Abstract
Background: Melanoma, the deadliest skin cancer, presents significant challenges globally. This study examines survival factors among patients treated at a high-complexity oncology center in Colombia's coffee-growing region. Methods: Records from 2010 to 2021 were analyzed, capturing socio-demographics, clinical variables and survival outcomes via Kaplan-Meier and Cox regression. Results: Among 766 patients, factors influencing survival included sex, TNM stage, diagnostic stage, ulceration, metastasis, Breslow thickness ≥1 mm and positive nodes. Age, ulceration, distant stage at diagnosis and Breslow thickness ≥1 mm were associated with mortality. Conclusion: Colombian melanoma patients exhibit lower survival rates compared with global trends. Key survival determinants align with international literature. Enhanced photoprotection and early detection initiatives are imperative.
Introduction
Global significance
Melanoma is the deadliest form of skin cancer, accounting for approximately 75% of skin cancer-related deaths.
Rising incidence
The incidence of melanoma has been steadily increasing, partly due to the depletion of the ozone layer and increased exposure to UV radiation.
Regional data
Limited information is available on melanoma behavior in different regions of Colombia, specifically in Risaralda, where no epidemiological studies existed prior to this research.
Materials & methods
Study design
This survival analysis study included patients diagnosed with melanoma between 2010 and 2021 at a high-complexity oncology institution in Colombia.
Data collection
Medical records from 766 patients were analyzed, capturing socio-demographic variables, clinical variables and survival outcomes.
Analysis techniques
Kaplan-Meier survival analysis and Cox proportional hazards regression were used to identify factors influencing survival and mortality.
Results
Patient demographics
Among 766 patients, factors influencing survival included sex, TNM stage, diagnostic stage, ulceration, metastasis, Breslow thickness ≥1 mm and positive nodes.
Survival rates
The overall patient survival rate was 67.1%, with a median survival of 2963 days.
Mortality predictors
Variables associated with increased mortality risk included age (HR:1.025), presence of ulceration (HR: 1.821), distant stage at diagnosis (HR: 4.543) and tumors with Breslow thickness ≥1 mm (HR: 4.385).
Discussion
Comparative survival rates
The five-year survival rate for cutaneous melanoma in this Colombian cohort was lower than reported in high-income countries but comparable to other South American countries.
Sex & stage impact
Women exhibited higher five-year survival rates compared with men. Higher TNM stages (III and IV) at diagnosis were associated with lower survival rates.
Ulceration & thickness
The presence of ulceration and Breslow thickness ≥1 mm were significant predictors of lower survival.
Anatomical location
The lower limbs were the most common anatomical location for melanoma in this cohort, consistent with other Latin American studies.
Conclusion
Key determinants
Age, ulceration, distant stage at diagnosis and Breslow thickness >1 mm were significant predictors of mortality in melanoma patients.
Lower survival rates
The study highlighted lower survival rates in Colombian patients compared with global trends.
Importance of early detection
Enhanced photoprotection and early detection initiatives are imperative to improve melanoma outcomes.
Need for further research
Additional multi-institutional studies are needed to better understand melanoma survival and guide interventions in low- and middle-income settings.
Keywords: :
1. Background
Skin cancer, comprising melanoma and non-melanoma skin cancer, is the most prevalent form of cancer in humans. Its incidence has been steadily rising in recent years, partly due to the depletion of the ozone layer and increased exposure to UV radiation [Citation1–4]. Among the various histological types, basal cell carcinoma is the most common (52.7%), followed by squamous cell carcinoma (22.6%) and melanoma (16.1%). However, despite having a lower incidence, melanoma exhibits the highest mortality rate among skin cancer subtypes, accounting for approximately 75% of skin cancer-related deaths [Citation5]. About 3% of melanomas are characterized by the absence of a detectable primary site, referred to as melanoma of unknown primary (MUP). This atypical subtype of melanoma lacks a clear biological definition compared with the conventional melanoma of known primary (MKP). A recent publication suggests that patients with MUP exhibit more favorable outcomes than those with MKP of similar stages, likely attributed to heightened immunogenicity leading to regression at the primary site via immunological mechanisms [Citation6].
