our target sample size was 40 participants in order to run the linear mixed effects model used in our main pre-registered analysis including sensory modality and ADHD group. The participants were recruited via advertisements on city and university bulletin boards, via local social media groups, and by word-of-mouth. Inclusion criteria were individuals at least 18 years old with normal or corrected-to-normal vision and hearing, and no history of brain injury or motor problems in the hands. Three participants out of the 51 participants were excluded due to fitting one of the exclusion criteria in their answers to the questionnaire. A further 3 participants were excluded during data processing due to technical errors. A formal diagnosis of ADHD was not a requirement for recruitment. Thus, the final sample included data from 45 individuals, with adequate power achieved. Twenty-eight of the participants were female and the average age was 26.57 years (SD = 8.69, range = 18–62). All of the participants reported normal or corrected-to-normal vision and hearing. None of the participants had colour-blindness or dyslexia. All participants spoke Swedish and/or English fluently. Two participants reported currently taking medication for ADHD. None of the included participants reported a neurological condition or brain injury. The data were collected between May and October 2022. This study followed the ethical standards of the Declaration of Helsinki and was approved by the Swedish Ethical Review Authority (DNS/registration number 2021-04623). Participants received a reward for participation in the form of a gift card of 99 Swedish Krona.
Stimuli and apparatus
The audio-visual oddball task was programmed and presented using PsychoPy v. 2020.2.10 (Peirce, Citation2007) on a computer running on Windows 10 Education. The visual stimuli were displayed using a computer with a 60 Hz refresh rate on a 17'' monitor, and the distance between the participant and the screen was approximately 50 cm. The visual stimuli were 72-point digits in Arial font on a grey background. The sounds used in the task were created in Audacity (Citation2022). Sound was presented simultaneously in both ears at an intensity of approximately 75 dB(A) using high-attenuated Peltor HTB79F headphones (SNR = 33 dB(A)), in order to block noise from the handles’ motors. The standard sound was a 200 ms sinewave tone (600 Hz) and the deviant sound was a 200 ms of white noise. Both sounds were normalised and edited to include 10 ms of rise and fall ramps. To supply vibrotactile stimuli a vibration device was used to supply vibrations to the hands by spinning an eccentric mass on a rotor. The motors were hosted within a pair of handles made from plastic tubes. The tubes were 30 mm in diameter and 136 mm long. A response button was located at the top of each of the handles. The motors and response buttons were connected via parallel port to the task computer which triggered the vibrations and recorded signals from the response buttons. A control box controlled the speed of the motors, in this case presenting vibrations simultaneously to both hands of 2.6 m/s2 amplitude and 33 Hz frequency and a deviant vibration of 61 m/s2 amplitude and 114 Hz frequency. The total duration of each vibration was 200 ms.
Procedure
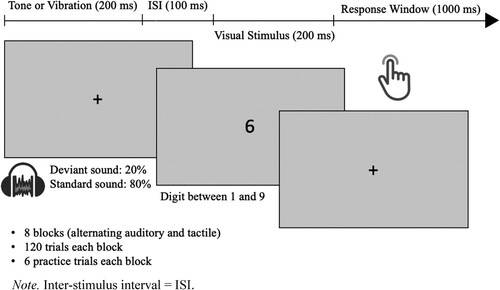
An experimenter was present in the same room at all times during the duration of the study. Participants filled in the demographic survey in a waiting area outside the lab either before or after completing the task. Verbal and on-screen instructions guided the participant before the start of the task. Participants were instructed to hold the vibrotactile handles in both hands throughout the task in a comfortable manner. The participants’ head position was not fixed, allowing for brief breaks of up to 3 min between each block. Likewise, the headphones were worn for the whole duration of the task, regardless of whether the block was auditory or tactile. The visual stimuli appeared in the centre of the screen following a fixation cross at each of the 960 trials across 8 blocks. There were 6 practice trials at the start of each of the 8 blocks. The visual stimuli were digits between 1 and 8 and appeared for 200 ms in the font Open Sans in the colour black on a grey background. Auditory and tactile distractors were present in intermittent blocks, always 300 ms before the appearance of the visual stimulus. Half of the participants began with the auditory block, while the other half were assigned a tactile block at the beginning. Reaction time and accuracy were measured using a button press on the vibrotactile device during a 1000 ms window. Participant responses were recorded 200 ms after the onset of the visual stimulus, in order to account for the amount of time needed for a visual stimulus to elicit a motor response (Ward et al., Citation1996). In 20 percent of the trials, a deviant distractor was presented in either the tactile or auditory modality, as opposed to the standard distractor presented in the remaining 80 percent of trials. The deviants were presented randomly within the standard trials, and no deviant was placed within two trials before or after another deviant. The participants were instructed to concentrate on the visual task only and avoid distraction from sounds or vibrations. The procedure of the oddball task is illustrated in .
Demographic data and information on ADHD symptoms were collected using paper questionnaires. Each participant was in the lab for approximately 1 h, including the time for completing the cognitive task and demographic survey.
