Abstract
The aim of this research project was to determine the rate at which oxygen depletes in enclosed spaces. Cargo holds, chain locker and double bottom tanks are only some of the enclosed spaces found on a ship. The project was conducted using oxygen depletion experiments on scrap metal in sealed and open vented containers. In addition, the speed of oxygen depletion in a replicated chain locker is investigated. It is necessary to highlight the speed of oxygen depletion in cargo hold situations and other enclosed spaces and prevent loss of life in these dangerous spaces.In the oxygen-depleted atmosphere within an enclosed space, there is no sensory indication to cause alarm regarding the dangers within that space. Therefore the results of the experiments must be seen as a significant step in raising awareness of the dangers within an enclosed space, in improving health and safety standards and preventing loss of lives at sea.
Background
This research raises awareness of the risks and hazards of entering enclosed spaces on ships by highlighting how quickly the oxygen level can drop to a dangerous level in an enclosed environment. Cargo spaces containing scrap metal or other bulk goods and other enclosed spaces are all suspect of being oxygen deficient. Once the hatch covers or tank lids are closed, the oxygen contained in the space immediately begins being consumed by the cargo and by rusting the space itself.
There are very few studies available to quantify the rapid depletion of oxygen in enclosed spaces where steel or scrap metal is being transported or in chain lockers. However, there are numerous publications and articles on formal investigation into accidents. Deadly accidents on the Saga Rose (MAIB Citation2009), the Viking Islay (MAIB Citation2008a) and the Sava Lake (MAIB Citation2008b) have been investigated. In more recent years, three persons died from asphyxiation on the cargo ship Appolo Kita (Safety4Sea Citation2018), whilst on the Sally Ann C two persons died in similar circumstances (IOM Citation2015). While guidance for entry into enclosed spaces is provided in publications by regulatory bodies such as the IMO (Citation2011) or the MCA (Citation2010), this doesn’t seem to prevent these accidents from happening.
Numerous studies have been carried out into the carriage of timber, logs and wood pellets and the dangers posed by these cargoes. Svedberg et al. studied five ocean vessels travelling between Canada and Sweden after a deadly incident occurred (Svedberg et al. Citation2008). A further study involved 41 shipments of wood products on 10 ships (Svedberg et al. Citation2009). In both studies, the measurements took place just before the hatch covers were opened, therefore giving an indication of danger level but not the speed in which a dangerous atmosphere is created. Another study by Hedlund and Hilduberg involved three ships where fatal accidents had occurred as a result of asphyxiation from off-gassing of wood pellets (Hedlund and Jarleivson Hilduberg Citation2017). First attempts have been made by Pa and Bi to model Off-Gas emissions from wood pellets during transportations based on data from previously mentioned studies (Pa and Bi Citation2010).
As opposed to the methodology used in other studies, our research aims to continuously monitor the oxygen depletion process in a model. This data allows us to determine the rate of oxygen depletion. Dalton’s Law of Partial Pressures states that for a mixture of gases in any container, the total pressure exerted is the sum of the partial pressures that each gas would exert if it were alone in that container. The standard atmosphere is a unit of pressure equal to 101.325 kPa. The partial pressures of each of the air components (in kPa) are equal to the percentage of that component. With the exception of oxygen and water vapour, all constituents of air are inert or unreactive gases; therefore oxygen and water vapour are the only gases which can be consumed in the rusting process. In a closed containment experiment if all the oxygen is used up by corrosion, the pressure in the container is 80.425 kPa which is equal to the atmospheric pressure minus the partial pressure of oxygen (20.9 kPa). Table shows the relationship between the Oxygen level, the absolute pressure within the container and the level of danger within the space.
Table 1. Oxygen levels, pressures and corresponding danger level (Standard Club Citation2017).
Results
Experimental setups
The parameters that affect oxygen depletion by a cargo such as scrap metal are surface area of material available for rusting, oxygen level, moisture and temperature. We investigated three different situations in our study: a completely closed containment, an open vented containment and a chain locker. Each experiment was repeated at least three times and the average as well as a standard deviation was calculated. The full data set can be found in the supporting information. The oxygen level was either calculated from the pressure inside the closed containment or measured by an oxygen sensor. The experiments for the closed containment must be carried out using an airtight experimental setup. Since atmospheric pressure changes during the experiment will affect the pressure readings, atmospheric pressure data were taken for Cork (Met Eireann) and Glasgow (Met Office) and the experimental data was corrected to reflect those changes.
Closed containment
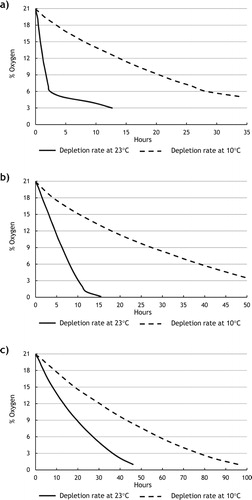
In closed containment, the time taken for oxygen level to fall to 19.5%, 10% and 6% at 10°C were 1.45, 18.08 and 25.3 h respectively whilst at 23°C the times were 0.3, 1.56 and 2.41 respectively. The difference in the rate of oxygen depletions between the sets of experiments at 10°C and 23°C can be explained by the fact that rusting takes place at a faster rate at higher temperatures. The results are alarming considering the fact that entering into enclosed spaces is one of the highest causes of death at sea. However, courses on entry into enclosed spaces are neither mandatory nor is there any general awareness how fast oxygen is depleted once a space is enclosed. The results were confirmed using an experimental setup including an oxygen sensor. (Figure a).