The Surveillance, Epidemiology and End Results (SEER) database in the United States provides relative 5-year survival rates for melanoma skin cancer, categorized into localized (confined to the skin of origin), regional (spread to nearby structures or lymph nodes) and distant (metastasized to distant body parts) stages, rather than utilizing the American Joint Committee on Cancer (AJCC) TNM staging system [Citation2]. The 5-year survival rates, according to SEER, are localized at 98%, regional at 64% and distant at 23%. However, there is limited information available on the behavior of melanoma across different regions in Colombia, specifically in Risaralda, where no epidemiological studies exist.
Globally, the standardized incidence rates of melanoma in men and women have increased from 2.3 and 2.2 per 100,000 individuals in 1990 to 3.1 and 2.8 per 100,000 individuals in 2008, respectively. In 2015, there were an estimated 351,880 melanoma cases worldwide, with an age-standardized incidence rate of 5 per 100,000 individuals per year [Citation3]. Melanoma predominantly affects individuals over 50 and is more prevalent in highly developed countries [Citation7]. Incidence and mortality rates are increasing globally, with predictive models suggesting a 50% rise in melanoma incidence, reaching approximately 510,000 new cases and a 68% increase in mortality, resulting in an estimated 96,000 deaths by 2040 [Citation2,Citation7]. A study conducted at the National Cancer Institute of Colombia revealed that the adjusted incidence of melanoma per 100,000 individuals varied between 0.4 and 9.3 for women and 0.1 and 6.9 for men across different regions [Citation5].
Factors associated with melanoma development include UV radiation exposure, low socioeconomic status, the presence of cutaneous nevi (typical, atypical, or congenital), nevus number and size, immunosuppression, alcohol consumption and genetic polymorphisms such as the presence of the MC1R gene, as well as comorbidity with other malignancies like prostate cancer [Citation8–14]. Some researchers suggest that the increased incidence of melanoma may be attributed to an upsurge in skin biopsies and variations in the histological interpretation of early evolving lesions, leading to increased melanoma screening and early detection of incipient lesions. However, this does not fully explain the escalating mortality rates, particularly among older men [Citation3].
Given the unique characteristics and global significance of melanoma in terms of mortality, numerous studies have established optimal treatment strategies based on the disease stage at diagnosis. Treatment options for advanced melanoma, including metastatic cases, encompass surgical metastasectomy, immunotherapy and radiotherapy for symptomatic metastatic sites. Moreover, with the deepening of the research on the PI3K/AKT/NF-κB pathway, its function in melanoma has gradually attracted wide attention. AKT family member mutations are often dysregulated in melanoma and have been identified in up to 43–60% of melanoma cases. PTEN, which classily dampens the PI3K/AKT/mTOR growth-promoting signaling cascade, is noted in 38% of patients with primary melanoma and 58% with metastatic disease. Changes in PTEN and BRAF pathways often co-exist, theoretically allowing dysregulation of both the MAPK and PI3K pathways simultaneously. Hence, it might be possible that PI3K inhibitors may afford some benefit to patients with PTEN and AKT-mutant melanomas [Citation15]. Primary melanoma management relies on accurate diagnosis through pathological analysis of biopsy specimens with 0–3 mm margins. Surgical interventions are the treatment of choice for most patients, except for those with advanced stages who may be offered chemotherapy or radiotherapy as adjuvant treatments. Immunotherapy with monoclonal antibodies, such as anti-PD1 alone or in combination with anti-CTLA4, has emerged as a promising alternative, improving progression-free survival in various studies. Additionally, combined treatment approaches have demonstrated superior efficacy to individual treatments [Citation16,Citation17]. In addition, myeloid-derived suppressor cells, T regulatory (Treg) cells and tumor-associated macrophages constitute immunosuppressive cells present within the tumor microenvironment, which release reactive oxygen species (ROS) among other factors, effectively inhibiting NK (natural killer) cell response. Higher levels of fibroblasts secrete more metalloproteinases, resulting in further shedding of ligands that could link to NK cells. Fibroblasts even have a more direct impact on NK cells by preventing cytokine-induced activating receptor upregulation. The presence of melanoma-derived exosomes also impairs NK cell function [Citation18]. Consequently, some of the characteristics that have been described that may serve as protection against melanoma include melanin pigmentation, which protects against melanoma development. However, it affects the outcome of developed diseases and therapy, as discussed in recent studies [Citation19].