ADHD symptoms
The Adult ADHD Self-report Scale (ASRS) was used to assess core ADHD symptoms within the last 6 months (Rodriguez et al., Citation2007). The ASRS shows high internal consistency (Cronbach’s alpha 0.88 for the patient-administered version and 0.89 for the rater-administered version; Adler et al., Citation2006). To categorise participants into low and high levels of ADHD symptoms groups, we used the 6-item screener of the ASRS scale, because it exhibits higher classification accuracy (97.9% compared to 96.2%) and sensitivity (68.7% compared to 56.3%) in contrast with the full 18-item version (Kessler et al., Citation2005). The official scoring guide for the 6-item screener was used to label participants as reporting high ADHD symptoms. For each of the 6 Likert-scale items on the screener, the official diagnostic symptom cut-offs for ADHD were used.
Analytic strategy
Data pre-processing occurred in the following steps: (1) removal of practice trials corresponding to the first 6 trials of each block, (2) removal of trials with reaction times outside of the 1000 ms response window, and (3) removal of trials with inaccurate responses.
A linear mixed model (LMM) analysis was used. We ran the analysis in R (version 4.1.2) using the lme4 package (Bates et al., Citation2015). We used Helmert-style contrasts, to incorporate categorical effects from factors with discrete levels into the LMMs. This coding scheme was applied to the categorical variables detailed later.
An LMM is a preferred method in this case, particularly because the data contains a nested or crossed random structure. An LMM accounts for the correlation within the nested structure by modelling the random effects and can better represent the data. LMM also allows for modelling of the effects of random predictors and independent residuals. For example, if you have multiple measurements within each subject, as is the case here, we have a hierarchical structure that benefits from the LMM analysis. In addition, it provides robust inferences in the presence of non-normality, unequal variances and outliers in the response variable (Schielzeth et al., Citation2020). LMMs are also robust when it comes to unbalanced designs and less sensitive to the presence of missing values (Carnero-Alcázar et al., Citation2022). A maximum random effects structure was used (Barr et al., Citation2013). The dependent variable was reaction time in milliseconds and fixed effects of sensory modality trial type and ADHD symptoms (ASRS) were added as fixed effects. Participant ID was included as a random effect. The model specification was logRT ∼ trialtype + modality + ADHD + trialtype*modality*ADHD (1|Participant). We followed Barr et al. (Citation2013) to find the best-fitting, most parsimonious model. Tukey HSD was used to correct for multiple comparisons. Estimation of degrees of freedom and statistical significance for the fixed effects were calculated using Satterthwaite’s approximations (Satterthwaite, Citation1941).
We performed two exploratory analyses: a sensitivity analysis of missing data and a Bayesian analysis for robustness of the null hypothesis. For missing data, in cases where a participant did not respond on a trial, and therefore did not provide an accurate response were excluded from the analysis, we performed a sensitivity analysis using the R package “nanair” to look for patterns in the missing trials. We used the “BayesFactor” package version 0.9.2 with the outcome variables of change scores of deviant minus non-post-deviant standard reaction times on accurate trials, for each modality separately.
Results
We measured the computerised visual task with the main outcome measure of reaction time in accurate trials according to the within-subject predictors: Modality (Auditory or Tactile), Trial type (Standard, Deviant, or Post-deviant), and ADHD symptoms (high or low symptom severity). shows mean task performance according to these predictors.
Figure 2. Reaction time for correct responses by sensory modality and trial type.
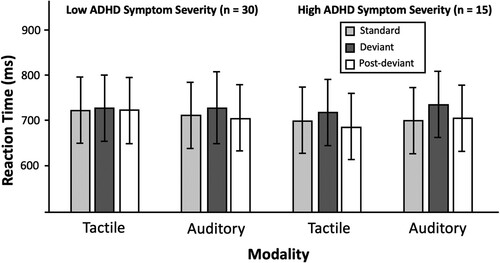
Fifteen participants met the cut-off for ADHD symptoms on the ASRS, and 30 participants scored within the non-ADHD symptom range. The two groups will be called low and high ADHD symptoms, and the term used to denote the difference between groups will be called symptom severity. The mean percent accuracy across participants was 92.30 and there were no between-groups differences in accuracy. A summary of descriptive values can be seen in .
Table 1. Oddball task reaction time performance of accurate responses.
Main analysis
For the LMM analysis, we added fixed effects of Trial type (Deviant vs. Post-deviant vs. Standard), Modality (Auditory vs. Tactile), and ADHD symptom group (Low vs. High), including interaction effects. The model formula for the full model is the following: RT ∼ 1 + Trialtype + Modality + ADHD + Trialtype:Modality + Trialtype:ADHD + Modality:ADHD + (1|subject).
A linear mixed effects model was fit using the restricted maximum likelihood (REML) method. The model's REML criterion at convergence was – 47125.44. The distribution of the residuals was approximately normal with a median of 0.016, and a standard deviation of 0.13.
The variance estimate of the random effect Participant was 0.01. The residual variance was estimated at 0.03. Fixed effects estimates are presented in . In this model, the effect of the deviant was significant (β = −0.008, SE = 0.001, t(576.74) = −7.76, p < 0.001), and the effect of the post-deviant was also significant (β = −0.005, SE = 0.002, t(50.18) = −2.67, p = 0.01), indicating that individuals, regardless of ADHD symptom group or sensory modality, took longer to respond on deviant and post-deviant trials compared to standard trials.
