ABSTRACT
Background
In this paper we focus on medical device development (MDD) in Industrial Design Engineering (IDE) academia. We want to find which methods our MDD-students currently use, where our guidance has shortcomings and where it brings added value.
Methods
We have analysed 19 master and 3 doctoral MDD-theses in our IDE curriculum. The evaluation focusses around four main themes: 1) regulatory 2) testing 3) patient-centricity and 4) systemic design.
Results
Regulatory aspects and medical testing procedures seem to be disregarded frequently. We assume this is because of a lack of MDD experience and the small thesis timeframe. Furthermore, many students applied medical-oriented systemic tools, which enhances multiperspectivism. However, we found an important lack in the translation to the List of Specifications and to business models of these medical devices. Finally, students introduced various participatory techniques, but seem to struggle with implementing this in the setting of evidence-based medicine.
1. Introduction
Traditional new product development (NPD) approaches inherently apply to a broad set of industries. This general nature may turn them insensitive to the specificities of demanding contexts. For healthcare, with its high level of regulations, complex economics and a very diverse set of stakeholders, the need for detailed additions to general NPD approaches has been recognised in literature (Medina et al., Citation2012; Ocampo & Kaminski, Citation2019). Accordingly, different NPD models specifically for medical device design (MDD) have been proposed, often elaborating on the complex regulatory context (Kaplan et al., Citation2004; Ogrodnik, Citation2019; Pietzsch et al., Citation2009). These MDD models mostly apply to industrial companies, whose products are heavily influenced by economic and regulatory conditions. For academia, specific MDD models and programmes have been proposed, such as Stanford’s BioDesign Process (Yock et al., Citation2011) and TU Delft’s Medisign programme (Goossens et al., Citation2004). These models usually show the development process, but do not specifically cover the practical tools and methods to apply in MDD.
In the Industrial Design Engineering (IDE) curriculum at our university, we noticed an increasing popularity of MSc theses in MDD in recent years. Here, students develop a medical device for (and together with) a hospital, care institution or medical device company. The end result mostly is a proof-of-concept prototype and a written report. Our MSc students are coached by their university promotor and the external institution. Furthermore, they can fall back on general design and engineering courses from earlier in the curriculum. Our IDE programme offers a broad polytechnical education with a specialisation in industrial design engineering; specifics can be found in the Addendum. However, we currently do not offer specific courses on MDD in our IDE curriculum, nor is there a specialisation track. We wish to improve the thesis guidance of our MDD students and wish to be more efficient as promotors. Therefore, we have analysed the MDD theses from the past 8 years in this study. By investigating which tools our MDD students currently use, we may find where our guidance and curriculum show shortcomings and where they bring added value.
Our teaching curriculum, research and thesis supervision are influenced by the fields of Industrial Design Engineering and Systems Thinking. Both fields have a strong implementation in the healthcare sector. Nevertheless, their individual approaches cannot fully serve every problem. We hypothesise that a synergy arises when combining them. In this study, we quantified the implementations of these fields and their tools in MDD-related theses to get a better understanding of their added values.
1.1. Systems thinking in healthcare
Healthcare is a classic example of a complex sociotechnical system, as a whole of interactions between interdependent parts (Jones, Citation2013). Systems thinking approaches are widespread in healthcare and medical device management. In risk and quality management particularly, different models have been introduced that investigate the different levels of a system and the relationships between its components. Frameworks such as Systems Engineering Initiative for Patient Safety (SEIPS) have contributed in enhancing patient safety in a holistic manner (Carayon et al., Citation2006). Many of these models stem from systems and Human Factors Engineering (HFE), such as the Cognitive Work Analysis (Naikar, Citation2017). Here, the human factor is not analysed standalone but in its systemic context.