Several studies have aimed to identify variables present at the time of melanoma diagnosis that could assist in establishing a prognosis and selecting therapeutic interventions that offer maximum benefit to patients regarding melanoma's prognostic and predictive factors. The use of LDH and S100 in prognostication and disease monitoring is well established. DNA markers, such as BRAF and NRAS, provide well-established associations with patient selection and predict response to target therapy. ctDNA and miRNAs or lncRNAs give a practical insight into tumors' genetics and help with understanding the pathophysiology of the disease and they hold the great advantage of allowing serial, non-invasive sampling for disease monitoring. Therapeutic targeting of miRNAs can impact the natural history of melanoma by enhancing sensitivity to standard therapies and immune checkpoint inhibitors. In particular, elevated levels of miRNA-221 have been identified in early melanomas compared with healthy individuals. Increased levels were also linked to the increased stage of the disease. Moreover, circulating miRNA-615–3p levels are consistently efficient in detecting melanoma patients who developed progressive disease while treated with immune checkpoint inhibitors [Citation20].
The literature highlights certain variables associated with mortality and survival, including age (worse outcomes in older individuals), male sex, melanoma located at the cephalic pole, histological subtypes like nodular or acral lentiginous melanoma, tumor thickness measured by the Breslow scale (≥1 mm), advanced invasion defined by the Clark scale, mitotic rate (>1 mitosis/field) and advanced diagnostic stages (regional and distant) [Citation21–24].
Early detection, timely diagnosis and access to early treatment modify the prognosis of the disease [Citation25]. Identifying individual and environmental factors associated with survival directly related to the stage, metastasis to other organs and type of treatment received is of utmost importance. This study identified factors associated with melanoma survival and mortality in a high-complexity oncology institution in Colombia, according to histological classification, status, socio-demographic characteristics and type of treatment between the years 2010–2021.
2. Materials & methods
2.1. Ethics approval
The bioethics committee of the Universidad Tecnológica de Pereira and Oncólogos de Occidente, Pereira, Colombia, approved this study. It was classified as no risk since the medical records database would be used.
The study corresponds to a survival analysis, a special analytical observational cohort study type. The time elapsed between the date of initiation of care and the date of death was considered a failure. Records that exceeded the study cut-off date (12/31/2021), deaths due to causes other than melanoma and patients who did not complete the follow-up time were censored and the time to the last consultation was calculated. The information on the records was obtained from the databases of clinical histories offered by the same institution; all the records of patients (766) with an anatomic-pathologic diagnosis of melanoma attended an institution of a high level of complexity specialized in oncologic pathology between the years 2010 to 2021 were included. Cutaneous melanoma was included in the analysis, but other types were excluded.
This institution treats patients from cities of origin (Armenia, Manizales and Pereira) in the coffee-growing region of Colombia. No sample was calculated. Socio-demographic variables (sex, age), clinical variables (diagnostic stage, anatomical location, pathology studies (histological subtype, presence of ulceration, Breslow, mitosis) and the type of treatment received were considered. The primary outcome was death and patient survival time. Death was determined by the information in the clinical history and by cross-checking the death databases of people in the region.