Table 2. Summary of fixed effects for the linear mixed effects model.
There was no significant effect of modality in this model, meaning that participants responded equally quickly with auditory and tactile stimuli. There were also no significant interaction effects in the model.
Exploratory analyses
Additional results that were not part of our main pre-registered analysis will now be presented. This includes (1) a Bayesian analysis and (2) a sensitivity analysis.
Bayes factor robustness checks for evidence in favour of the null hypothesis
Since the LMM did not provide evidence in favour of our alternative hypotheses regarding the influence of ADHD and sensory modality, we performed two independent samples Bayes factor (BF10) tests for difference scores for deviant vs. non-post-deviant standard trials with accurate responses for the low and high ADHD groups, for each sensory modality. This is an exploratory check that was not part of our pre-registered analysis plan. The purpose of this Bayesian analysis is to perform a Bayes factor robustness checks for evidence in favour of the null hypothesis. This test can be thought of as a similar design to an independent sample t-test using frequentist methods, but the benefit is that it can give us an idea of how sure we can be that the null hypothesis is true. The following details provide information that aids in the reproducibility of Bayesian modelling. Two MCMC chains with 500 burn-ins, which give the Markov chain time to reach its equilibrium distribution, and 10,000 iterations (steps in the chain) were used for a test in auditory and tactile modalities separately. There was moderate evidence for the null hypothesis that the ADHD symptom group was not associated with a deviant effect in the auditory modality (BF10 = 0.25, Cauchy prior width = 1.0, median = −0.099, error % = 0.017). The 95% credibility interval (CI) for the low ADHD group was 2–39 milliseconds in the auditory modality. The 95% CI for the high ADHD group was 10–41 milliseconds in the auditory modality. There was also moderate evidence in favour of the null hypothesis that ADHD symptom group was not associated with a deviant effect in tactile modality (BF10 = 0.23, Cauchy prior width = 1.0, median = −0.008, error % = 0.02). The 95% CI for the low ADHD group was 2–22 milliseconds in the tactile modality. The 95% CI for the high ADHD group was 9–38 milliseconds in the tactile modality. This result, combined with the result of the LMM, increases the confidence that there is no relationship between the deviant effect and ADHD symptom group in this sample, in neither the auditory nor tactile modality. That being said, the results of the sensitivity analysis for missingness indicate that these results should be interpreted with caution and future studies should clarify the findings.
Sensitivity analysis
Missing data is comprised of times when participants responded outside of the 1000 millisecond time window. We performed a sensitivity analysis to determine whether or not data was missing completely at random. The results of the missing data analysis showed a significant difference in missing trials between the high-symptom ADHD (1.7 percent missing) and low-symptom ADHD groups (1.2 percent missing; p < 0.001). This amounts to a difference of 326 trials between groups. However, this difference alone does not warrant including missingness as an explanatory variable in our main analysis, because of the difference in sample sizes between the ADHD and non-ADHD groups. We further examined missingness in relation to the other explanatory variables in our model. Missing data was significantly more likely to occur earlier within the 8 blocks (p < 0.001). Missing data was significantly more likely to occur in deviant compared (1.9 percent missing) to standard trials (1.5 percent missing; p = 0.004). This difference is expected due to the low rate of deviant trials (20% of all trials), but may also be explained by the impact of the unexpected sound or vibration. We therefore also tested for differences in missingness between sensory modalities. There was no significant difference between missingness in auditory and tactile modalities (1.5 percent missing in each; p = 0.986). Missing trials were removed from the main analysis. Further details about the sensitivity analysis can be found in the Supplementary Materials document.
Habituation to the deviant effect
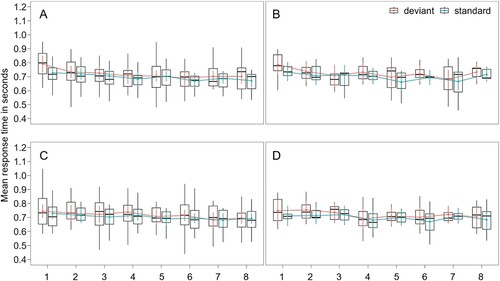
In our final exploratory analysis, we aimed to study habituation to the deviant effect across the entire task. Selective attention in the orienting response to to-be-ignored deviants in the visual oddball paradigm, including in cross-modal vibrotactile auditory oddball research, decreases in response to the repeated presentation over the course of a single session (Sörqvist et al., Citation2012). We calculated the habituation rate by taking the quotient of the magnitude of the deviation effect, represented by reaction time, and the number of trial, chronologically from start to finish throughout the task, across blocks. Regarding our sample as a whole, as expected, habituation in the magnitude of the deviant effect was present (a rate different from zero) across the experiment, t(4767) = −2.24, p < 0.05, with an effect size of – 0.06 Cohen’s d (SE = 0.03) in the direction of lower deviation effects later in the task, in both the auditory (M = 0.003, SD = 0.007) and tactile (M = 0.004, SD = 0.009) modalities. Habituation of the post-deviant effect was likewise present, t(6019) = −24.49, p < 0.001, with an effect size of – 0.32 Cohen’s d (SE = 0.01), indicating that habituation in this sample was present to a larger degree in post-deviant compared to deviant trials. We repeated a linear mixed effects model with the same specifications as our main analysis, but excluding trial type as a predictor and including habituation rate in the magnitude of the deviation effect as the dependent variable. This resulted in a model without any significant fixed effects and the ADHD symptom group (low or high) did not significantly predict the habituation rate (F(1449) = 0.19, p = 0.67). There is no significant interaction between group (high vs. low ADHD) and deviant modality. See for a comparison of the ADHD group and sensory modality reaction times across trial blocks. Testing for the impact of sensory modality, a significant difference was found in the latter 4 blocks of the task (F(2844) = 2.42, p = 0.01) but only in the low ADHD group and not in the high ADHD group.