1.2. Industrial design engineering in healthcare
In IDE, user-centred approaches (e.g., Human Centred Design) often take a central role in the development process, making sure the user desires the product and is able to use it properly. In most MDD, user research is also structurally embedded via HFE, but this mostly is for a different reason. Here, usability tends to be investigated for patient safety and risk mitigation reasons, pushed by the high level of regulations (Benker, Citation2015). Although these are valid motives, the user acceptance aspect may be overlooked this way (Karsh, Citation2004). This is unfortunate, as it is crucial to detect and mitigate use errors and user rejection as early as possible, in order to reduce development costs. On the other hand, user-centred design often lacks a systemic perspective (Santos & Wauben, Citation2014; Sevaldson, Citation2010).
It is therefore suggested that the user-centred, empathic and pragmatic approaches of IDE may overcome the shortcomings of HFE in MDD (Barbero & Pallaro, Citation2017; Privitera, Citation2017; Privitera et al., Citation2015). Similarly, the HFE methods may tackle the lack of a systemic approach of user-centred approaches. Therefore, we believe it is useful to investigate how students implement these various approaches in MDD.
2. Materials and methods
We have collected and analysed all MDD-related theses from the Industrial Design Engineering specialisation, submitted between 2014 and 2021 at our local university. These 19 MSc and 3 PhD theses are listed in and are available throughout the institutional repository. Projects were identified as dealing with MDD if they met the EU Medical Device Regulations’ (REGULATION (EU) 2017/745, 2017) definition of a medical device. In brief, a medical purpose such as prevention, diagnosis, monitoring or treatment was required for the device. Projects that fell into the broader healthcare spectrum without medical purpose (e.g., commercial health wearables) were withdrawn from this selection.
Table 1. List of the analysed theses. In the second column, a short description of the thesis is given. The third column describes whether this was a MSc or a PhD thesis. In the fourth column, the type of client is given. We divided these into 3 main categories: medical device manufacturers, universities or university hospitals, and care institutions. The fifth column shows the academic year in which the thesis was submitted. In the sixth column, the anonymised code of the promotor is given. In the seventh column, the reference to the university website is given to find more details on the thesis.
For this qualitative study we followed the QUAGOL guideline, derived from Grounded Theory Approach (Dierckx de Casterle et al., Citation2012) In the first part of QUAGOL, documents are analysed iteratively and rich information is clustered into concepts. In this case, a list of MDD-related theses was composed. The four researchers then individually scanned the theses for tools, methods and guidelines where medical specificities were indispensable. The full methodological list was shared and discussed amongst the investigators. To structure the coding into concepts, the researchers together came up with 4 common themes after the first iteration: 1) regulatory aspects, 2) testing methods, 3) patient-centricity and 4) systemic design. In the second part of QUAGOL, the concepts are critically analysed by the group of reviewers. With the final concepts structured, documents are analysed again for conclusions. In this case, the MDD-related theses were divided amongst the investigators and read thoroughly. The investigators coded the information and finally discussed the outcomes for conclusions.
shows three general methodologies that we teach to students. Although they are general, this combination of methodologies is not taught in every IDE curriculum and is thus typical to our institution. We embedded them in as they presumably influence the work of our students, and as they are used to further structure the tools and methods in the Results section.
Table 2. Overview of general methodology in our IDE programme. Our curriculum and research combine three typical methodologies: research-through-design, co-design and systems thinking. In the third column, we linked each of these general methodologies to the main themes that were identified in this analysis: 1) regulatory aspects, 2) testing methods, 3) patient-centricity and 4) systemic design. This can also be found in .
3. Results
The quantitative results can be found in , supported by qualitative information in and . provides a schematic overview of the methodologies, tools and guidelines that were discussed in this analysis. This figure serves as a guide to see the relationships between the examined methods and tools. provides a structured summary of the different methods and tools that appeared (at least once) in the analysis. shows the frequency of the different methods and tools. The data of is analysed below.