Univariate analysis: frequency distribution tables, percentages, rates and ratios were used for qualitative variables. Measures of central tendency (mean, median, mode) and dispersion (standard deviation, interquartile range, minimum and maximum values) were used for quantitative variables. Bivariate analysis: the variables of interest (alive-dead state, tumor stage, histologic type) and the socio-demographic and clinical variables were crossed to look for statistically significant associations. For nominal variables, Pearson's chi-square or Fisher's exact test was used when frequencies were low (less than 5); for quantitative variables that met the assumptions of normality, parametric statistics such as Student's t-test or ANOVA were used; for those that did not meet the assumptions of normality, non-parametric tests such as Mann Whitney U test were used.
Survival analysis was performed from the time elapsed between time t0 to time tk, where survival is the difference of tk –t0. The variable of interest or outcome is death attributable to cancer. Hazard or h(t) was calculated as the instantaneous potential for a specific time to occur, calculated through a log-rank test. Multivariate analysis was performed through Cox proportional hazards regression analysis and confounder adjustment was performed. All tests were run at a 95% confidence level and p-value < 0.05.
3. Results
The medical records of 766 patients diagnosed with melanoma were analyzed from 2010 to 2021. summarizes the distribution of the patients' socio-demographic and clinical variables.
Table 1. Socio-demographic and clinical variables.
The overall mortality of patients during the observation period was 19.4% (). The variables that were statistically significantly associated with mortality were sex, primary melanoma and diagnostic stage ().
Table 2. Variables associated with mortality.
The patient's overall survival (A) was 67.1%, with a median survival of 2963 days. The variables statistically associated with survival were sex (B), SEER stage (C), TNM stage at diagnosis (A), Breslow thickness (B), presence of ulceration (C) and presence of positive nodes. All variables data are shown in .
Figure 1. Kaplan Meier curves by overall survival, sex and SEER stage.
(A) Overall survival expressed in days.
(B) Survival by sex expressed in days.
(C) Survival by SEER stage expressed in days.
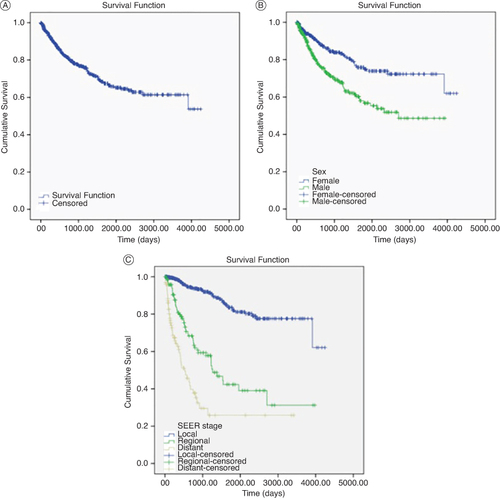
Figure 2. Kaplan Meier curves by TNM stage, Breslow thickness and Ulceration.
(A) Survival by TNM stage at diagnosis expressed in days.
(B) Survival by Breslow thickness expressed in days.
(C) Survival by Ulceration presence expressed in days.
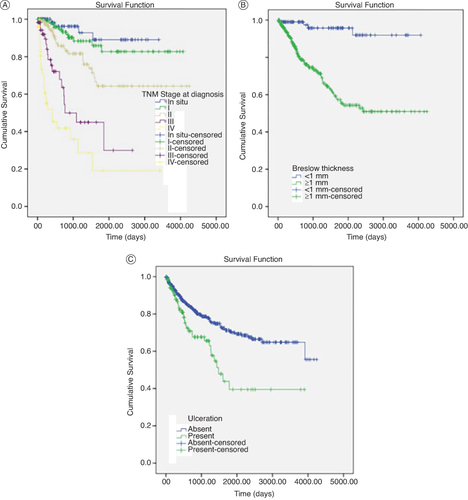
Table 3. Variables associated with survival.
In Cox regression analysis, variables associated with mortality were age (HR:1.025, CI: 1.008–1.042, p: 0.003), presence of ulceration reported in pathology studies (HR: 1. 821, CI: 1.142–2.902, p: 0.012), distant stage at diagnosis (HR: 4.543, CI: 2.607–7.916, p: <0.001) and tumors ≥1 mm according to the Breslow classification (HR: 4.385, CI: 1.562–12.311, p: 0.005) .
