Abstract
Background
Severe acute malnutrition (SAM) affects nearly 20 million children worldwide and is responsible for up to 1 million deaths per year in children under the age of 5 years. Current WHO guidelines recommend oral amoxicillin for children with uncomplicated malnutrition and parenteral benzylpenicillin and gentamicin for those with complicated malnutrition. Because of cost pressures and increasing antimicrobial resistance, the administration of empirical antibiotics for children with SAM has recently been debated.
Methods
A systematic review of the current published literature was undertaken to assess the efficacy, safety, cost-effectiveness and pharmacokinetics of antimicrobial treatment of children with SAM in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
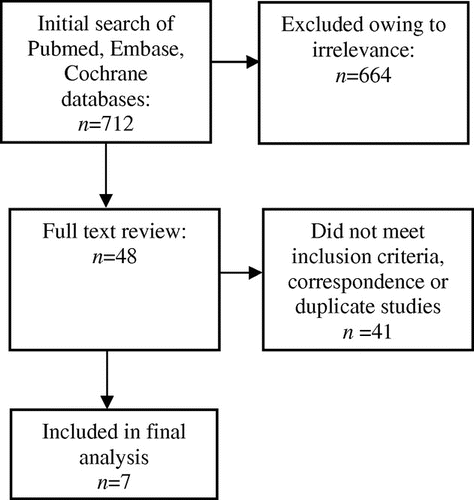
The initial search found 712 papers, eight of which met the inclusion criteria. Quality assessment of the studies was performed as per the Grading of Recommendations Assessment, Development and Evaluation guidelines. International guidelines and clinical data registries were also reviewed which identified inconsistencies in current first- and second-line therapies and dosing regimens.
Conclusion
Current evidence supports the continued use of broad-spectrum oral amoxicillin for treating children with uncomplicated SAM as outpatients. There is no strong evidence to justify changing the current parenteral therapy guidelines for children admitted with complicated SAM, although they should be clarified to harmonise the dosage regimen of amoxicillin for the treatment of SAM to 40 mg/kg twice daily, and to continue parenteral antimicrobials beyond 2 days if indicated by the clinical condition.
Introduction
Severe acute malnutrition (SAM) affects nearly 20 million children under 5 years and causes up to 1 million deaths annually by increasing susceptibility to death from severe infection [Citation1]. The most vulnerable age for malnutrition is 6–18 months when growth velocity and brain development are especially high. However, with the introduction of solid food in many low-income settings to children as young as 2 months, it is increasingly recognised that SAM may occur in infants aged <6 months [Citation2].
SAM is defined by two distinct clinical entities: (i) severe wasting [marasmus, defined as middle upper-arm circumference (MUAC) < 115 mm in children aged 6–59 months or a weight-for-height/length <–3 Z-scores according to the 2006 WHO growth standards in children aged 0–59 months]; and (ii) nutritional oedema (kwashiorkor, defined as bilateral pitting oedema) [Citation3–5]. Children with SAM are further classified according to the presence or absence of medical complications [Citation4]. Uncomplicated SAM includes children who are clinically well, i.e. without signs of infection and with a retained appetite (‘passed the appetite test’) which is regarded as indicative of the absence of severe metabolic disturbance. Complicated SAM includes children with clinical features of infection, metabolic disturbance, severe oedema, hypothermia, vomiting, severe dehydration, severe anaemia or a lack of appetite who require inpatient treatment.
Traditionally, all children with malnutrition were managed as inpatients with empirical broad-spectrum parenteral antibiotics, regardless of whether clinical features of infection (or other complications) were present [Citation6]. However in the past decade, the advent of clinically effective ready-to-use therapeutic foods (RUTF) has resulted in the recommendation that children with uncomplicated SAM (>80% of paediatric SAM cases) be treated as outpatients, following the WHO–UNICEF community-based model for the management of malnutrition [Citation3,6]. This has occurred concurrently with changes to the nutritional and clinical profile of children diagnosed with (and treated for) SAM following the publication of the 2006 WHO Child Growth Standards which resulted in significant changes to the measurement of nutritional status and a large increase in the number of children classified as having SAM [Citation7,8].
The rationale behind antibiotic treatment for children with SAM lies in the view that malnourished children may not show signs of clinical infection [Citation9]. Older clinical trials suggested evidence for improved growth and decreased mortality in malnourished children treated with antibiotics [Citation10]. The mechanism behind this clinical improvement has been postulated to be secondary to the treatment of underlying covert infection, prevention of colonising micro-organisms, minimisation of nutrient diversion by dampening inflammatory responses and a reduction in enteropathy via alterations in the gut microbiome [Citation10,11]. Indeed, several epidemiological studies have documented a high prevalence of covert pneumonia, bacteraemia and urinary tract infections in children with malnutrition caused by a variety of Gram-positive and Gram-negative organisms, including Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Klebsiella spp, Salmonella spp and other enterobacteriaceae [Citation12–17].
With most SAM treatment now being in the outpatient setting, broad-spectrum oral antibiotics for uncomplicated SAM continue to be recommended by WHO and UNICEF. For complicated SAM, intravenous therapy followed by oral therapy (including a prolonged course of an aminoglycoside) is recommended; however, the evidence base for this is weak [Citation4,18,19]. Increasing antibiotic resistance is of international concern [Citation20], as are the cost and logistical considerations of empirical antimicrobial treatment and its possible side effects. High rates of non-susceptibility to first- and second-line therapies have been documented in several epidemiological studies in children with SAM [Citation12,13,15,21–25], and the need for a routine course of oral antibiotics in children with uncomplicated SAM has been questioned with some resource-constrained clinics choosing not to prioritise their administration [Citation9,26].
