Abstract
Background: While bubble continuous positive airway pressure (bCPAP) is commonly used in low- and middle-income countries (LMIC) to support neonates with respiratory distress, there are limited non-invasive support options for non-neonatal children.
Aim: To demonstrate safety of a new device designed to support children during respiratory distress in LMIC.
Methods: A paediatric bCPAP device was designed called SEAL-bCPAP (Simplified Ear-plug Adapted-bCPAP). SEAL-bCPAP is constructed from inexpensive, easily obtainable materials. The nasal prong interface was modified from previously described neonatal bCPAP set-ups using commercial ear-plug material to improve nasal seal. A prospective interventional study was conducted to evaluate safety in children with respiratory distress treated with SEAL-bCPAP. Patients aged 30 days to 5 years presenting to a hospital in northern Uganda from July 2015 to June 2016 were screened. Those with moderate–severe respiratory distress and/or hypoxia despite nasal cannula oxygen were eligible for study. Enrolled patients were supported with SEAL-bCPAP until respiratory improvement or death. Complications attributable to SEAL-bCPAP were recorded. Clinical outcomes were compared with historical control pre-trial data.
Results: Eighty-three of 87 enrolled patients were included in the final analysis. No patients had significant SEAL-bCPAP complications. Five patients had mild complications which resolved (four with nasal irritation and one with abdominal distention). Trial patients had significant (p < 0.0001) improvement in their TAL score, respiratory rate and O2sat after 2 h of SEAL-bCPAP. Fifty-two of 64 patients (62.7%) with severe illness at Time1 did not have severe illness at Time2 (after 2 h of SEAL-bCPAP) (p < 0.0001). Unadjusted mortality rates were 12.2% (6/49) and 9.6% (8/83), respectively, for pre-trial (historical control) and trial patients (p = 0.64); the study was not powered to show efficacy.
Conclusions: The SEAL-bCPAP device is safe for treatment of respiratory distress in non-neonatal children in LMIC. There is a trend toward decreased mortality that should be evaluated with adequately powered clinical trials.
Abbreviations:
- ACU, acute care unit
- bCPAP, bubble continuous positive airway pressure
- BUBBLES, bCPAP used beyond babies in low economic settings
- cmH2O, cm of water
- CPAP, continuous positive airway pressure
- LMIC, low- and middle-income countries
- OR, odds ratio
- O2sat, oxygen saturation
- RR, respiratory rate
- SD, standard deviation
- SEAL-bCPAP, simplified ear-plug adapted low-cost bCPAP
- TAL score, modified TAL clinical score
Introduction
Pneumonia, the number one cause of mortality worldwide in children under age of five years, is estimated to cause two million paediatric deaths annually [Citation1]. Ninety-seven per cent of pneumonia cases occur in low- and middle-income countries (LMIC) [Citation2]. Respiratory support is central to reducing mortality from respiratory illnesses [Citation3]. In LMIC, respiratory support is often limited to low-flow nasal cannula or face mask. While these devices deliver an enriched oxygen concentration, they do not support respiratory effort. In contrast, in high-income countries, many technologically advanced treatment options are available to support respiratory effort. The study aimed to address the LMIC respiratory support gap. Bridging this gap ideally involves development of inexpensive devices that do not rely on electricity or intensive monitoring/skilled personnel.
Continuous positive airway pressure (CPAP) ventilation is widely used in treating respiratory insufficiency in paediatrics [Citation4–Citation6]. CPAP devices function optimally if there is adequate seal at the nose and/or mouth interface. ‘Bubble’ CPAP (bCPAP) is a form of CPAP previously used exclusively in neonates. bCPAP uses short nasal prongs as its interface [Citation7]. The expiratory resistance providing alveolar and airway stenting is delivered via a tube whose distal aperture is under water. The distance the tube is submerged is approximately equal to the pressure generated in the circuit [i.e. a tube aperture under 5 cm of water (H2O) creates about 5 cmH2O pressure]. When the patient exhales, the water bubbles, hence the name ‘bubble’ CPAP [Citation8,9]. bCPAP has been shown to be a viable, safe alternative to mechanical ventilation in neonates [Citation8,10–13], including in LMIC [Citation14]. Adverse events have included nasal tissue injury, abdominal distension and rarely pneumothorax [Citation8].