Open vented containment
In acargo hold air may enter from outside or from adjacent linked areas into the cargo space, to better simulate open vented containment, experiments utilising a oxygen transducer were carried out. The permeability of space was calculated as the difference between the total space and the space occupied by the simulated cargo. In this case, the permeability or free air space within the containment of the experiment space was 80%. The time taken for oxygen level to drop to 19.5%, 10% and 6% at 10°C were 1.91, 24.06 and 38.84 h respectively whilst at 23°C the times were 0.84, 5.68 and 8.03 h respectively. (Figure b). This experiment was designed with a single small pipe leading to outside the containment that allows air to enter to maintain atmospheric pressure; however, this will not displace the oxygen-depleted air within the space. Therefore the time that is needed for the oxygen levels to drop is longer than in an enclosed containment but the same dangerous oxygen levels are reached after a short amount of time.
Chain locker
A chain locker is generally an open vented space and without forced air ventilation. The scale model chain locker size has been constructed on a scale comparable to the chain locker on the MV Cill Airne. This experiment utilises steel chain as the corrodible material. The air to steel ratio is approx. 20.35:1 by volume. The time taken for oxygen level to reduce to 19.5%, 10% and 6% at 10°C were 4.53, 38.48 and 58.17 h respectively whilst at 23°C the times were 2.05, 17.25 and 27.17 h respectively. (Figure c). The higher permeability of space in this experiment of 95% combined with a greater air to steel ratio causes oxygen depletion to slow compared to the open vented experiment.
Summary
This rapid reduction of oxygen level is a significant factor that should be highlighted in trying to prevent deaths in cases of entry into enclosed spaces. Looking at the results from all three types of experiments (Table ) it is clear that once the hatch covers or tank lids are closed, the oxygen contained within the space is immediately being absorbed and that enclosed spaces must at all times be treated as unsafe. Minor changes in temperature, pressure, condition of the cargo used and other experimental conditions can influence the speed of oxygen depletion. To account for this effect standard deviations have been calculated for each experiment. Taking this into account after 2 or 3 days oxygen levels are reached in both cargo holds and chain lockers that are fatal within minutes of entry into the space. Ballast Tanks, Fresh Water Tanks and cofferdam’s will react slower but will deplete over time and thereafter will be completely devoid of oxygen. We are currently investigating other types of closed containments and a variety of different cargoes to deepen our understanding of oxygen depletion in the shipping industry.
Table 2. Summary of oxygen depletion under different experimental scenarios.
Acknowledgements
We would like to specially thank Paul Little, Principal and CEO of City of Glasgow College, for acting as patron of this research. Without the support of Principal Little and his team at City of Glasgow College, this would not have been possible.
We are very thankful to Linus Reichenbach for his guidance and contribution to writing and editing this publication.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Daniel Burke
Daniel C Burke is a former Head of the Nautical Studies Department at Cork Institute of Technology, and former Head of the National Maritime College of Ireland. After retiring in 2006 he became a consultant in Marine Engineering, working on the new build of the Angolan Maritime College in Sumbe. He has been the Secretary of the Stena Association of Maritime Institutes (STAMI) for several years.
Manhal Alnasser
Dr. Manhal Alnasser is an experienced lecturer and researcher in engineering and biotechnology. He is a post-graduate of Heriot Watt University, holding a PhD in Chemical Engineering. he also holds TQFE from Dundee University , Scotland and a BA in Chemical Engineering. Manhal is also an External Verifier with the SQA.
References
- Hedlund FH, Jarleivson Hilduberg Ø. 2017. Fatal accidents during marine transport of wood pellets due to off-gassing – experiences from Denmark. In: Tumuluru J. S., editor. Biomass volume estimation and valorization for Energy. London, UK: Intech Open; p. 73–97.
- [IMO] International Maritime Organization. 2011. Revised recommendations for entering enclosed spaces Aboard Ships, A.1050(27).
- [IOM] Isle of Man Ship Registry. 2015. Mar. Isle of man registered “Sally Ann C” enclosed space fatalities and near fatality. Casualty investigation Report No. CA121.
- [MAIB] Marine Accident Investigation Branch. 2008. Viking Islay Report. 12/2008.
- [MAIB] Marine Accident Investigation Branch. 2008. Sava Lake Report. 15/2008.
- [MAIB] Marine Accident Investigation Branch. 2009. Saga Rose Report. 1/2009.
- [MCA] Marine and Coastguard Agency. 2010. Entry into dangerous spaces. Marine Guidance Note 423 (M).
- Met Eireann. 2017-2019 [accessed 2019 Feb 6]. http://www.met.ie
- Met Office. 2017-2019 [accessed 2019 Feb 6]. http://www.metoffice.gov.uk
- Pa A, Bi X. 2010. Modelling of off-gas emissions from wood pellets during marine transportation. Ann Occup Hyg. 54(7):833–841.
- Safety4Sea. 2018. Three crewmen die after enclosed space entry on cargo ship. [accessed 2019 Feb 6]. https://safety4sea.com/three-crewmen-die-after-enclosed-space-entry-on-cargo-ship/.
- The Standard Club. 2017. A master guide to enclosed space entry. Aug. p 9, London, UK: Charles Taylor & Co. Limited.
- Svedberg U, Petrini C, Johanson G. 2009. Oxygen depletion and formation of Toxic gases following Sea transportation of logs and wood chips. Ann Occup. Hyg. 53(8):779–787.
- Svedberg U, Samuelsson J, Melin S. 2008. Hazardous off-gassing of carbon monoxide and oxygen depletion during ocean transportation of wood pellets. Ann Occup. Hyg. 52(4):259–66.