Figure 1. Overview of methodologies and tools used in MDD at the Industrial Design Engineering specialisation at our university. The first row shows a simplified version of Pahl and Beitz’ Systematic Approach. This is our design workflow of choice at Ghent University, but as shown by Ocampo (Ocampo & Kaminski, Citation2019) different general design workflows are applicable in MDD. The second row shows the general approaches we actively teach that also may show added value to MDD. The 3rd row shows the 4 main aspects we focused on. Next to these aspects, the initials of the general approaches from the 2nd row are placed, showing which approach is typically used in the given aspect. In the 4th row, we placed the different tools and guidelines that were found in the analysis, sorted underneath the proper main aspect.
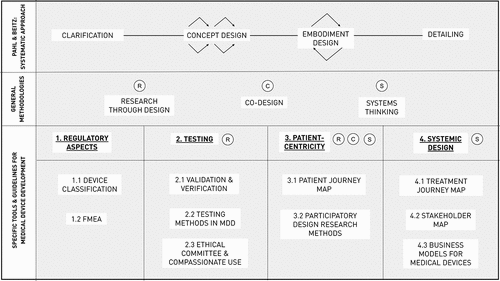
Table 3. Summary of the methods and tools. Each of these appeared at least once in the analysed theses. Column 1 shows the main theme to which the tool or method can be linked. This allocation was discussed amongst the researchers and is not exhaustive. Column 2 shows the specific tool, method (or guideline) that was analysed. A structured overview of the themes and their tools can be found in Figure 1. Column 3 offers a short description. Column 4 refers to related methods and tools, that were not found in the student theses but are also relevant to MDD. This non-exhaustive list was composed by researchers with experience in MDD.
Table 4. Count of the different methods and tools. We divided both the MSc and the PhD theses in 3 subgroups, differentiated by the client type. For the methodologies, tools and guidelines, we used the same structure as found in Figure 1.
3.1. Analysis
Medical regulatory aspects (device classification, MDR, FMEA, …) are often disregarded in the analysed MSc-theses. If the project gets picked up further (e.g., PhD-thesis or industrialisation), this may result in important and costly problems later on, as explained in the design paradox. Similar tendencies were found for medical testing. Little medical testing protocols were applied in the analysed cases.
Both Patient Journey Maps, Treatment Journey Maps and Stakeholder Maps were commonly used in the theses. We consider this as systemic MDD tools that support multiperspectivism. Nonetheless, we noticed that the wishes and demands of stakeholders, uncovered in these tools, were regularly overlooked in the List of Specifications.
Rather classic systemic tools such as causal loops and function-structure diagrams were used in a minority of theses. As for business models, we found a large frequency in theses for medical device companies whilst business models were underexposed in theses for universities, hospitals and care institutions.
3.2. Examples
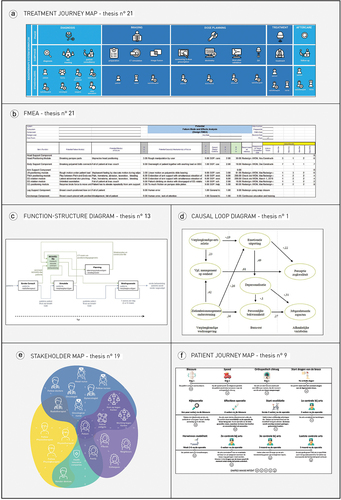
shows a selection of valid examples of different tools that were used in the analysed theses. gives further details on these examples and explains their added value.
Table 5. Thesis examples of different methods and tools for MDD. In this table, we highlighted some good examples of tools used in the analysed MDD theses. In the 4th column we explain the added value of this method or tool for the end result of this specific thesis, addressed by the student (in the thesis) or the reviewers. In the 5th column we explain the potential pitfalls of these tools, addressed by the reviewers. The examples are visualised in .
4. Discussion
By quantifying the tools and methods that our students use in MDD-related theses, we wanted to find trends and opportunities that could enhance the guidance of thesis students. With the results listed, we can now explain the observed trends and make recommendations for MDD-related theses.