While commercially manufactured bCPAP devices cost about US$6000 [Citation11], simplified versions can be constructed inexpensively. These bCPAP devices are ideal in LMIC because they do not require electricity, attach to regular oxygen port or compressed air sources, are easy to assemble and do not require extensive training or monitoring [Citation7].
bCPAP is not used outside the neonatal period in high-income countries. However, in LMIC, because of a paucity of respiratory support options, it has occasionally been used to rescue older children, despite a lack of well demonstrated safety and efficacy. Anecdotal reports note that this therapy seems limited by the difficulty of creating a secure intranasal seal in older children. Clinical research on bCPAP beyond the neonatal period is limited to a few small observational studies showing efficacy in older infants with bronchiolitis [Citation15,16] and a handful of small clinical trials published in the last three years [Citation17–Citation20]. One randomised control trial in Bangladesh showed lower mortality with bCPAP than with low-flow oxygen in children with severe pneumonia [Citation21]. There have been no comprehensive studies of safety in this age group, but adverse events reported are similar to those in neonates.
The basis of the study was the hypothesis that a bCPAP device with a nasal interface modified for use in older children would provide hospitals in LMICs with a safe respiratory support option otherwise unavailable. Simple Ear-Plug Adapted Low-Cost bCPAP, ‘SEAL-bCPAP’, a bCPAP device with modified nasal prongs was designed. SEAL-bCPAP can be easily assembled on site in LMIC using readily available materials by local medical staff, costs under US$5 and can be run using any oxygen or compressed air source. The BUBBLES (bCPAP used beyond babies in low-economic setting) study was designed as a clinical trial to demonstrate the safety of SEAL-bCPAP. Additionally, trends in mortality rates using SEAL-bCPAP were evaluated as a pilot study for a larger, adequately powered efficacy study.
Methods
Approval
The study protocol and consent forms were approved by the institutional review boards of the University of Minnesota, Hennepin County Medical Center, Gulu Regional Referral Hospital and St Mary’s Lacor Hospital (affiliated with Gulu University and operates on behalf of the Uganda National Council for Science and Technology).
Consent
Patients carers were guided through an informed consent process. Consent forms were translated into the predominant local language. If a child needed imminent stabilisation, abbreviated verbal consent was obtained with written informed consent reviewed after stabilisation. Carers could remove the child from the study at any time.
Study design
BUBBLES was a prospective, non-blinded, non-randomised interventional study to evaluate the safety of SEAL-bCPAP, the modified nasal interface bCPAP device, during clinical use in children with respiratory distress in a low-resource setting. The study was powered to evaluate safety. Secondarily, clinical and mortality data were recorded and compared with historical data to evaluate efficacy.
Population
The trial was conducted in the paediatric acute care unit (ACU) at Gulu Regional Referral Hospital, a public government referral hospital in northern Uganda.
Time-frame
Historical data were collected from April 2015 to June 2015. Trial enrollment was from July 2015 to June 2016.
Inclusion criteria
Children aged 30 days to five years with respiratory distress were screened. Those with moderate or severe respiratory distress based on a calculated respiratory score (TAL score > 3) () [Citation22,23] or hypoxia [oxygen saturation (O2sat)<92%] despite low-flow oxygen were eligible for enrollment.
Table 1. Modified TAL clinical score [Citation22,23].
Exclusion criteria
Patients were excluded if they had a pneumothorax, congenital lung disease, cyanotic heart disease or nasal tissue injury/facial trauma/congenital anomaly that made the nasal interface unusable, active epistaxis, recent abdominal surgery, significant abdominal distension, agonal (inadequate gasping) respirations, Glasgow coma scale < 4 or in whom death was considered imminent.
Statistical analysis
The sample size was based on the safety analysis with a desired prevalence of <2% occurrence of the combined significant adverse event composite. For 1.5% precision (0.5–3.5% actual rate) and a confidence interval of 80%, the total number of patients needed to evaluate safety was estimated to be 87.
Unadjusted comparisons of pre-trial and trial patient demographic characteristics, morbidity rates, illness severity and clinical measures [respiratory rate (RR), TAL score, O2sat] were undertaken using χ2 tests for categorical variables and two-sample t-tests for continuous variables.
Paired t-tests were used to test the null hypothesis of no change from baseline (‘Time1’) to 2 h later (‘Time2’) in mean TAL score, RR and O2sat in the trial patients. McNemar’s χ2 test was used to test for change in categorical variables over time. Unadjusted associations between the binary outcome measures of death and severe illness with demographic and clinical measures were evaluated using χ2 tests for categorical variables and logistic regression models for continuous variables. Multiple logistic regression models were used to estimate odds ratios (comparing trial with pre-trial) for mortality and morbidity adjusted for age, gender, baseline RR and baseline morbidity.