Table 4. Cox regression analysis.
Although melanoma is known for its metastatic potential and severity, advances in early diagnosis and treatment options have significantly improved survival rates in recent years. However, long-term survival can vary considerably depending on factors such as stage at diagnosis, tumor location and thickness and response to treatment. Patients diagnosed at earlier stages have a better chance of survival, while those with advanced disease face significant challenges in terms of treatment and prognosis. Continuous monitoring, access to innovative therapies and comprehensive care are critical to improving melanoma patients' survival and quality of life. Five-year survival of patients with melanoma is a crucial aspect in the evaluation of prognosis and treatment efficacy; in our study, as can be seen in the graphs, those patients who made it to year 5 were less likely to die from melanoma, data that are congruent with those reported in the international literature. Analyzing by subgroup in our study, similar to that described in the international literature, we found that men and people with distant disease at diagnosis had a worse prognosis and shorter survival than those who did not. —. A higher TNM stage (III, IV) at diagnosis was associated with a lower probability of survival. In addition, a Breslow>1 at diagnosis was associated with lower survival, perhaps because this is where a higher probability of distant seeding has been described compared with a lower Breslow. Finally, histologic ulceration at diagnosis was associated with higher mortality.
The present study investigated the survival rates and associated factors in patients diagnosed with melanoma and the variables related to mortality. The findings revealed that the distribution of the clinical variables of interest in the studied population exhibited a pattern similar to what has been reported in the existing literature for most of these variables. However, regarding the anatomical location of the melanoma, our results differed from the descriptive studies conducted with the SEER database, as reported by Yuan et al. Alternatively, studies by Chevalier et al. and Wee et al. focusing on European and Asian populations found that the trunk was the primary anatomical location for melanoma, with other locations showing significant heterogeneity in order of importance [Citation26–28].
In this study, the location was distributed as follows: lower limbs (33.7%), cephalic pole (26.9%) and trunk (20.5%). These findings are consistent with several descriptive studies conducted in the Latin American population, where the lower limbs were found to be a more common location for melanoma, as reported by Iribarren et al. and Pérez et al. in Chile and Uribe et al. in Colombia [Citation29–31].
Although superficial extensive melanoma remained the predominant histological subtype in our study (39.5%), its prevalence was not as high as reported in the literature, where it can reach up to 70% [Citation19,Citation32]. Conversely, subtypes typically considered uncommon, such as lentigo maligna (14.2%) and acral lentiginous (14.1%), exhibited a higher prevalence in our study.
Surgical treatment remained the primary management approach, utilized in 90.5% of cases. However, a noteworthy proportion of patients (8.1%) received immunotherapy treatments, which have emerged in recent years as an effective treatment option for improving patient survival, as reported in previous studies [Citation17,Citation21,Citation32–36].
4. Discussion
Variables associated with mortality were age (HR:1.025, CI: 1.008–1.042, p: 0.003), presence of ulceration reported in pathology studies (HR: 1.821, CI: 1.142–2.902, p: 0. 012), metastatic status at diagnosis (HR: 4.543, CI: 2.607–7.916, p: <0.001). Additionally, thick tumors defined by Breslow ≥1 mm were linked to a higher mortality risk, with an HR of 4.385 (CI: 1.562–12.311, p = 0.005). These findings align with other studies investigating mortality in melanoma.
However, several variables did not show statistically significant differences in the present study. These variables include male sex, site of melanoma localization, histological subtype and the number of mitoses reported in pathology. Although these factors have been previously associated with mortality in the literature [Citation7,Citation21,Citation37,Citation38], they did not exhibit significant correlations in this study.
In this study, the overall five-year survival rate for cutaneous melanoma was 67.1%. This value differs significantly from the reported 97.3% in the United States and studies conducted in Europe [Citation39]. Conversely, similar survival outcomes have been documented in Colombia and other South American countries, with an estimated 5-year survival of 68.7% and 67.6%, respectively [Citation40,Citation41].