In many theses (n° 1–13, 15, 18–19), we encountered an important lack in anticipating regulatory requirements. We assume that the short timeframe (10 months at 40% of their total workload) and limited experience of students may evoke other priorities. A big regulatory lack was found in theses for hospitals, presumably as they’re not primarily focussed on marketable products. Students often referred to the slow nature of clinical trials, their large administrative workload and the difference in priorities between the student and the medical researchers. In theses with medical device manufacturers however, the regulatory side wasn’t highlighted that often either. We presume that this is because these companies have in-house knowledge to further tackle regulatory issues. Altogether, we would suggest a short introduction of the most important regulatory aspects to thesis students and a structural integration in their List of Specifications. This is important, as projects may suffer from regulatory flaws in further development stages.
As for the use of systemic design, we noticed that some medical-oriented systemic tools (e.g., Stakeholder Mapping) were popular (n° 1, 3–5, 8–15, 17, 19, 21–22), but that general systems thinking tools (e.g., function-structure diagrams) were not widely applied (n° 1–2, 8, 13, 21–22). Systemic tools may be useful in complex settings for different applications such as problem finding, problem reframing, future forecasting and value communication (Peters, Citation2014). However, understanding and interacting with systems can take a lot of time and effort. For our master theses, the timeline may be too limited to elaborate on systemic interactions. We should therefore aim for time-efficient systemic tools in MDD, like Treatment Journey Mapping.
Furthermore, we found that participatory design methods were applied often (n° 1–2, 4–6, 9–10, 12–13, 16–22), but classic medical testing procedures were applied less (n° 2, 10–12, 14, 18, 20–22) . For industrial design engineers, these different research approaches may be difficult to cope with (Groeneveld et al., Citation2018). Whilst medical testing relies on strict protocols and quantitative data, participatory techniques are less defined and open to the researcher. It may be difficult to apply these two opposed ways of thinking, and also to convince medical staff of their added value as they’re not used to these rather subjective methods. Nevertheless, both research methods are of important value in MDD. Participatory techniques support in obtaining end user acceptance, while traditional medical testing (“evidence-based medicine”) is vital to obtain an effective, safe end product that also surpasses the regulatory requirements. For the students, it can be useful to anticipate on further medical testing in the future work section of the thesis. is an adapted model of the Verification and Validation model, that originates from the FDA’s Design Control. Here, it is shown how this regulatory framework can serve as a template for designerly tools, bridging both approaches. shows a list of MDD testing methods, adapted from Stanford’s Biodesign process (Yock et al., Citation2015). This summary may help IDE students to efficiently overcome differences in both approaches.
Figure 2. Validation & Verification (Design Control) as a template for designerly tools in MDD (adapted from (Privitera et al., Citation2015).The work of professor Privitera shows the possibility of using the classical Design Control figure as a template to map different design techniques upon. It is noteworthy that next to the clinical and technical requirements, participatory techniques can play a useful role in uncovering user needs. This may lead to a usable and desirable product, as checked by the Validation process. Furthermore, the active testing of prototypes is an important step to valorise the overall functioning of the product, as checked by the Verification process. For this process, we have described the benefits of using a Research through Design approach in a co-design trajectory.
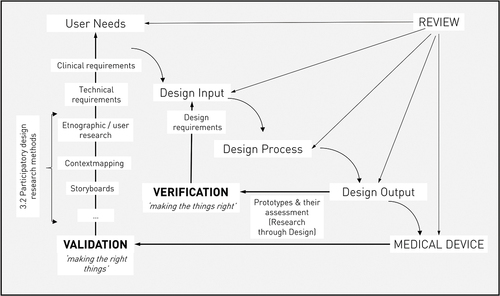
Figure 3. Testing methods in MDD (adapted from (Yock et al., Citation2015)). The first row shows the simplified “Levels of evidence in healthcare design”, which give students notice of the ways healthcare professionals determine evidence. The second row shows the “Testing continuum”, a schematic overview of the different testing procedures in MDD and their respective order. The “Testing continuum” is not limited to medical devices, whereas the “Key R&D” milestones (3rd row) provides a chronological order of important testing milestones that relate to medical devices.