This review was therefore undertaken to evaluate the recent international literature for evidence (or otherwise) pertaining to the clinical efficacy of antibiotic treatment in children with SAM. Current recommendations by WHO (Table ) are published in the 2013 Pocketbook for Hospital Care for Children [Citation5, 27] but, with the global threat of increasing antimicrobial resistance and recently published new data evaluating the efficacy and safety profiles of antimicrobials in children presenting with uncomplicated SAM, a review of the evidence to ensure that current recommendations remain appropriate is warranted.
Table 1. Current WHO inpatient and outpatient management guidelines for severe acute malnutrition.
Methods
A search for systematic reviews, meta-analyses, multi-centre studies and randomised controlled trials (RCTs) was undertaken using the search terms outlined in Table . The databases EMBASE, Cochrane Database of Systematic Reviews and Pubmed were searched. Trials were limited to those conducted in humans and published since 2010 in English or French to update the research which informed the 2013 Guidelines [Citation28]. Inclusion and exclusion criteria are outlined in Table . International clinical practice guidelines were also reviewed, including the Infectious Diseases Society of America (IDSA), the European Society for Clinical Medicine (ESCMID), BMJ Clinical Evidence, the American Academy of Pediatrics, Therapeutic Guidelines (Australia), Action Contre le Faim, Médicins Sans Frontières, Valid International and national guidelines in high-burden countries in Asia and Africa. Clinical trial registries including www.clinicaltrials.gov and http://www.who.int/ictrp/en/ were searched for ongoing trials relevant to antibiotic treatment of SAM.
Table 2. Search terms used in search strategy.
Table 3. Inclusion and exclusion criteria.
Results
The initial search produced 712 papers (Figure 1), 48 of which qualified for full text review. Ultimately, seven studies met the inclusion criteria which were abstracted as detailed in Appendix 1. Quality assessment of the studies was performed as per the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines [Citation29].
Characteristics of the included studies
Since 2010, four systematic reviews and/or meta-analyses (conducted across an international setting) [Citation28,30–32] and three double-blind, placebo-controlled trials conducted in Malawi [Citation33], Niger [Citation26] and Kenya [Citation34] evaluating children with SAM have been published. All papers assessed children aged 6–59 months, apart from one recent RCT which extended the intervention group to severely malnourished children aged ≥2 months [Citation34]. The definition of complicated and uncomplicated SAM was the same in all the studies.
The single meta-analysis was classified as GRADE A (high-quality evidence) and the remaining systematic reviews and RCTs were classified as GRADE level B (moderate-quality evidence, Appendix 1). Study heterogeneity in interventions and populations prevent further pooling of the results for this review. There was one further review of pharmacokinetics of antimicrobials in children with SAM [Citation35].
Evidence for current guidelines for uncomplicated SAM
A systematic review which informed the current WHO guidelines documented evidence from two studies assessing the clinical efficacy of amoxicillin in children with uncomplicated SAM [Citation28]. The first, an unblinded RCT in Sudan (n = 458), demonstrated that oral amoxicillin (40 mg/kg/day twice daily) for 5 days was as effective as IM ceftriaxone for 2 days [Citation36]. Intention-to-treat analysis in the study showed that 53.5% (123/230) in the amoxicillin group and 55.7% (127/228, difference 2.2%, 95% CI –6.9–11.3) in the ceftriaxone group had a weight gain of at least 10 g/kg/day during a 14-day period. The recovery rate was not significantly different [70.0% (161/230) in the amoxicillin group and 74.6% (170/228) in the ceftriaxone group (p = 0.27)], nor were the case fatality rates [3.9% (9/230) and 3.1% (7/228), respectively (p = 0.67)] with most deaths occurring in the first 2 weeks of admission [Citation36].
A retrospective study in Malawi compared oral amoxicillin (60 mg/kg/day, n = 498) for 7 days with no antibiotics (n = 1955). The recovery rate at 4 weeks was poorer in children receiving amoxicillin (39.8% vs. 70.8%, p < 0.001), but was similar at 12 weeks with similar rates of death and default [Citation37]. However, the research was acknowledged to be at risk of bias, not just because of its retrospective data collection but also because of the different district locations of the cohorts of patients who were not stratified by risk of HIV status (with a high prevalence in the study population) [Citation38].
The current search identified two further systematic reviews [Citation30,31] and two RCTs [Citation7,39] assessing the efficacy of amoxicillin in SAM, as well as a meta-analysis combining their results [Citation32]. While excluded from the inclusion criteria, three relevant observational studies were also found in the literature and for the provision of their epidemiological data, these studies are described in Appendix 2 [Citation14,40,41].
A systematic review in 2012 [Citation31] did not find any interventional studies apart from those already documented in the review outlined above. A 2013 systematic review and meta-analysis (of observational data only) [Citation30] favoured amoxicillin over co-trimoxazole for cumulated susceptibilities of all isolated bacteria [median 42% (IQR 27–55%) vs. 22% (IQR 17–23%); population-weighted means 52.9% (IQR 23–57%) vs. 35.4% (IQR 6.7–42%)], yet the authors noted that the evidence from intervention studies (discussed below) revealed conflicting results regarding the efficacy of amoxicillin in children with SAM, which is especially difficult to interpret when patients were not stratified by HIV infectivity status.