A significance level (α) of 0.05 was used for all statistical tests, with p-values between 0.05 and 0.10 indicating marginally significant results. SAS version 9.4 was used for all statistical analyses.
Ethics
The study was designed and conducted in accordance with the current revision of the Declaration of Helsinki and the guidelines for protection of human subjects [Citation24]. The study population was vulnerable owing to poverty/limited resources. SEAL-bCPAP provided a level of respiratory support unavailable in this hospital and was designed specifically for this population. Ethics approval was granted by the Lacor Hospital Institutional Research and Ethics Committee (LHRIEC 063/09/14).
Equipment
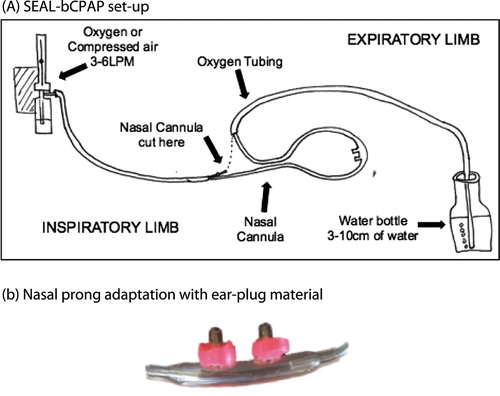
SEAL-bCPAP was constructed using inexpensive materials available in most LMIC. Materials included a simple nasal cannula, oxygen tubing, disposable water bottles, ‘superglue’, tape and commercial compressible ear-plugs (3 M™ tekk protection, Disposable Earplugs) ((A)). The oxygen source was cylinder or concentrator. The nasal interface was modified by stretching a small cut ring of compressible ear-plug material over the end of each nasal prong and attaching it with superglue ((B)). This material was pinched and held for a few seconds on the nasal prong. The prongs, with the compressed material, were slipped into the nares and allowed to expand to create a more occlusive intranasal seal. SEAL-bCPAP was designed to not rely on electricity or require intensive monitoring and cost about US$5. If needed, the apparatus could be cleaned and re-used, except the earplug material which was removed and replaced.
Figure 1. (A) SEAL-bCPAP set-up (device constructed from oxygen tubing, nasal cannula, plastic water bottle and tape/glue); (B). Nasal prong adaptation with ear-plug material.
Continuous pulse oximetry monitors (Nellcor™ N-395) and suction machines (DeVilbiss™ Vacu-Aide-Compact Suction Unit, 7310 Series) were donated for the study. This degree of monitoring was used for the purposes of the study but is not mandatory for routine use of bCPAP.
Nurse training
A dedicated BUBBLES trial team of six Ugandan nurses received interactive training. All nurses demonstrated SEAL-bCPAP assembly and troubleshooting. Scores between nurses varied by a maximum of one point during evaluation of respiratory scoring.
Definitions and scoring
Respiratory score definition
The ‘modified TAL clinical score’ (TAL score), a clinical scoring system not requiring laboratory data, was used to categorise the severity of respiratory distress. Four variables are evaluated: wheezing, retractions, RR and cyanosis. Each variable is scored on a scale of 0 to 3. The scores are added for a total of 0–12 points. The total score determines the severity of distress: ‘mild’ (0–3 points), ‘moderate’ (4–8 points) or ‘severe’ (9–12 points) [Citation22,23] ().
Adverse event scoring
Any clinical findings considered secondary to SEAL-bCPAP were documented and graded. Grade I findings were warning signs requiring intervention to avoid significant adverse events. Grade II findings were considered significant adverse events. Grade I signs included nasal tissue redness, mild readily controlled epistaxis, incidentally identified small pneumothorax without respiratory status change, and abdominal distension relieved by tube decompression. Grade II events included nasal tissue erosion beyond the epidermis, difficult to control epistaxis, clinically significant pneumothorax or pneumothorax > 10% of lung volume (diagnosis based on detection of unequal breath sounds and/or rapid decline in patient’s respiratory status and confirmed by chest radiograph), abdominal distension not relieved by tube decompression, aspiration, peritoneal signs and device fragmentation if tubing completely disconnected or ear-plug material dislodged into the nares.