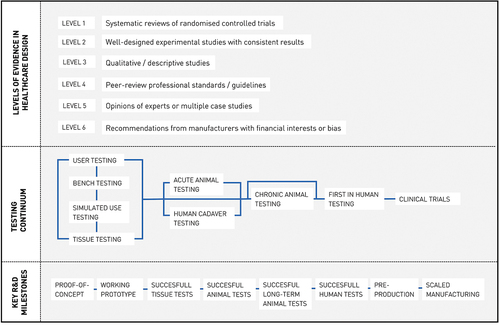
Figure 4. Visual examples of tools used in the examined theses. The letters (A-F) 406 correspond with the detailed explanation given in .
By implementing co-design and participatory design methods, we believe students facilitate multiperspectivism in a medical context with different types of stakeholders. This may support the user acceptance and usability. However, we also found that the requirements of these various stakeholders are not all translated into the List of Specifications. This is a point of attention, as not only the patient and other main stakeholders have a large influence on the medical product and its underlying processes. Furthermore, we found that only students at medical device companies paid attention to the business model behind the product. This seems evident, as these companies focus on marketable products whereas hospitals and care institutions do not. Also, it can be difficult to envision the financial mechanisms as a lot depends on the reimbursement of the device, which is not yet clear in the development phase. Nevertheless, we think it’s unfortunate that students take the effort of portraying all stakeholders, but do not put them together in a business model. This not only involves the financial aspects but also other forms of value exchange: who handles and owns the medical data, can used products be remanufactured, …
We could not distinguish any clear trends over time, except for the Patient Journey Map that became more widespread, whilst the Stakeholder Map was the overall most frequent tool. Furthermore we also examined the influence of the promotor on the use of tools. We did not find any clear differences, but we found that some promotors guided increasingly more MDD-related theses over time. This experience presumably adds to the efficiency of the guidance. A last comparison can be made between MSc and PhD theses, although the sample size of the PhD theses is too low for generalisations. We found that the PhD theses used a larger variety of tools, which is presumably facilitated by the larger timeframe (>4 years versus 1 year). The impact of design implementations could also be better evaluated as there is a larger timespan. PhD students are also expected to spend more time on scientific work, which is noticeable in the theses. Here, the experiments were better performed, based with less subjective and more scientific outcomes. The creative R&D-process was more pronounced in the MSc-theses.
Finally, we want to discuss the shortcomings of this analysis. The sample size (n = 22) was rather small, and the analysis may be prone to bias as our curriculum logically influences the theses results and ourselves as researchers. As for the future, it would be useful to incorporate the findings of this manuscript in guiding new thesis students. Furthermore, we would like to continue this evaluation to obtain bigger sample sizes, and to compare it to analyses of other (design) engineering curricula. Also, we would like to include interviews with students, to uncover why certain tools were (not) applied.
5. Conclusions
In this case-study based analysis, we screened 22 MDD-related theses of industrial design engineers for the methods and techniques that were applied. In the end, we wanted to find the added value and pitfalls of different methods that demand extra attention from our part as promotors.
Regulatory aspects and medical testing procedures seem to be disregarded frequently in theses, although these are of great importance to a sound MDD. We assume the small thesis timeframe plays an important role, next to limited experience with MDD. Furthermore, many students applied medical-oriented systemic tools such as treatment journey mapping, which enhance multiperspectivism. However, we found an important lack in the translation of these multidisciplinary insights to the list of specifications and business models for medical devices. Finally, students introduced various participatory techniques, which enhances the usability aspect of different stakeholders. However, it seems that students working for hospitals struggled to fully implement these participatory techniques in settings of evidence-based medicine. The insights of this paper may be of interest to other academic curricula, including but not limited to: mechanical engineering, biomedical engineering and medicine curricula.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Barbero, S., & Pallaro, A. (2017). Systemic Design for Sustainable Healthcare. Design Journal, 20(sup1), S2473–S2485. https://doi.org/10.1080/14606925.2017.1352762
- Benker, A. (2015). Design for Usability of Complex Medical Devices: Leading a Technology-Push Innovation Towards User Acceptance. Journal of Design, Business & Society, 1(1), 29–42. https://doi.org/10.1386/dbs.1.1.29_1
- Boru, A., Joore, P., Smulders, F., Dijkstra, A., & Goossens, R. (2015). Visualization of Systems and Stakeholders in Health Care Innovation by means of a Multilevel Design Model. September, 13–16.