A 2013 double-blind, three-armed RCT [Citation42] (GRADE level B) compared the third-generation oral cephalosporin cefdinir with amoxicillin and placebo in Malawi (n = 2767). The overall case fatality rate was 5.4% and it was significantly higher in children receiving placebo (7.4%) than in those receiving amoxicillin (4.8%, p = 0.02) or cefdinir (4.1%, p = 0.003). This corresponds to a 36% (95% CI 7.0–55) reduction in mortality when given amoxicillin and a 44% (95% CI 18.0–62.0) reduction when given cefdinir. Children who received either antibiotic agent also had greater increases in MUAC than those who received placebo. The authors concluded that the results provide clear evidence to support the recommendation of routine oral antibiotics as part of the outpatient management of SAM. However, in considering the potential benefit of any policy move towards the widespread use of oral cephalosporin, this would need to be weighed against the risk of promoting community-based antimicrobial resistance [Citation9].
In contrast, a 2016 RCT of 2399 children aged 6–59 months with uncomplicated SAM in four rural treatment centres in Niger found no significant difference in the likelihood of recovery between those treated with amoxicillin and those who received placebo (RR with amoxicillin 1.05, 95% CI 0.99–1.12) [Citation26]. Amoxicillin significantly accelerated early gains in weight and MUAC (week 1 RR 3.8, 95% CI 3.1–4.6, p < 0.001) but had no significant effect on overall weight or height gain by week 4. Among children who recovered, time to recovery was significantly shorter with amoxicillin (by only 2 days, however) than with placebo (mean treatment 28 vs. 30 days, p < 0.001); amoxicillin tended to reduce the risk of death in children who were >24 months (RR 0.24, 95% CI 0.02–2.12), but not in younger children (<24 months, RR 3.04, 95% CI 0.61–15.01). Notably, however, amoxicillin decreased the risk of transfer to inpatient care, (RR 0.86, 95% CI 0.76–0.98, p = 0.02), for acute gastroenteritis in particular (RR 0.67, 95% CI 0.48–0.94, p = 0.02). This is surprising because the causative organisms primarily responsible for gastroenteritis in young children tend to be less sensitive to amoxicillin [Citation43]. Gastrointestinal side effects are common with amoxicillin so this clinical improvement may in fact reflect the antibiotic’s effect on reducing small bowel flora, modifying the composition of the gut microbiome [Citation44]. Overall, amoxicillin significantly reduced hospital admission in those transferred for inpatient care (RR 0.76, 95% CI 0.62–0.92, p = 0.005).
This trial challenged the view that antibiotic therapy is always necessary or beneficial in the management of SAM and concluded that, by eliminating the routine prescription of antibiotics in children with uncomplicated SAM, treatment could be simplified (with associated cost savings, and limiting the spread of antibiotic resistance) [Citation26]. The international literature response to these conclusions was mixed. Some supported the view that the use of broad-spectrum mass antibiotics has unintended consequences which outweigh the benefits of routine administration [Citation45], emphasising the importance of ensuring that essential and effective antimicrobials are available to treat all clinical infections in children [Citation45]. Others noted that a lack of clinical improvement in children receiving amoxicillin should not be extrapolated to mean that antibiotics are not beneficial in children with SAM as it may simply be that amoxicillin is no longer the most appropriate antibiotic [Citation46].
These two RCTs [Citation26,42] were subsequently analysed in a 2016 meta-analysis (GRADE A high-quality evidence) [Citation32] which highlighted limitations in the RCT undertaken in Niger, notably its failure to include children with oedema, a potential selection bias since the WHO definition of uncomplicated SAM includes mild-to-moderate bilateral oedema. This meta-analysis (total events 1610 amoxicillin, 1535 placebo) revealed an overall benefit to survival in children with all three clinical forms of SAM (kwashiorkor, marasmic kwashiorkor and marasmus, summary risk ratio 1.03, 95% CI 1.00–1.06, p = 0.03) and survival benefits of amoxicillin in children with marasmus (summary risk ratio 1.05, 95% CI 1.00–1.11, p = 0.05). Minimal inconsistency was observed between the two studies in tests for heterogeneity (I2 = 0%) and the authors concluded that the benefits of antibiotics demonstrated in their analysis should reaffirm continuity of the current WHO recommendations.
Despite the limitations of its methodology, one additional observational study deserves comment. A retrospective cohort study of 628 children with uncomplicated SAM managed by an outpatient therapeutic programme in rural Ethiopia found that children who received amoxicillin recovered significantly more quickly than children who did not and with a higher rate of recovery (HR 1.95, 95% CI 1.17–3.23) [Citation40]. However, the methodology was unclearly described and children were not observed in the administration of their medication (which also included a package of interventions such as RUTF, vitamin A and deworming tablets).
Evidence for current guidelines for complicated SAM
A systematic review in 2011 [Citation28] found only one interventional study (completed in 1996) which assessed the clinical efficacy of ampicillin and gentamicin in 300 children, reporting a case fatality reduction in children receiving antibiotics from 20 to 6% (OR 4.0, 95% CI 1.7–9.8) [Citation47]. However, these antibiotics were administered alongside a new protocol for the treatment of hypoglycaemia, and benefits therefore cannot be attributed to the antibiotic regimen alone. No other interventional trials assessing empirical parenteral therapy in children with complicated SAM were identified in this updated review, although a 2013 systematic review found susceptibilities of >80% to combined amoxicillin–gentamicin and gentamicin in blood, urine and CSF cultures (Table ) [Citation30].