Figure 3. Habituation across the task.
Discussion
The aim of the present study was to investigate a possible relationship between self-reported ADHD symptom severity and deviance distraction in a visual selective attention task in auditory and vibrotactile modalities. We hypothesised that having ADHD symptoms would be associated with sensory differences in both of these modalities in the form of differences in reaction time between deviant and non-deviant trials.
Our results showed that individuals from both the low and high ADHD symptom groups demonstrated a deviant effect, where the reaction time is on average slower in trials where a deviant sound or vibration is presented. Additionally, a post-deviant effect was found, meaning that participants were on average also slower in trials that immediately followed deviant trials compared to standard trials that were not immediately after deviant trials, irrespective of group symptom severity. The presence of both of these effects is in line with previous research in this same task paradigm, including the similarities in auditory and vibro-tactile conditions, in studies on adults with typical development (Ljungberg & Parmentier, Citation2012; Parmentier et al., Citation2011). Regarding our hypothesis that there will be a difference between low and high ADHD symptom groups, we found moderate evidence in favour of the null hypothesis that no such difference in deviant effects exists in this sample in either sensory modality.
While the field of vibrotactile distraction is narrow, research has generally shown similar higher cognitive processes to underpin perception in both auditory and tactile distraction. For example, researchers have argued that perceptual grouping of stimuli occurs similarly in both modalities (Gallace & Spence, Citation2011). A series of studies in a short-term memory paradigm including both auditory and tactile distraction concluded that the content of the distraction, not the modality of the distraction, governs the level of disruption from the task (Marsh et al., Citation2024). The pattern of both auditory and tactile distraction in individuals with low or high ADHD symptoms is similar, and we can be moderately certain that there are no differences depending on sensory modality. In the Bayes factor robustness check, the finding of moderate evidence in favour of the null hypothesis is an interpretation of a Bayes factor of 0.25, in other words indicating that the findings show four times more evidence for the null hypothesis compared to the alternative hypothesis.
As a result of our exploratory sensitivity analysis, we found differences between ADHD symptom groups and the amount of missing data (1.7% missing in the high symptom group and 1.2% missing in the low symptom group), a finding that could be explained in a number of different ways. First, this between-groups difference could be due to a higher incidence of mind wandering, or focus away from the task, in the group with high ADHD symptoms. This visual oddball task demands a high level of focus and sustained attention, so participants who are more inclined to mind wandering or daydreaming might skip trials due to these lapses in attention (Bozhilova et al., Citation2021). Mind wandering and sustained attention have been studied in individuals with ADHD (Lanier et al., Citation2021), so it is possible that this oddball paradigm might be used in future studies on these concepts. However, another explanation could be that participants with high ADHD symptoms were more likely to take more than the 1000 ms time window to respond to the trials. In order for this explanation to hold true, one should consider that higher overall reaction times in individuals with high symptom severity were not observed in this study, indicating that some other dysfunction besides slower cognitive speed is at play. The evidence to support this explanation is the significant small difference between missing trials in deviant vs. standard trials (1.5% missing standard trials compared to 1.9% missing deviant trials). But a simpler explanation might be the large difference between the amount of trials of each type. Another finding was a higher likelihood of missing trials earlier in the task compared to later in the task. This indicates that missing data was not due to fatigue effects, but rather that a likely explanation is that – despite the practice trials – it could take some time to get accustomed to the task, evidenced by a failure to respond. One limitation of this paradigm is that it cannot include a forced-choice response, so there can be missing data. In most populations, this is not a problem, because of the simplicity of the task. That being said, results from a sample of university students showed a distraction effect in the first block of a similar visual-auditory oddball paradigm (Ljungberg et al., Citation2014), perhaps pointing to the explanation that surprise leads to a delay in processing speed due to the novelty of the stimuli especially at the beginning of the task. Future studies could try to find a relationship between missed trials in this paradigm and other measures of mind wandering in ADHD populations.