Severity of illness
Severity of illness was assessed by the TAL score and O2sat, defined as mild (TAL score 4–5 and O2sat >90%), moderate (TAL score 6–7 or O2sat >85% but < 90%) or severe (TAL score ≥8 or O2sat <85%) ().
Table 2. Severity of illness.
Study protocol
Patients identified by the medical officer as having respiratory distress during standard acute care evaluation had their TAL score and O2sat checked by a BUBBLES nurse to determine clinical eligibility. If criteria were met and the carer consented, support with SEAL-bCPAP was commenced. All other medical therapy was provided by the hospital team. Patients were monitored by continuous pulse oximetry. Vital signs, TAL score and examination were recorded before initiation of therapy (Time1), two hours after SEAL-bCPAP initiation (Time2) and every six hours thereafter. Examinations specifically evaluated nasal tissue injury, epistaxis, abdominal distension, signs of pneumothorax and device fragmentation. Device function was checked at least every two hours. SEAL-bCPAP level started at 5 cmH2O and incrementally changed to a maximum of 10 cmH2O based on evaluation by a medical officer. SEAL-bCPAP was continued until the TAL score was <3 with ≤5 cmH2O, hypoxia had resolved and the medical officer agreed that the patient was stable. Patients were monitored in the ACU until discharge, transfer or death. Data were collected on outcomes and any acute adverse events possibly related to SEAL-bCPAP.
Historical data were collected three months before study enrollment when bCPAP was not yet available. The BUBBLES nurses monitored pre-trial patients who would have met the inclusion criteria and these patients were enrolled as historical controls.
Results
Study analysis
Of 101 patients considered for enrollment, 87 were enrolled and 83 were included in the final analysis. No patients refused enrollment. Fourteen patients met the exclusion criteria. Four patients were excluded after enrollment owing to subsequent diagnosis of cyanotic heart disease or chronic lung disease. Oxygen ran out for six patients part-way through their treatment; although SEAL-bCPAP was then terminated they were still included in the study analysis.
Enrolled patient characteristics
Patient characteristics are shown in and supplementary Tables 1 and 2. The study enrolled 44 boys and 39 girls. Mean age was 1.3 years (1 month to 4.8 years). Mean (SD) Time1 TAL score was 7 (1.5). Time1 illness was severe in 64/83 (77.1%) patients. Mean (SD) days receiving SEAL-bCPAP was 2.3 (1.4, range 2 h to 7 days).
Table 3. Baseline (Time1*) characteristics.
Primary outcome
Evaluation of safety
None of the 83 patients developed significant complications related to SEAL-bCPAP. Four developed mild (grade I) nasal tissue irritation which resolved with SEAL-bCPAP removal. Irritation was noted after two or more days on SEAL-bCPAP. One patient had mild abdominal distension that resolved with nasogastric tube decompression. All six patients who ran out of oxygen survived. Charts of deceased patients were reviewed with the nurse present at the time of death and deaths were unrelated to SEAL-bCPAP.
Mortality
The unadjusted trial mortality was 8/83 (9.6%) and seven of those who died had admission diagnoses of severe pneumonia. All the deceased met the criteria for severe illness and none had SEAL-bCPAP complications (Supplementary Table 3).
Secondary analysis
The power to detect associations with mortality was low because only eight patients died. Trial patients with a severe Time1 TAL score (≥8) had a higher unadjusted risk of death than those with a Time1 TAL score <8 (17.7% vs 4%, P = 0.04). Adjusted for age and gender, patients with a severe Time1 TAL score had marginally significant higher odds of death than patients with a Time1 TAL <8 (OR 4.5, P = 0.08).
Time1 to Time2 analysis
Trial patients had significant (P < 0.0001, paired t-test of mean change) improvements in TAL score, RR and O2sat after 2 h of SEAL-bCPAP. The severity of illness score also improved after 2 h of SEAL-bCPAP. Fifty-two of 64 patients (62.7%) with severe illness at Time1 did not have severe illness at Time2 (p < 0.0001). The net improvement in TAL scores from ‘severe’ to ‘not severe’ was significant (p < 0.0001) ().
Table 4. Trial patients Time1 (before SEAL-bCPAP) vs Time2 (two hours after SEAL-bCPAP commenced).
Pre-trial vs trial comparisons
Patient characteristics
Pre-trial database patient characteristics are shown in and Supplementary Table 1. Age and gender were not significantly different; however, severity of illness was significantly lower (p < 0.0001) for pre-trial patients ().