Table 4. Bacterial antibiotic susceptibilities (%) for common first- and second-line therapies for treating children with SAM: results of a meta-analysis of 767 children from Uganda, Kenya, Turkey, Nigeria, Kenya and South Africa [Citation30].
Recent observational evidence includes a prospective cohort study published in 2015 of 407 children with respiratory compromise and radiological pneumonia admitted to the Dhaka Hospital of ICDDR,B between 2011 and 2012 [Citation41]. It evaluated patients treated with parenteral ampicillin and gentamicin, and, in children assessed as having treatment failure, antibiotics were changed to second-line agents, ceftriaxone plus levofloxacin, in accordance with hospital protocol. Eighteen children (4.4%) had bacteraemia, and 111 (27%) of those admitted exhibited WHO-defined ‘danger signs’ of severe pneumonia (hypoxaemia, cyanosis, grunting, convulsions, inability to drink or persistent vomiting) [Citation5]. These children were significantly more likely to exhibit treatment failure (RR 3.14, 95% CI 2.30–4.29, p < 0.001) and death (RR 2.78, 95% CI 2.06–3.75, p < 0.001). The authors postulated that the bacterial aetiology of pneumonia in these children with SAM might be more likely to be owing to Gram-negative bacteria, and the unique combination of severe infection, bacterial endotoxin and small bowel overgrowth often observed in children with SAM (resulting in oxidative stress and endogenous nitric oxide production) might contribute to the high levels of treatment failure and mortality [Citation48]. Consistent with previous publications [Citation49], there was a low yield of positive blood cultures in children with pneumonia and SAM, although 16% of the study population had received prior antibiotic therapy. The few cultures which were positive demonstrated better in vitro susceptibility to fluoroquinolones and extended-spectrum cephalosporins than ampicillin and gentamicin. Only six children had a blood culture isolate that was not susceptible to ampicillin and gentamicin, and three of 407 children had blood culture isolates that were not susceptible to ceftriaxone and only one was not susceptible to ciprofloxacin [Citation41].
Another observational study in Niger [Citation14] undertook clinical and biological characterisation of infections in 311 children aged 6–59 months admitted during 2007/2008 with complicated SAM who received parenteral amoxicillin or ceftriaxone for suspected severe or complicated infections, with subsequent treatment targeted towards the suspected type of infection. Gentamicin was not listed as an administered medication. Gastroenteritis was the most frequent clinical diagnosis on admission, followed by respiratory tract infections and malaria. Blood cultures were positive in 17% of cases, more than half of which were considered to be contaminants. The majority of isolates were Gram-negative bacilli (most frequently Salmonella spp.), followed by (in order of frequency) S. aureus, E. coli, K. pneumonia, S. typhi, S. pneumonia, E. faecium, E. faecalis and S. pyogenes. Most enterobacteriaceae isolated were resistant to amoxicillin and co-trimoxazole but susceptible to ceftazidime/ceftriaxone, gentamicin and quinolones. These results concur with a recent epidemiological study in Niger [Citation50] in which faecal carriage of extended-spectrum β-lactamase-producing enterobacteriaceae (ESBL-E) in 55 children aged 6–59 months in a paediatric nutrition centre was 31% (n = 17/55) on admission, with an acquisition rate of 94% (n = 15/16) among those who were not carriers on admission and were resampled on discharge. Of note, the CTX-M-15 gene was found in >90% of carriers. All children had received antibiotic treatment while in hospital, with the majority (75%) receiving multiple antimicrobial therapies including amoxicillin, ceftriaxone and ciprofloxacin [Citation50].
Intestinal carriage of ESBL-E is a significant concern for the dissemination of multidrug-resistant bacterial infections as it might leave few therapeutic options for the treatment of sepsis. Furthermore, the CTX-M gene is of particular concern because of its known spread in hospital and community settings [Citation51]. Previous research has detected a link between β-lactam exposure and intestinal colonisation by enterobacteriaceae resistant to cephalosporins [Citation52], which is of concern when monitoring ongoing resistance patterns in children with SAM. If the spread of ESBL results in the narrowing of clinically effective antimicrobial therapy to carbapenems, the consequences of further dissemination would be of extreme concern given their expense and general lack of availability in low-income settings. These observational studies highlight the importance of continued monitoring of ESBL-producing organisms in children admitted with SAM.
Current guidelines support a prolonged (7-day) course of gentamicin despite no previous supporting RCT evidence of efficacy [Citation9]. The ototoxicity and nephrotoxicity effects should be considered in children with SAM who may have reduced renal function or dehydration. The clinical efficacy of gentamicin, an aminoglycoside antibiotic distributed in the extracellular fluid and eliminated by the kidneys, is determined by the relationship between peak concentration and minimal inhibitory concentration (MIC), with similar pharmacokinetic parameters in malnourished and eutrophic children [Citation53]. Gentamicin has advantages in covering many Gram-negative organisms including pseudomonas spp. (not covered by ceftriaxone), and convenient once-daily dosing. However, no research has investigated the possible adverse effects caused by a prolonged course, and its safety depends on children having normal renal function. Most international guidelines require the serum gentamicin concentration to be monitored after the third or fourth dose to avoid nephrotoxicity, which is expensive and logistically difficult in the low-income settings in which SAM is usually treated [Citation54]. Therefore, in these settings, the implications of prolonged gentamicin require consideration.