As a result of our exploratory habituation analysis, we found that those with low levels of ADHD symptoms demonstrated the typical habituation to deviants in the different sensory modalities in the last half of the task, as expected according to prior findings (Sörqvist et al., Citation2012). However, the individuals in the high ADHD symptom group showed an equal amount of habituation between sensory modalities. We can only speculate on the reasons behind this novel finding. It seems in-line with research on individuals with ADHD having sensory difficulties across different modalities. However, since we did not find differences in habituation rates between those with high and low ADHD symptoms, further research should investigate habituation in a task with a longer duration or with a lower proportion of deviant stimuli. A task with a lower density of deviants could naturally produce larger effect sizes since a rarer deviant tends to be more distracting. A diagnosed clinical population with severe symptomatology might also shed more light on habituation or learning compared to the effect sizes present in this general population.
The findings of this study add to the theoretical debate on the aetiology of adult ADHD. One aspect of ADHD symptomatology is sensitivity to environmental stimuli (Panagiotidi et al., Citation2018). Specifically, the findings support the idea that distractibility is not related to sensory modality, in this case comparing tactile and auditory distraction. The implication is that managing inattention is oftentimes a multimodal process, such as using tactile methods like fidgeting to attempt to limit auditory distraction from the environment. A lack of between group differences in the sensory modality of deviance distraction supports the theory that the executive functions are a modal at higher levels of organisation regardless of high or low ADHD symptoms. One modality is not impacted by ADHD symptoms much more than the other, implying that the management of ADHD symptoms, including behavioural treatments of this disorder, could incorporate multiple modalities. In the present study, one unknown theoretical implication is if this lack of differences between modalities would be reflected in other cognitive tasks, such as those requiring voluntary control of attentional resources (see for example, Karch et al., Citation2010). The null findings regarding ADHD symptoms in this study should not be inflated to suggest that there might also be null findings in studies of selective attention, where individuals use decision-making rather than involuntary attention distraction by environmental deviants.
Statistical power for linear mixed-effects models is difficult to estimate. For this study, we performed a simulation power analysis on data previously collected with the same study paradigm. The simulation power analysis did not include an ADHD sample. One downside is that, since we didn’t have any expectations for effect size differences between the low and high ADHD groups, our findings in favour of the null hypothesis rely on the assumption that this study is correctly powered for spotting even small differences in effect size. It should be emphasised that there could be a very tiny between group difference that this study did not have the power to spot. But then we are left wondering if a difference of less than 10 or 20 milliseconds is practically important.
Although this study does not include a clinical sample, the ASRS screening tool has psychometric properties that allow it to be used for epidemiological purposes in the general population (Kessler et al., Citation2005). The use of the screening tool for separating participants into high- and low-symptom groups for the purpose of this study is therefore justified. However, one avenue for further research could be analysing impacts on detailed aspects of ADHD symptomatology using a clinical sample and more comprehensive questionnaires. The participants in the high-symptom group based on the ASRS screening tool did not all have a formal diagnosis of ADHD. Therefore, caution should be used when interpreting the generalizability of the findings to clinical settings.
One aspect that would be interesting to investigate further in a paradigm with increased difficulty is post-error slowing. Post-error slowing is implicated as part of a re-orienting response and a re-appraisal of future actions (Parmentier et al., Citation2019), two processes for which there is increasing evidence of differences for individuals who have ADHD compared to those who don’t (Gumenyuk et al., Citation2023). A recent study found that 9 individuals with ADHD were more prone to distraction from irrelevant auditory stimuli, specifically in re-orienting attention, compared to 9 control participants (Gumenyuk et al., Citation2023) and importantly that an ERP component was a marker of this deficit. In this visual task with auditory distractors, participants could press a left or right button to respond to stimuli on the screen, or they could leave no response, classified as a “miss”. Participants with ADHD made fewer correct responses and more missed responses compared to control participants. This is in-line with the findings of the present study that participants with high ADHD symptoms had more missing trials compared to the neuro-typical control group. Future studies should incorporate a more difficult higher cognitive functioning task that allows for more errors to occur.
In conclusion, the main finding of this study is that there is moderate evidence of no differences in deviance distraction comparing individuals with low or high ADHD symptoms in auditory or tactile modalities, combining evidence from a linear mixed effects model and a Bayes factor robustness check. Adults with differing levels of ADHD symptoms possibly don’t differ in the likelihood of getting attention diverted from a focal visual task, lending further support that ADHD impacts only higher levels of attentional processes, irrespective of sensory modality. Another finding of this study is differences between groups regarding the average number of missed responses, as evidenced by our exploratory analysis. Those with high ADHD symptoms were more likely to miss responding compared to those who scored low in ADHD symptoms. This difference should be explored more in future studies. A further observed difference is in habituation to the deviation effect. Our exploratory findings suggest that there may be a lack of differential habituation between sensory modalities in individuals with high ADHD symptoms. Overall, this study shows that there are behavioural differences between those with high and low ADHD symptoms, but these differences are not evident in lower-level distraction by deviants from a visual focal task in either tactile or auditory modality.