Mortality
Unadjusted mortality was 12.2% (6/49) for pre-trial patients and 9.6% (8/83) for trial patients (χ2 test, P = 0.64).
Time1 to Time2 analysis
Severity of illness at Time1 and Time2 were compared between pre-trial and trial patients (). At Time1, 77% of trial patients and 24.5% of pre-trial patients had severe illness. At Time2, 14.5% of trial patients and 18.2% of pre-trial patients had severe illness. Adjusted for Time1 illness severity, RR, age and gender, the odds of severe illness at Time2 were significantly lower for trial patients than for pre-trial patients (OR 0.19, P = 0.02) (Supplementary Table 4).
Table 5. Illness severity at Time1 and Time2 in trial and pre-trial patients.
Discussion
In LMIC, minimal paediatric respiratory support options exist. In affluent areas, non-invasive respiratory support with CPAP devices has become a standard treatment for children with respiratory distress. bCPAP, including low-cost versions, has been used safely and effectively for infant respiratory distress [Citation7,9–12], but has not been extensively used or studied in older children.
SEAL-bCPAP, the device constructed for this study, was similar to modified neonatal bCPAP except for the nasal interface. A material that was tissue-tested was chosen (commercial ear-plugs) and used to modify the nasal prongs to improve the seal. The primary objective in this study was to show safety of this new modification. The primary hypothesis was that SEAL-bCPAP complication rate would be similar to that noted with conventional CPAP and neonatal bCPAP. Study results confirmed this hypothesis. No significant adverse events, with only mild nasal tissue irritation and abdominal distension amenable to tube decompression were observed. As predicted, longer use of SEAL-bCPAP increased likelihood of nasal tissue injury.
The complication rate in this BUBBLES study was similar to those reported in the few other published studies [Citation17,20]. Specifically, during an H1N1 outbreak in India, bCPAP was used in 36 patients aged 5–36 months [Citation14] and for bronchiolitis in 35 patients aged <15 months in a tertiary care centre in New Zealand, with no adverse events noted in either study [Citation16]. As in this BUBBLES study, abdominal distension was noted in 9/229 (4%) children aged <5 years with severe pneumonia in a study comparing bCPAP, high-flow nasal cannula and low-flow nasal cannula [Citation21].
While this BUBBLES study was not powered to demonstrate efficacy, collected clinical data showed a trend towards decreased morbidity and mortality. Illness became significantly less severe after two hours of SEAL-bCPAP and there was a lower adjusted odds of death in patients treated with SEAL-bCPAP. This trend is not surprising. Before the study began, the highest level of respiratory support available at the hospital was low-flow oxygen. CPAP, whether bCPAP or conventional CPAP, works by maintaining a positive airway pressure throughout the breathing cycle, resulting in decreased effort in breathing and improved oxygenation [Citation4–Citation6]. It is predicted that this higher level of respiratory support improves outcome, and a few recent studies have demonstrated the clinical efficacy of similar bCPAP devices in this older age group [Citation18–Citation20]. One of the only randomised trials of bCPAP in children was terminated early owing to a significant decrease in mortality in children treated with bCPAP versus low-flow cannula [Citation21]. This BUBBLES study shows that after two hours of SEAL-bCPAP children had significantly improved respiratory scores and less severe illness. The modification of the nasal interface appeared to improve the nasal seal which created less leakage and would predictably improve CPAP delivery and lower the necessary oxygen flow rates needed.
Several factors in this study may have affected the degree of mortality/morbidity benefit. It is important to note that the trial patients had significantly more severe illness than pre-trial patients. The trend in decreased mortality in more severely ill patients and thus the benefit to overall mortality might be under-estimated. The greater severity of illness in trial patients is not fully understood. Despite the aim to collect data on all pre-trial patients with respiratory distress, there is concern that the more severely ill pre-trial patients may not have been captured owing to the severity of their illness. Without respiratory support, pre-trial children with severe illness may have died while awaiting evaluation/stabilisation or fled seemingly futile care. In this study’s trial phase, severely ill patients were screened and SEAL-bCPAP was commenced; thus, the enrolled patients were more severely ill and the pre-trial mortality rate might have been higher than documented.