Evidence for alternative antibiotic therapies
A meta-analysis in 2013 of 2767 children with all grades of SAM pooled from observational data assessed the antibiotic resistance of blood, urine and CSF cultures [Citation30]. Apart from the improved susceptibility of amoxicillin over co-trimoxazole (as described above), the analysis also documented that the susceptibilities of chloramphenicol and amoxicillin–clavulanate were 73.7% and 30.7%, respectively. Gentamicin, amoxicillin–gentamicin, ceftriaxone and ciprofloxacin had the highest rates of susceptibility (>80%). These aggregated data document the generally high resistance to first-line antibiotics in a population of mixed, moderate and severely malnourished children in sub-Saharan Africa and Turkey (Table ).
Metronidazole
Metronidazole has anti-anaerobic and anti-protozoal activity and is effective against small bowel bacterial overgrowth and Clostridium difficile colitis. Its antimicrobial effect depends on peak concentration and there is a significant ‘post-antibiotic’ killing effect. The 2013 WHO guidelines state “Metronidazole 7.5 mg/kg every 8 h for 7 days may be given in addition to broad-spectrum antibiotics; however, the efficacy of this treatment has not been established in clinical trials” [Citation5]. Small cohort studies suggest that metronidazole has benefits for nutritional recovery in SAM, and improved nutrition is associated with improved survival. Metronidazole is highly effective against protozoa such as Giardia, which has been shown (by microscopy) to be prevalent in more than 30% of children with SAM in a Nairobi slum [Citation55], and up to 60% of malnourished children in Rwanda (by PCR) [Citation56]. In Jamaica, half of a population of children admitted for nutritional rehabilitation had evidence of small bowel anaerobic bacterial overgrowth on breath hydrogen testing associated with reduced appetite and increased stool frequency, with breath hydrogen normalising after a 5-day course of oral metronidazole [Citation57].
However, metronidazole can cause nausea and anorexia, potentially impairing recovery from malnutrition and, rarely, liver and neurological toxicity (Table ). In Mexico, a small pharmacokinetic study of metronidazole in children with SAM reported significantly prolonged clearance, suggesting that it may be warranted to reduce dosing frequency [Citation58]. Therefore, current evidence is insufficient to be conclusive or to alter policy, and, pending the results of a current pharmacokinetic study and clinical trial (https://clinicaltrials.gov/ct2/show/NCT02746276), the routine use of metronidazole should be avoided [Citation9].
Amoxicillin–clavulanate
There have been no trials or pharmacokinetic studies of amoxicillin–clavulanate in children with SAM, although its routine use is often prescribed with the anecdotal intention of tackling systemic infection and small intestinal bacterial overgrowth [Citation9].
Ciprofloxacin
Ciprofloxacin could be a suitable alternative for the management of sepsis in severely malnourished children, and absorption is not affected by the simultaneous administration of feeds [Citation59]. As with third-generation cephalosporins, however, the risk of causing resistance to this important antimicrobial needs to be weighed against its clinical efficacy. To target its use, considering the high rates of gastrointestinal presentations in children with complicated SAM, future clinical studies could investigate the option of oral ciprofloxacin as first- or second-line therapy for children with complicated SAM presenting with gastrointestinal symptoms together with broad-spectrum parenteral therapy. A 2013 systematic review documented ciprofloxacin susceptibilities of IQR 82–93% (median 93%) [Citation30].
Ceftriaxone
The potential value of oral third-generation cephalosporins (cefdinir) in uncomplicated SAM has been documented above [Citation39]. In view of the favourable susceptibility data (median 84, IQR 80–94%) identified by the above 2013 systematic review (Table ), parenteral ceftriaxone should in future be a focus of clinical trials for children with complicated SAM. Ceftriaxone has a broad spectrum of activity, is effective in short courses, is logistically simple in its daily dose administration (which may be intramuscular) and has a wide therapeutic index which increases its safety and efficacy [Citation28].
Azithromycin
Since mortality benefits were observed after its mass distribution for trachoma control in Ethiopia, azithromycin has been considered a promising possible alternative for uncomplicated SAM [Citation60]. However, no pharmacokinetic studies have been undertaken in children with SAM, although they should be considered in the future.
Co-trimoxazole as prophylaxis
Although replaced by amoxicillin in the current SAM treatment guidelines, a recent multi-centre, double-blind RCT [Citation34] in four sites in Kenya assessed co-trimoxazole as prophylaxis in the same way it is used for children with HIV infection for which it has reduced all-cause mortality [Citation61]. A total of 1778 HIV-negative children aged 2–59 months with complicated SAM were randomly assigned to receive daily co-trimoxazole prophylaxis or a matched placebo for 6 months after clinical nutritional stabilisation. There was no significant impact (p = 0.429) on growth or mortality in those receiving cotrimoxazole [Citation34].