Supplemental Material
Download MS Word (696.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adler, L. A., Faraone, S. V., Spencer, T. J., Berglund, P., Alperin, S., & Kessler, R. C. (2017). The structure of adult ADHD. International Journal of Methods in Psychiatric Research, 26(1), e1555–e1555. https://doi.org/10.1002/mpr.1555
- Adler, L. A., Spencer, T., Faraone, S. V., Kessler, R. C., Howes, M. J., Biederman, J., & Secnik, K. (2006). Validity of pilot adult ADHD self-report scale (ASRS) to rate adult ADHD symptoms. Annals of Clinical Psychiatry, 18(3), 145–148. https://doi.org/10.1080/10401230600801077
- Alexander, D. M., Hermens, D. F., Keage, H. A. D., Clark, C. R., Williams, L. M., Kohn, M. R., Clarke, S. D., Lamb, C., & Gordon, E. (2008). Event-related wave activity in the EEG provides new marker of ADHD. Clinical Neurophysiology, 119(1), 163–179. https://doi.org/10.1016/j.clinph.2007.09.119
- Anbarasan, D., Kitchin, M., & Adler, L. A. (2020). Screening for Adult ADHD. Current Psychiatry Reports, 22(12), 1–15. https://doi.org/10.1007/s11920-020-01194-9
- Audacity. (2022). Audacity (3.2) [Computer software]. Audacity. https://www.audacityteam.org.
- Barr, D. J., Levy, R., Scheepers, C., & Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. https://doi.org/10.1016/j.jml.2012.11.001
- Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., Bolker, M. B. (2015). Package ‘lme4’. Convergence, 12(1), 2.
- Ben-Sasson, A., Soto, T. W., Heberle, A. E., Carter, A. S., & Briggs-Gowan, M. J. (2017). Early and concurrent features of ADHD and sensory over-responsivity symptom clusters. Journal of Attention Disorders, 21(10), 835–845. https://doi.org/10.1177/1087054714543495
- Bozhilova, N., Michelini, G., Jones, C., Kuntsi, J., Rubia, K., & Asherson, P. (2021). Context regulation of mind wandering in ADHD. Journal of Attention Disorders, 25(14), 2014–2027. https://doi.org/10.1177/1087054720956714
- Brevik, E. J., Lundervold, A. J., Haavik, J., & Posserud, M.-B. (2020). Validity and accuracy of the Adult Attention-Deficit/Hyperactivity Disorder (ADHD) Self-Report Scale (ASRS) and the Wender Utah Rating Scale (WURS) symptom checklists in discriminating between adults with and without ADHD. Brain and Behavior, 10(6), e01605–e01605. https://doi.org/10.1002/brb3.1605
- Carnero-Alcázar, M., Montero-Cruces, L., & Maroto-Castellanos, L. (2022). Mixed models: An essential tool for non-independent data analysis. European Journal of Cardio-Thoracic Surgery, 62(4), ezac462–ezac462. https://doi.org/10.1093/ejcts/ezac462
- Corbetta, M., Patel, G., & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. https://doi.org/10.1016/j.neuron.2008.04.017
- Dellapiazza, F., Michelon, C., Vernhet, C., Muratori, F., Blanc, N., Picot, M.-C., & Baghdadli, A. (2021). Sensory processing related to attention in children with ASD, ADHD, or typical development: Results from the ELENA cohort. European Child & Adolescent Psychiatry, 30(2), 283–291. https://doi.org/10.1007/s00787-020-01516-5
- Escera, C., Alho, K., Winkler, I., & Näätänen, R. (1998). Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience, 10(5), 590–604. https://doi.org/10.1162/089892998562997
- Faraone, S. V., Banaschewski, T., Coghill, D., Zheng, Y., Biederman, J., Bellgrove, M. A., Newcorn, J. H., Gignac, M., Al Saud, N. M., Manor, I., Rohde, L. A., Yang, L., Cortese, S., Almagor, D., Stein, M. A., Albatti, T. H., Aljoudi, H. F., Alqahtani, M. M. J., Asherson, P., … Wang, Y. (2021). The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neuroscience & Biobehavioral Reviews, 128, 789–818. https://doi.org/10.1016/j.neubiorev.2021.01.022
- Gallace, A., & Spence, C. (2011). To what extent do Gestalt grouping principles influence tactile perception? Psychological Bulletin, 137(4), 538–561. https://doi.org/10.1037/a0022335
- Green, P., & MacLeod, C. J. (2016). SIMR: an R package for power analysis of generalized linear mixed models by simulation. Methods in Ecology and Evolution, 7(4), 493–498. https://doi.org/10.1111/2041-210X.12504
- Gumenyuk, V., Korzyukov, O., Tapaskar, N., Wagner, M., Larson, C. R., & Hammer, M. J. (2023). Deficiency in Re-orienting of attention in adults with attention-deficit hyperactivity disorder. Clinical EEG and Neuroscience, 54(2), 141–150. https://doi.org/10.1177/15500594221115737
- Janssen, T. W. P., Geladé, K., van Mourik, R., Maras, A., & Oosterlaan, J. (2016). An ERP source imaging study of the oddball task in children with Attention Deficit/Hyperactivity Disorder. Clinical Neurophysiology, 127(2), 1351–1357. https://doi.org/10.1016/j.clinph.2015.10.051
- Karch, S., Thalmeier, T., Lutz, J., Cerovecki, A., Opgen-Rhein, M., Hock, B., Leicht, G., Hennig-Fast, K., Meindl, T., Riedel, M., Mulert, C., & Pogarell, O. (2010). Neural correlates (ERP/fMRI) of voluntary selection in adult ADHD patients. European Archives of Psychiatry and Clinical Neuroscience, 260(5), 427–440. https://doi.org/10.1007/s00406-009-0089-y
- Keating, J., Gaffney, R., Bramham, J., & Downes, M. (2022). Sensory modulation difficulties and assessment in children with attention deficit hyperactivity disorder: A systematic review. European Journal of Developmental Psychology, 19(1), 110–144. https://doi.org/10.1080/17405629.2021.1889502