When analyzing survival by sex, women exhibited a higher five-year survival rate of 76.5% compared with men, who had a survival rate of approximately 56.8% at the same cut-off point. This disparity is consistent with findings in the United States, where women had a calculated survival rate of 95.4% compared with 92.4% for men. A similar study in Colombia also reported higher 5-year survival rates for women (74%) than men (53.5%) [Citation42]. Additionally, an Australian population-based study with a 20-year follow-up demonstrated higher overall survival rates in women [Citation22]. The histologic characteristics of the patients were not discussed since, having a wide range of analyses in terms of dates, the data are heterogeneous and incomplete. For this reason and because the data did not exist in clinical reports, melanoma was not classified according to cumulative solar damage (CSD).
Regarding diagnostic stage, survival outcomes from those reported in Europe, where stage I patients exhibited higher survival rates [Citation39]. Similarly, the results differed from studies conducted in Colombia and Brazil [Citation40,Citation43] and contrasted with the findings in Mexico, where early-stage patients showed lower survival rates, 85.5% for stage I and 65% for stage II, compared with 68.9% and 54.3% in Mexico, respectively [Citation43]. Concerning the localized, regional and distant classification, there is evidence of lower survival in each category compared with North America.
Significantly, variables such as ulceration and Breslow thickness demonstrated substantial differences in survival rates. Ulceration was associated with lower survival rates, consistent with previous reports in Colombia [Citation40,Citation42] Mexico [Citation43] and Brazil [Citation41]. Likewise, the difference in survival outcomes between patients with Breslow thickness <1 mm and ≥1 mm aligns with other studies conducted in Latin America [Citation40,Citation43].
This study has potential limitations. Focusing on a single center limits generalizability, and its retrospective design introduces potential selection bias. More studies across different institutions are needed to better understand the factors influencing survival in low-income countries.
5. Conclusion
This study identified age, ulceration, metastasic status and Breslow thickness >1 mm as significant predictors of mortality in melanoma patients. These findings align with existing literature, although sex, site, histologic type and mitosis did not show substantial associations regarding this end point. The observed 5–year survival rate (67.1%) was lower than reports in high-income countries but comparable to South America. Further, multi-institutional studies are needed in low—and middle-income settings to understand melanoma survival and guide interventions better.
Author contributions
A Mauricio: conceptualization, supervision, writing- original draft preparation.
M Germán: conceptualization, methodology, writing- original draft preparation.
A Mateo: conceptualization, methodology, data curation, writing- review & editing.
L Isaac: conceptualization, resources, visualization.
M Juanita: resources, writing- original draft preparation.
M Mateo: resources, writing- original draft preparation.
R Juan: resources, writing- original draft preparation.
Financial disclosure
The authors declare no potential conflicts of interest.
Ethical conduct of research
The bioethics committees of the Universidad Tecnológica de Pereira and Oncólogos de Occidente approved this study and classified it as safe. Study Approval number: MELANOMA.V1.3feb2020.
Consent to use the information contained in the medical records could not be obtained from all patients because many of them died. Their review was done under oath of confidentiality.
Acknowledgments
Research Office of Oncólogos de Occidente, Pereira, Risaralda, Colombia.