Pharmacokinetics in SAM
When available, the pharmacokinetics of the above therapies in children with SAM (including comparison with well nourished controls) has been detailed in previous reviews [Citation28,35]. In 2010, a pharmacokinetic review of 34 drugs including non-antibiotics in children with SAM concluded that the available data did not allow firm conclusions to be drawn on the effects of SAM on drug absorption rates [Citation35]. Several drugs have reduced protein-binding — chloramphenicol, (flu)cloxacilllin, penicillin and sulphamethoxazole — and clearance is decreased for drugs metabolised in the liver (chloramphenicol and metronidazole), which is of concern because of potential toxicity. However, clearance appears largely unchanged for drugs renally metabolised (cefoxitin, penicillins, gentamicin and amikacin) [Citation35]. Two papers have identified the need for adjustment of chloramphenicol dosage in children with SAM [Citation28,35].
A 2011 population pharmacokinetic study in Kenya of ciprofloxacin in 52 children with SAM reported that 10 mg/kg thrice daily (30 mg/kg/day) rather than 10 mg/kg twice daily (20 mg/kg/day) might be a suitable alternative antibiotic for sepsis in severely malnourished children, and absorption was unaffected by the simultaneous administration of feeds [Citation59]. In 2016, a population pharmacokinetic study of gentamicin in 26 children with SAM in Mexico reported that an intravenous dose of 7.5–15 mg/kg once daily in children with SAM and normal renal function has a high probability of efficacy and low risk of nephrotoxicity [Citation44].
Synopsis of international guidelines
A summary of the available international guidelines for the use of antibiotics in SAM is documented in Table . Currently, all guidelines recommend amoxicillin as the first-line therapy in uncomplicated SAM, although there is variation in recommended dosages (from 50 to 100 mg/kg/day) and the duration of therapy (5–7 days).
Table 5. Synopsis of international guidelines on antimicrobial therapy for children with severe acute malnutrition.
For complicated SAM, there is inconsistency in the first-line therapy recommended, including ampicillin/amoxicillin, gentamicin and alternatives that comprise a wide spectrum of antibiotics including third-generation cephalosporins, ciprofloxacin, co-amoxiclav, metronidazole and even amikacin. Dosages of medications also differ with gentamicin recommendations ranging from 5 to 7.5 mg/kg, although β-lactam dosage guidelines are consistent throughout.
Review of harms and toxicity: summary of evidence on safety
Of the studies which included data on safety and adverse events, no significant rate of adverse events was documented for any antibiotic intervention group [Citation26,28,33,34]. Side effects and relevant interactions between the currently recommended therapies for SAM, and those which may be considered in future clinical trials are documented in Table .
Table 6. Common adverse reactions to antibiotics used in severe acute malnutrition in children [Citation55].
Discussion
On the basis of a meta-analysis of two clinical trials which indicate an overall survival benefit and reduction in admission rates, the current evidence supports the continued use of broad-spectrum oral antibiotics for treating children with uncomplicated SAM (26, 32, 42). Ideally, the choice of antibiotic should be dictated by local resistance patterns and common pathogens [Citation5]. However, where malnutrition is common, microbiological data are rarely available and may be misleading if laboratories are not externally quality-controlled. The choices of antibiotic are therefore influenced by cost, availability, ease of administration and local susceptibility profiles [Citation10].
Amoxicillin is relatively safe with minimal serious adverse side effects and reaches therapeutic plasma levels after oral administration in malnourished children; it has been proven to improve outcomes in children with SAM [Citation62,63]. Currently available evidence supports the continued routine administration of amoxicillin for children with uncomplicated SAM treated in the community at a clarified and harmonised dose of 80 mg/kg/day in two divided doses for 7 days. This should also be the regimen for children with complicated SAM after they have stabilised.
For complicated SAM, there is limited evidence suggesting that third-generation cephalosporins might be more effective than ampicillin/gentamicin as parenteral therapy during stabilisation. However, cephalosporins carry an increased risk of exacerbating antimicrobial resistance and therefore recommendations should not be changed until further clinical trials have been conducted. Parenteral treatment should be continued beyond 2 days if indicated by the clinical condition, such as in severe pneumonia or sepsis. The rationale for a 7-day course of gentamicin in children with complicated SAM, who commonly have dehydration and compromised renal function, needs ongoing consideration because of the potential ototoxic and nephrotoxic adverse events in this population. However, the risks of toxicity have not been well characterised and there are limited affordable alternative choices with a similar spectrum of cover.
There is increasing evidence of non-susceptibility to commonly used antimicrobials in children with SAM, including high rates of nosocomially and community-acquired ESBL. Monitoring antimicrobial resistance including distinguishing community from nosocomial infections should be routinely undertaken when empirical broad-spectrum antibiotics are used for any community treatment of children with a life-threatening illness, including SAM. Future clinical trials adhering to CONSORT (Consolidated Standards of Reporting Trials) guidelines should investigate alternative options such as azithromycin, ciprofloxacin and oral third-generation cephalosporins which have been shown to exhibit benefit, while improving the available pharmacokinetic data for children with SAM and assessing the impact on local resistance rates where these antimicrobials are used.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Phoebe C. M. Williams, MBBS(Hons.), received her medical degree from The University of Sydney and a Masters in Global Health Science from The University of Oxford. She is a paediatric registrar and dual trainee in Infectious Diseases at Sydney Children’s Hospital, Australia. She is a DPhil candidate through The University of Oxford, with her research focusing on antimicrobial resistance in paediatric patients.