- Kessler, R. C., Adler, L., Ames, M., Demler, O., Faraone, S., Hiripi, E., Howes, M. J., Jin, R., Secnik, K., Spencer, T., Ustun, T. B., & Walters, E. E. (2005). The World Health Organization adult ADHD self-report scale (ASRS): A short screening scale for use in the general population. Psychological Medicine, 35(2), 245–256. https://doi.org/10.1017/S0033291704002892
- Kumle, Leah, Võ, Melissa L.-H, & Draschkow, Dejan. (2020). Simulation-based solutions for power analyses for mixed models considering by-subject and by-item variability. Journal of Vision, 20(11), 696. https://doi.org/10.1167/jov.20.11.696
- Lanier, J., Noyes, E., & Biederman, J. (2021). Mind wandering (internal distractibility) in ADHD: A literature review. Journal of Attention Disorders, 25(6), 885–890. https://doi.org/10.1177/1087054719865781
- Lin, H.-Y., Hsieh, H.-C., Lee, P., Hong, F.-Y., Chang, W.-D., & Liu, K.-C. (2017). Auditory and visual attention performance in children With ADHD: The attentional deficiency of ADHD Is modality specific. Journal of Attention Disorders, 21(10), 856–864. https://doi.org/10.1177/1087054714542004
- Ljungberg, J. K., & Parmentier, F. B. R. (2012). Cross-Modal distraction by deviance. Experimental Psychology, 59(6), 355–363. https://doi.org/10.1027/1618-3169/a000164
- Ljungberg, J. K., Parmentier, F. B. R., Jones, D. M., Marsja, E., & Neely, G. (2014). ‘What’s in a name?’ ‘No more than when it's mine own’. Evidence from auditory oddball distraction. Acta Psychologica, 150, 161–166. https://doi.org/10.1016/j.actpsy.2014.05.009
- Ljungberg, J. K., Parmentier, F. B. R., Leiva, A., & Vega, N. (2012). The informational constraints of behavioral distraction by unexpected sounds: The role of event information. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38(5), 1461–1468. https://doi.org/10.1037/a0028149
- Marsh, J. E., Vachon, F., Sörqvist, P., Marsja, E., Röer, J. P., Richardson, B. H., & Ljungberg, J. K. (2024). Irrelevant changing-state vibrotactile stimuli disrupt verbal serial recall: Implications for theories of interference in short-term memory. Journal of Cognitive Psychology, 36(0), 78–100. https://doi.org/10.1080/20445911.2023.2198065
- McLoughlin, G., Asherson, P., Albrecht, B., Banaschewski, T., Rothenberger, A., Brandeis, D., & Kuntsi, J. (2011). Cognitive-electrophysiological indices of attentional and inhibitory processing in adults with ADHD: Familial effects. Behavioral and Brain Functions, 7(1), 1–9. https://doi.org/10.1186/1744-9081-7-26
- Mueller, A., Hong, D. S., Shepard, S., & Moore, T. (2017). Linking ADHD to the neural circuitry of attention. Trends in Cognitive Sciences, 21(6), 474–488. https://doi.org/10.1016/j.tics.2017.03.009
- Orinstein, A. J., & Stevens, M. C. (2014). Brain activity in predominantly-inattentive subtype attention-deficit/hyperactivity disorder during an auditory oddball attention task. Psychiatry Research: Neuroimaging, 223(2), 121–128. https://doi.org/10.1016/j.pscychresns.2014.05.012
- Panagiotidi, M., Overton, P. G., & Stafford, T. (2018). The relationship between ADHD traits and sensory sensitivity in the general population. Comprehensive Psychiatry, 80, 179–185. https://doi.org/10.1016/j.comppsych.2017.10.008
- Parmentier, F. B. R. (2014). The cognitive determinants of behavioral distraction by deviant auditory stimuli: A review. Psychological Research, 78(3), 321–338. https://doi.org/10.1007/s00426-013-0534-4
- Parmentier, F. B. R., Ljungberg, J. K., Elsley, J. V., & Lindkvist, M. (2011). A behavioral study of distraction by vibrotactile novelty. Journal of Experimental Psychology: Human Perception and Performance, 37(4), 1134–1139. https://doi.org/10.1037/a0021931
- Parmentier, F. B. R., Vasilev, M. R., & Andrés, P. (2019). Surprise as an explanation to auditory novelty distraction and post-error slowing. Journal of Experimental Psychology: General, 148(1), 192–200. https://doi.org/10.1037/xge0000497
- Pedersen, A., & Ohrmann, P. (2018). Impaired behavioral inhibition in implicit sequence learning in adult ADHD. Journal of Attention Disorders, 22(3), 250–260. https://doi.org/10.1177/1087054712464392
- Peirce, J. W. (2007). PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods, 162(1), 8–13. https://doi.org/10.1016/j.jneumeth.2006.11.017
- Puts, N. A. J., Harris, A. D., Mikkelsen, M., Tommerdahl, M., Edden, R. A. E., & Mostofsky, S. H. (2017). Altered tactile sensitivity in children with attention-deficit hyperactivity disorder. APSselect, 4(10), 2568–2578. https://doi.org/10.1152/[email protected]
- Rienäcker, F., Jacobs, H. I. L., Van Heugten, C. M., & Van Gerven, P. W. M. (2018). Practice makes perfect: High performance gains in older adults engaged in selective attention within and across sensory modalities. Acta Psychologica, 191, 101–111. https://doi.org/10.1016/j.actpsy.2018.09.005
- Roberts, M., Ashinoff, B. K., Castellanos, F. X., & Carrasco, M. (2018). When attention is intact in adults with ADHD. Psychonomic Bulletin & Review, 25(4), 1423–1434. https://doi.org/10.3758/s13423-017-1407-4
- Rodriguez, A., Ginsberg, Y., Nyberg, L., & Fernholm, A. (2007, May 2). ADHD svårt att diagnostisera hos vuxna. Läkartidningen. https://lakartidningen.se/klinik-och-vetenskap-1/2007/05/adhd-svart-att-diagnostisera-hos-vuxna/.