Study limitations
Evaluation for complications was based on clinical evidence and therefore small pneumothoraces might not have been identified. The study was not a randomised controlled trial, nor was it powered or designed to evaluate the effectiveness of bCPAP in children outside the neonatal period. The pre-trial versus trial group differences raise questions for those evaluating the study despite the obvious clinical improvement seen at the bedside by the researchers. Patients with all-cause respiratory distress were enrolled. Because the study was not limited to respiratory distress caused by lung disease (i.e. pneumonia, bronchiolitis, asthma), there are more variables which could have contributed to patients’ overall course. This BUBBLES study was not designed to eliminate other factors (i.e. antibiotic use, nutritional status or blood products) which could contribute to differences in mortality since these should not significantly affect safety. Additionally, mortality data could be confounded by care provided by the dedicated BUBBLES team; however, the same nurses contributed to care of the pre-trial patients who were monitored for data collection purposes.
Additional contribution
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conclusions
To conclude, owing to the high mortality rate from respiratory diseases in LMIC, there is a great need for simple, low-cost, safe and effective respiratory support. SEAL-bCPAP safely provided respiratory support to children aged 1 month to 5 years. A larger, randomised control trial is required to truly demonstrate efficacy, but, based on the results of this pilot study, decreased mortality with SEAL-bCPAP use is expected.
Declarations
Availability of data and material: The datasets used and/or analysed in this study are maintained in a REDcap database and are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Thrasher Research Fund (Salt Lake City, UT); University of Minnesota; Pediatric Device Innovation Consortium (PDIC); Office of Discovery and Translation (ODAT) (Minneapolis, MN) and Masonic Children’s Hospital (Minneapolis, MN).
Notes on contributors
Ashley Bjorklund is currently the Medical Director of the Walter Reed National Military Medical Center pediatric intensive care unit. At the time of the study, she was completing a pediatric critical care fellowship at the University of Minnesota.
Beatrice Odongkara is a pediatric endocrinologist and the department head of paediatrics at Gulu Regional Referral Hospital in Uganda.
Marie Steiner is a pediatric intensivist, hematologist/oncologist and is the program director of the Pediatric Critical Care fellowship at the University of Minnesota.
Gwyneth Fischer is a pediatric intensivist at the University of Minnesota and is the founder of the Pediatric Device Innovation Consortium.
Cynthia Davey is a biostatistician at the University of Minnesota.
Tina Slusher is a pediatric intensivist at Hennepin County Medical Center and is a professor of pediatrics in the Pediatric Residency Global Health Program at the University of Minnesota. She has done extensive research on low-cost therapy for neonatal jaundice in Nigeria.
Supplemental data
Supplemental material for this article can be accessed https://doi.org/10.1080/20469047.2018.1474698.
Acknowledgments
We should like to thank our dedicated BUBBLES team study coordinator, Gloria Omoko Amongi, and study nurses Tonny Okino, Judith Aceng, Florence Amito, Geoffrey Okello and Babra Adong; also the administration and all the physicians/medical officers, nurses and support staff at Gulu Regional Referral Hospital, Gulu, Uganda without whom this study would not have been possible. We are grateful to the following who donated supplies: suction machines by Glen Kosirog (Kosirog Rexall Pharmacy and Homeware, Chicago, IL); pulse oximeters by Andrew Larson (Biomedical Engineering, Hennepin County Medical Center, Minneapolis, MN); and nasogastric tubes, nasal cannulas, gloves by Global Health Ministries (Minneapolis, MN). Finally, we should like to thank Joseph Hale, engineering consultant, University of Minnesota Medical Device Center, for assistance in device design, Abigail Faulman for assistance in nurse training and Hellen Aanyu-Tukamuhebwa, pulmonary consultant, Mulago Hospital and Makerere University, Kampala, Uganda.