James A. Berkley FRCPCH, MD is a professor of Paediatric Infectious Diseases at The University of Oxford based at the KEMRI-Welcome Trust Research Programme in Kilifi, Kenya. He is the principal investigator of the CHAIN network with a research focus on serious infection and survival in highly vulnerable groups of infants and children.
Funding
This work was supported by The World Health Organization; The Nuffield Department of Medicine (University of Oxford); General Sir John Monash Foundation; The Wellcome Trust; Bill and Melinda Gates Foundation.
Acknowledgments
We are grateful to the WHO Department of Newborn, Child and Adolescent Health for their valuable input to the conclusions arising from this review.
References
- Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451.
- Kerac M, Mwangome M, McGrath M, et al. Management of acute malnutrition in infants aged under 6 months (MAMI): current issues and future directions in policy and research. Food Nutr Bull. 2015;36;Suppl 1:S30–S34.
- Community-based management of severe acute malnutrition: a joint statement. Geneva: WHO, World Food Programme, United Nations System Standing Committee on Nutrition and United Nations Children’s Fund; 2007.
- World Health Organization. Updates on the management of severe acute malnutrition in infants and children. Geneva: WHO; 2013.
- World Health Organization. Pocket book of hospital care for children. 2nd ed. Geneva: WHO; 2013.
- World Health Organization. Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva: WHO; 1999.
- Isanaka S, Villamor E, Shepherd S, et al. Assessing the impact of the introduction of the world health organization growth standards and weight-for-height z-score criterion on the response to treatment of severe acute malnutrition in children: secondary data analysis. Pediatrics. 2009;123:e54–e59.
- World Health Organization, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children’s Fund. Geneva: WHO, 2009.
- Jones KD, Berkley JA. Severe acute malnutrition and infection. Paediatr Int Child Health. 2014;34;Suppl 1:S1–S29.
- Jones KD, Thitiri J, Ngari M, et al. Childhood malnutrition: toward an understanding of infections, inflammation, and antimicrobials. Food Nutr Bull. 2014;35(Suppl 2):S64–S70.
- Manary MJ, Maleta K, Trehan I. Randomized, double-blind, placebo-controlled trial evaluating the need for routine antibiotics as part of the outpatient management of severe acute malnutrition. Washington, DC: US Aid, 2012; FHI360/FANTA-2 Bridge.
- Reed RP, Rothberg AD. Bacteraemia in malnourished rural African children. Ann Trop Paediatr. 1996;16:61–68.
- Reed RP, Wegerhoff FO. Urinary tract infection in malnourished rural African children. Ann Trop Paediatr. 1995;15:21–26.
- Page AL, de Rekeneire N, Sayadi S, et al. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS ONE. 2013;8:e68699.
- Çaksen H, Cesur A, Uner S, et al. Urinary tract infection and antibiotic susceptibility in malnourished children. Int Urol J. 2000;32:245–247.
- Bahwere P, Levy J, Hennart P, et al. Community-acquired bacteraemia among hospitalised children in rural central Africa. Int J Infect Dis. 2001;5:180–188.
- Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47.
- Ashworth A, Khanum S, Jackson A, et al. Guidelines for the inpatient treatment of severely malnourished children. Geneva: WHO; 2003 . Available from: http://www.who.int/nutrition/publications/guide_inpatient_text.pdf
- Tickell KD, Denno DM. Inpatient management of children with severe acute malnutrition: a review of WHO guidelines. Bull WHO. 2016;94:642–651.
- Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance – the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098.
- Bachou H, Kaddu-Mulindwa DH, Tumwine JK, et al. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis. 2006;6:3–3167.
- Mulholland EK, Corrah PT, Omosigho C, et al. A randomized trial of chloramphenicol vs. trimethoprim-sulfamethoxazole for the treatment of malnourished children with community-acquired pneumonia. Pediatr Infect Dis J. 1995;14:959–964.
- Noorani N, Oyatsi D, Revathi G. Bacterial isolates in severely malnourished children at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005;82:343–348.
- Thame M, Wilks R, Forrester TE. The appropriateness of the current antibiotic empiric therapy based on the bacteria isolated from severely malnourished Jamaican children. West Indian Med J. 2001;50:140–143.
- Isaack H, Hirji KF. Nosocomial bacterial infections among children with severe protein energy malnutrition. East Afr Med J. 1992;69:433–436.
- Isanaka S, Langendorf C, Berthé F, et al. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med. 2016;374:444–453.
- World Health Organization. Recommendations for management of common childhood conditions: evidence for technical update of pocket book recommendations: newborn conditions, dysentery, pneumonia, oxygen use and delivery, common causes of fever, severe acute malnutrition and supportive care. Geneva: WHO; 2012.
- Lazzerini M, Tickell D. Antibiotics in severely malnourished children: systematic review of efficacy, safety and pharmacokinetics. Bull WHO. 2011;89:594–607.
- Balshem H, Helfand M, Schünemann H, et al. Grade Guidelines 3: Rating the quality of the evidence – introduction. J Clin Epidemiol. 2011;64:401–406.
- Alcoba G, Kerac M, Breysse S, et al. Do children with uncomplicated severe acute malnutrition need antibiotics? A systematic review and meta-analysis. PLoS ONE. 2013;8:e53184.
- Picot J, Hartwell D, Harris P, et al. The effectiveness of interventions to treat severe acute malnutrition in young children: a systematic review. Health Technol Assess. 2012;16:1–316.