- Santangelo, V., Fagioli, S., & Macaluso, E. (2010). The costs of monitoring simultaneously two sensory modalities decrease when dividing attention in space. NeuroImage, 49(3), 2717–2727. https://doi.org/10.1016/j.neuroimage.2009.10.061
- Satterthwaite, F. E. (1941). Synthesis of variance. Psychometrika, 6(5), 309–316. https://doi.org/10.1007/BF02288586
- Schielzeth, H., Dingemanse, N. J., Nakagawa, S., Westneat, D. F., Allegue, H., Teplitsky, C., Réale, D., Dochtermann, N. A., Garamszegi, L. Z., & Araya-Ajoy, Y. G. (2020). Robustness of linear mixed-effects models to violations of distributional assumptions. Methods in Ecology and Evolution, 11(9), 1141–1152. https://doi.org/10.1111/2041-210X.13434
- Schmidt, S. L., Simões, E. N., & Novais Carvalho, A. L. (2019). Association between auditory and visual continuous performance tests in students With ADHD. Journal of Attention Disorders, 23(6), 635–640. https://doi.org/10.1177/1087054716679263
- Schulz, S. E., Luszawski, M., Hannah, K. E., & Stevenson, R. A. (2023). Sensory gating in neurodevelopmental disorders: A scoping review. Research on Child and Adolescent Psychopathology, 51(7), 1005–1019. https://doi.org/10.1007/s10802-023-01058-9
- Senderecka, M., Grabowska, A., Gerc, K., Szewczyk, J., & Chmylak, R. (2012). Event-related potentials in children with attention deficit hyperactivity disorder: An investigation using an auditory oddball task. International Journal of Psychophysiology, 85(1), 106–115. https://doi.org/10.1016/j.ijpsycho.2011.05.006
- Sokhadze, E. M., Lamina, E. V., Casanova, E. L., Kelly, D. P., Opris, I., Tasman, A., & Casanova, M. F. (2018). Exploratory study of rTMS neuromodulation effects on electrocortical functional measures of performance in an oddball test and behavioral symptoms in autism. Frontiers in Systems Neuroscience, 12, 20–25. https://doi.org/10.3389/fnsys.2018.00020
- Sörqvist, P., Nöstl, A., & Halin, N. (2012). Working memory capacity modulates habituation rate: Evidence from a cross-modal auditory distraction paradigm. Psychonomic Bulletin & Review, 19(2), 245–250. https://doi.org/10.3758/s13423-011-0203-9
- Stevens, M. C., Pearlson, G. D., & Kiehl, K. A. (2007). An fMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. American Journal of Psychiatry, 164(11), 1737–1749. https://doi.org/10.1176/appi.ajp.2007.06050876
- Tegelbeckers, J., Brechmann, A., Breitling-Ziegler, C., Bonath, B., Flechtner, H.-H., & Krauel, K. (2022). Neural mechanisms underlying the effects of novel sounds on task performance in children with and without ADHD. Frontiers in Human Neuroscience, 16, 878994–878994. https://doi.org/10.3389/fnhum.2022.878994
- Ward, R., Duncan, J., & Shapiro, K. (1996). The slow time-course of visual attention. Cognitive Psychology, 30(1), 79–109. https://doi.org/10.1006/cogp.1996.0003
- Wetzel, N. (2015). Effects of the short-term learned significance of task-irrelevant sounds on involuntary attention in children and adults. International Journal of Psychophysiology, 98(1), 17–26. https://doi.org/10.1016/j.ijpsycho.2015.06.003
- Wetzel, N., & Schröger, E. (2014). On the development of auditory distraction: A review. PsyCh Journal, 3(1), 72–91. https://doi.org/10.1002/pchj.49