References
- Simões MCF, Sousa JJS, Pais AACC. Skin cancer and new treatment perspectives: a review. Cancer Lett. 2015;357(1):8–42. doi:10.1016/j.canlet.2014.11.001
- Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. 2018;392(10151):971–984. doi:10.1016/S0140-6736(18)31559-9
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
- Carr S, Smith C, Wernberg J. Epidemiology and risk factors of melanoma. Surg Clin North Am. 2020;100(1):1–12. doi:10.1016/j.suc.2019.09.005
- Pozzobon FC, Acosta ÁE, Castillo JS. Cáncer de piel en Colombia: cifras del Instituto Nacional de Cancerología [Skin Cancer in Colombia: Statistics from the National Cancer Institute]. Rev la Asoc Colomb Dermatología y Cirugía Dermatológica. 2018;26(1):12–17. Spanish. doi:10.29176/2590843X.25
- Boussios S, Rassy E, Samartzis E, et al. Melanoma of unknown primary: new perspectives for an old story. Crit Rev Oncol Hematol. 2021;158. doi:10.1016/j.critrevonc.2020.103208
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158(5):495. doi:10.1001/jamadermatol.2022.0160
- Pozzobon FC, Acosta AE. Epidemiological profile of primary cutaneous melanoma over a 15-year period at a private skin cancer center in Colombia. Rev Salud Publica (Bogota). 2018;20(2):226–231. doi:10.15446/rsap.V20n2.65616
- Li WQ, Qureshi AA, Ma J, et al. Personal history of prostate cancer and increased risk of incident melanoma in the United States. J Clin Oncol. 2013;31(35):4394–4399. doi:10.1200/JCO.2013.51.1915
- Sitenga JL, Aird G, Ahmed A, et al. Socioeconomic status and survival for patients with melanoma in the United States: an NCDB analysis. Int. J. Dermatol. 2018;57(10):1149–1156. doi:10.1111/ijd.14026
- Vernali S, Waxweiler WT, Dillon PM, et al. Association of incident amelanotic melanoma with phenotypic characteristics, MC1R status, and prior amelanotic melanoma. JAMA Dermatology. 2017;153(10):1026–1031. doi:10.1001/jamadermatol.2017.2444
- Vourc'H-Jourdain M, Martin L, Barbarot S, et al. Large congenital melanocytic nevi: therapeutic management and melanoma risk: a systematic review. J Am Acad Dermatol. 2013;68(3):493–498; e1–e14. doi:10.1016/j.jaad.2012.09.039
- Reddy KK, Farber MJ, Bhawan J, et al. Atypical (dysplastic) nevi: outcomes of surgical excision and association with melanoma. JAMA Dermatology. 2013;149(8):928–934. doi:10.1001/jamadermatol.2013.4440
- Kubica AW, Brewer JD. Melanoma in immunosuppressed patients. Mayo Clin Proc. 2012;87(10):991–1003. doi:10.1016/j.mayocp.2012.04.018
- Revythis A, Shah S, Kutka M, et al. Unraveling the wide spectrum of melanoma biomarkers. Diagnostics. 2021;11(8):1341. doi:10.3390/diagnostics11081341
- Schadendorf D, Fisher DE, Garbe C, et al. Melanoma. Nat Rev Dis Prim. 2015;1:15003. doi:10.1038/nrdp.2015.3
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060
- Li K, Shi H, Zhang B, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362. doi:10.1038/s41392-021-00670-9
- Slominski RM, Sarna T, Płonka PM, et al. Melanoma, melanin, and melanogenesis: the Yin and Yang relationship. Front Oncol. 2022;12:842496. doi:10.3389/fonc.2022.842496
- Janka EA, Várvölgyi T, Sipos Z, et al. Predictive Performance of Serum S100B Versus LDH in melanoma patients: a systematic review and meta-analysis. Front Oncol. 2021;11. doi:10.3389/fonc.2021.77216
- Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021. J Natl Compr Canc Netw. 2021;19(4):364–376. doi:10.6004/jnccn.2021.0018
- Green AC, Baade P, Coory M, et al. Population-based 20-year survival among people diagnosed with thin melanomas in Queensland, Australia. J Clin Oncol. 2012;30(13):1462–1467. doi:10.1200/JCO.2011.38.8561
- Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29(16):2199–2205. doi:10.1200/JCO.2010.31.5812
- Tseng WH, Martinez SR. Tumor location predicts survival in cutaneous head and neck melanoma. J Surg Res. 2011;167(2):192–198. doi:10.1016/j.jss.2010.10.008
- Johansson M, Brodersen J, Gøtzsche PC, et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6(6):CD012352. doi:10.1002/14651858.CD012352.pub2
- Yuan TA, Lu Y, Edwards K, et al. Race-, age-, and anatomic site-specific gender differences in cutaneous melanoma suggest differential mechanisms of early- and late-onset melanoma. Int J Environ Res Public Health. 2019;16(6):908. doi:10.3390/ijerph16060908
- Chevalier V, Barbe C, Le Clainche A, et al. Comparison of anatomical locations of cutaneous melanoma in men and women: a population-based study in France. Br. J. Dermatol. 2014;171(3):595–601. doi:10.1111/bjd.13052
- Wee E, Wolfe R, Mclean C, et al. The anatomic distribution of cutaneous melanoma: a detailed study of 5141 lesions. Australas J Dermatol. 2020;61(2):125–133. doi:10.1111/ajd.13223
- Iribarren O, Sepúlveda M, Hidalgo J, et al. Epidemiological study of malignant melanoma in IV Region of Chile. Cuad cir. 2005;33–38.