- Milion M, Lagier J, Raoult D. Meta-analysis on efficacy of amoxicillin in uncomplicated severe acute malnutrition. Microb Pathog. 2016;106:76–77.
- Trehan I, Goldbach HS, LaGrone LN, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med. 2013;368:425–435.
- Berkley JA, Ngari M, Thitiri J, et al. Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, randomised placebo-controlled trial. Lancet Glob Health. 2016;4:e464–e473.
- Oshikoya KA, Sammons HM, Choonara I. A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol. 2010;66:1025–1035.
- Dubray C, Ibrahim SA, Abdelmutalib M, et al. Treatment of severe malnutrition with 2-day intramuscular ceftriaxone vs 5-day amoxicillin. Ann Trop Paediatr. 2008;28:13–22.
- Trehan I, Amthor R, Maleta K, et al. Evaluation of the routine use of amoxicillin as part of the home-based treatment of severe acute malnutrition. Trop Med Int Health. 2010;15:1022–1028.
- Lazzerini M, Tickell D. Antibiotics in severely malnourished children: systematic review of efficacy, safety and pharmacokinetics. Bull WHO. 2011;89:594–607.
- Trehan I, Goldbach H, LaGrone L, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med. 2013;368:425–435.
- Yebyo H, Kendall C, Nigusse D, et al. Outpatient therapeutic feeding program outcomes and determinants in treatment of severe acute malnutrition in Tigray, Northern Ethiopia: a retrospective cohort study. PLoS ONE. 2013;8:e65840.
- M Chisti, M Salam, P Bardhan, et al. Treatment failure and mortality amongst children with severe acute malnutrition presenting with dough or respiratory difficulty and radiological pneumonia. PLoS One. 2015;10:e0140327. DOI:10.1371/journal.pone.0140327
- Trehan I, Maleta KM, Manary MJ. Antibiotics for uncomplicated severe malnutrition. N Engl J Med. 2013;368:2436–2367.
- Kotloff K, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222.
- Laterza L, Ianiro G, Scoleri I, et al. Rifaximin for the treatment of diarrhoea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2015;16:607–615.
- Rawson TM, Moore LS, Holmes A. Amoxicillin for severe acute malnutrition in children. N Engl J Med. 2016;375:190–191.
- Saha A. Does routine antibiotic therapy benefit children with severe acute malnutrition? Indian Pediatr. 2016;53:329–331.
- Wilkinson D, Boyd N. Reduction in in-hospital mortality of children with malnutrition. J Trop Pediatr. 1996;42:114–115.
- Fechner A, Böhme C, Gromer S, et al. Antioxidant status and nitric oxide in the malnutrition syndrome Kwashiorkor. Pediatr Res. 2001;49(2):237–243.
- Carrol ED, Mankhambo LA, Guiver M, et al. PCR improves diagnostic yield from lung aspiration in malawian children with radiologically confirmed pneumonia. PLoS ONE. 2011;6:e21042.
- Woerther P, Angebault C, Jacquier H, et al. Massive increase, spread and exchange of extended spectrum beta-lactamase-encoding genes among intestinal Enterobacteriaceae in hospitalised children with severe acute malnutrition in Niger. Clin Infect Dis. 2011;53:677–685.
- Pitout J, Laupland K. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166.
- Cremieux AC, Muller-Serieys C, Panhard X, et al. Emergence of resistance in normal human aerobic commensal flora during telithromycin and amoxicillin-clavulanic acid treatments. Antimicrob Agents Chemother. 2003;47:2030–2035.
- Moore RD, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;15:593–599.
- British National Formulary for Children. London: British Medical Journal Publishing Group and Royal Pharmaceutical Society; 2016. Available from: http://bnf.nice.org.uk/
- Jones KD, Hunten-Kirsch B, Laving AM, et al. Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial. BMC Med. 2014;12:357.
- Ignatius R, Gahutu JB, Klotz C, et al. High prevalence of Giardia duodenalis Assemblage B infection and association with underweight in Rwandan children. PLoS Negl Trop Dis. 2012;6:e1677.
- Heikens GT, Schofield WN, Christie CD, et al. The Kingston Project. III. The effects of high energy supplement and metronidazole on malnourished children rehabilitated in the community: morbidity and growth. Eur J Clin Nutr. 1993;47:174–191.
- Lares-Asseff I, Cravioto J, Santiago P, et al. A new dosing regimen for metronidazole in malnourished children. Scand J Infect Dis. 1993;25:115–121.
- Thuo N, Ungphakorn W, Karisa J, et al. Dosing regimens of oral ciprofloxacin for children with severe malnutrition: a population pharmacokinetic study with Monte Carlo simulation. J Antimicrob Chemother. 2011;66:2336–2345.
- Porco T, Gebre T, Ayele B, et al. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomised trial. JAMA. 2009;302:962–968.
- Church JA, Fitzgerald F, Walker AS, et al. The expanding role of co-trimoxazole in developing countries. Lancet Infect Dis. 2015;15:327–339.
- Bolme P, Eriksson M, Paalzow L, et al. Malnutrition and pharmacokinetics of penicillin in ethiopian children. Pharmacol Toxicol. 1995;76:259–262.
- Zerihun G, Ashton M, Eriksson M. Oral absorption of amoxicillin in ethiopian children with respiratory symptoms and different nutritional status. Acta Paediatr. 1991;80:972–974.