References
- World Health Organization, UNICEF and Gavi Alliance. Pneumonia still responsible for one fifth of paediatric deaths, 2013. Available from: http://www.who.int/mediacentre/news/releases/2013/world-pneumonia-day-20131112/en/#.U07twPgptN8.email
- Schluger N. Pneumonia Global Impact. The Acute Respiratory Infections Atlas. 1st ed. World Lung Foundation; 2013. Available from: http://www.ariatlas.org/understanding_aris/pneumonia
- World Health Organization. MDG 4: Reduce Child mortality. 2013. Available from: http://www.who.int/topics/millennium_development_goals/child_mortality/en
- Brett A, Sinclair DG. Use of continuous positive airway pressure in the management of community acquired pneumonia. Thorax. 1993;48:1280–1281.10.1136/thx.48.12.1280
- Lindner KH, Lotz P, Ahnefeld FW, et al. Continuous positive airway pressure effect on functional residual capacity, vital capacity and its subdivisions. Chest. 1987;92:66–70.10.1378/chest.92.1.66
- Wilson P, Morris M, Biagas K, et al. A randomized clinical trial evaluating nasal continuous positive airway pressure for acute respiratory distress in a developing country. J Pediatr. 2013;162:988–992.10.1016/j.jpeds.2012.10.022
- Slusher T, Vaucher Y, Greenwood C, et al. Newborn care. In: Kamat DM, Fischer PR, editors. Textbook of global child health. Elk Grove Village, IL. American Academy of Pediatrics, 2012. p. 499.
- Rajiv PK. CPAP bedside application in the newborn. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2011.
- Manilal-Reddy PI, Al-Jumaily AM. The use of continuous oscillating positive airway pressure (bubble CPAP) to treat neonatal respiratory disease: an engineering approach. J Med Eng. 2009;33:214–222.
- Tagare A, Kadam S, Vaidya U, et al. Bubble CPAP versus ventilator CPAP in preterm neonates with early onset respiratory distress – a randomized controlled trial. J Trop Pediatr. 2013;59:113–119.10.1093/tropej/fms061
- Boyd J. Clinical study finds “bubble CPAP” boosts neonatal survival. Rice University News and Media. 2014. Available from: http://news.rice.edu/2014/01/29/clinical-study-finds-bubble-cpap-boosts-neonatal-survival-rates-2/
- De Klerk AM, De Klerk RK. Nasal continuous positive airway pressure and outcomes of preterm infants. J Paediatr Child Health. 2001;37:161–167.10.1046/j.1440-1754.2001.00624.x
- Kamath BD, MacGuire ER, McClure EM, et al. Neonatal mortality from respiratory distress syndrome: lessons for the low-resource countries. Pediatrics. 2011;127:1139–1146.10.1542/peds.2010-3212
- Kawaza K, Machen HE, Brown J, et al. Efficacy of a low-cost bubble CPAP system in treatment of respiratory distress in a neonatal ward in Malawi. PLoS One. 2014;9:e86327.10.1371/journal.pone.0086327
- Kinikar A, Kulkarni R, Valvi C, et al. Use of indigenous bubble CPAP during swine flu pandemic in Pune, India. Indian J Pediatr. 2011;78:1216–1220.10.1007/s12098-011-0389-x
- Pirret AM, Sherring CL, Tai JA, et al. Local experience with the use of nasal bubble CPAP in infants with bronchiolitis admitted to a combined adult/paediatric intensive care unit. Intensive Crit Care Nurs. 2005;21:314–319.10.1016/j.iccn.2005.06.009
- Kortz TB, Herzel B, Marseille E, et al. Bubble continuous positive airway pressure in the treatment of severe paediatric pneumonia in Malawi: a cost-effectiveness analysis. BMJ Open. 2017;7:e015344.10.1136/bmjopen-2016-015344
- Jayashree M, KiranBabu HB, Singhi S, et al. Use of nasal bubble CPAP in children with hypoxemic clinical pneumonia – report from a resource limited set-up. J Trop Pediatr. 2016;62:69–74.10.1093/tropej/fmv063
- Machen HE, Mwanza ZV, Brown JK, et al. Outcomes of patients with respiratory distress treated with bubble CPAP on a pediatric ward in Malawi. J Trop Pediatr. 2015;61:421–427.
- Walk J, Dinga P, Banda C, et al. Non-invasive ventilation with bubble CPAP is feasible and improves respiratory physiology in hospitalised Malawian children with acute respiratory failure. Paediatr Int Child Health. 2016;36:28–33.10.1179/2046905514Y.0000000166
- Chisti MJ, Salam MA, Smith JH. Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh; an open, randomised controlled trial. Lancet. 2015;386:1057–1065.10.1016/S0140-6736(15)60249-5
- Tal A, Bavilski C, Yohai D, et al. Dexamethazone and salbutamol in the treatment of acute wheezing in infants. Pediatrics. 1983;71:13–18.
- Shete S, Nagori G, Nagori P, et al. Relation between pulse oximetry and Clinical Score in Infants with acute bronchiolitis. Natl J Physiol Pharm Pharmacol. 2014;4:114–117.
- World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310:2191–2194.