- Pérez ME, Bley C, Cárdenas C, et al. Epidemiology of melanoma in the O'Higgins region during the years 2012 and 2015. Rev Chil Dermatol. 2017;33(4):43–48.
- Ortiz PAU, Villanueva JAN, Mejia CCC, et al. Characteristic features of cutaneous melanoma in two institutions of Bogotá, Colombia: analysis, 2012–2016. Rev Colomb Cancerol. 2021;25(4):188–195. doi:10.35509/01239015.692
- Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment - Update 2022. Eur J Cancer. 2022;170:256–284. doi:10.1016/j.ejca.2022.04.018
- Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, et al. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2(2):CD011123. doi:10.1002/14651858.CD011123.pub2
- Wheatley K, Wilson JS, Gaunt P, et al. Surgical excision margins in primary cutaneous melanoma: a meta-analysis and Bayesian probability evaluation. Cancer Treat Rev. 2016;42:73–81. doi:10.1016/j.ctrv.2015.10.013
- Goepfert RP, Myers JN, Gershenwald JE. Updates in the evidence-based management of cutaneous melanoma. Head Neck. 2020;42(11):3396–3404. doi:10.1002/hed.26398
- Curti B, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. 2021;384(23):73–74. doi:10.1056/NEJMra2034861
- Behbahani S, Malerba S, Samie FH. Acral lentiginous melanoma: clinicopathological characteristics and survival outcomes in the US National Cancer Database 2004–2016. Br J Dermatol. 2020;183(5):952–954. doi:10.1111/bjd.19211
- Mejbel HA, Torres-Cabala CA, Milton DR, et al. Prognostic significance of acral lentiginous histologic type in T1 melanoma. Mod Pathol. 2021;34(3):572–583. doi:10.1038/s41379-020-0641-x
- Svedman FC, Pillas D, Taylor A, et al. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe - a systematic review of the literature. Clin Epidemiol. 2016;8:109–122. doi:10.2147/CLEP.S99021
- Rodríguez-Betancourt JD, Arias-Ortiz N. Cutaneous melanoma incidence, mortality, and survival in Manizales, Colombia: a population-based study. J Int Med Res. 2022;50(6):3000605221106706. doi:10.1177/03000605221106706
- Vazquez VDL, Silva TB, Vieira MDA, et al. Melanoma characteristics in Brazil: demographics, treatment, and survival analysis. BMC Res Notes. 2015;8(1):4. doi:10.1186/s13104-015-0972-8
- Reyes E, Uribe C, de Vries E. Population-based incidence and melanoma-specific survival of cutaneous malignant melanoma in a Colombian population 2000–2009. Int J Dermatol. 2018;57(1):21–27. doi:10.1111/ijd.13839
- Lino-Silva LS, Domínguez-Rodríguez JA, Aguilar-Romero JM, et al. Melanoma in Mexico: clinicopathologic features in a population with predominance of acral lentiginous subtype. Ann Surg Oncol. 2016;23(13):4189–4194. doi:10.1245/s10434-016-5394-x