- Carayon, P., Schoofs Hundt, A., Karsh, B. T., Gurses, A. P., Alvarado, C. J., Smith, M., & Brennan, P. F. (2006). Work system design for patient safety: The SEIPS model. Quality & Safety in Health Care, 15(SUPPL. 1), i50–i58. https://doi.org/10.1136/qshc.2005.015842.
- Dierckx de Casterle, B., Gastmans, C., Bryon, E., & Denier, Y. (2012). QUAGOL: A guide for qualitative data analysis. International Journal of Nursing Studies, 49(3), 360–371. https://doi.org/10.1016/j.ijnurstu.2011.09.012
- Durfee, W. K., & Iaizzo, P. A. (2018). The medical device innovation process. In Engineering in Medicine: Advances and Challenges. Elsevier Inc. https://doi.org/10.1016/B978-0-12-813068-1.00019-1
- Goossens, R. H. M., Lange, J. F., & Kleinrensink, G. J. (2004). MEDISIGN: Educating designers for the operating room. Minimally Invasive Therapy and Allied Technologies, 13(3), 185–190. https://doi.org/10.1080/13645700410033544
- Greig, M., Salustri, F., Village, J., Zolfaghari, S., & Neumann, W. P. (2014). Adapting Engineering Design Tools to Include Human Factors. IIE Transactions on Occupational Ergonomics and Human Factors, 2(1), 1–14. https://doi.org/10.1080/21577323.2014.905884.
- Griffioen, I. P. M., Rietjens, J. A. C., Melles, M., Snelders, D., Homs, M. Y. V., van Eijck, C. H., & Stiggelbout, A. M. (2021). The bigger picture of shared decision making: A service design perspective using the care path of locally advanced pancreatic cancer as a case. Cancer Medicine, 10(17), 5907–5916. https://doi.org/10.1002/cam4.4145
- Groeneveld, B., Dekkers, T., Boon, B., & Olivo, P. D. (2018). Challenges for design researchers in healthcare. Design for Health, 2(2), 1–22. https://doi.org/10.1080/24735132.2018.1541699
- Jones, P. (2013). Design for care: innovating healthcare experience. Louis Rosenfield. https://rosenfeldmedia.com/books/design-for-care/.
- Jones, P., Shakdher, S., & Singh, P. (2017). Synthesis maps: Visual knowledge translation for the canIMPACT clinical system and patient cancer journeys. Current Oncology, 24(2), 129–134. https://doi.org/10.3747/co.24.3452
- Kaplan, A. V., Baim, D. S., Smith, J. J., Feigal, D. A., Simons, M., Jefferys, D., Fogarty, T. J., Kuntz, R. E., & Leon, M. B. (2004). Medical device development: From prototype to regulatory approval. Circulation, 109(25), 3068–3072. https://doi.org/10.1161/01.CIR.0000134695.65733.64
- Karsh, B. T. (2004). Beyond usability: Designing effective technology implementation systems to promote patient safety. Quality & Safety in Health Care, 13(5), 388–394. https://doi.org/10.1136/qshc.2004.010322.
- Kleinsmann, M., Sarri, T., & Melles, M. (2018). Learning histories as an ethnographic method for designing teamwork in healthcare CoDesign International Journal of CoCreation in Design and the Arts Learning histories as an ethnographic method for designing teamwork in healthcare Maaike Kleinsmann , Tom. CoDesign, 00(00), 1–19. https://doi.org/10.1080/15710882.2018.1538380
- McCarthy, S., O’Raghallaigh, P., Woodworth, S., Lim, Y. L., Kenny, L. C., & Adam, F. (2016). An integrated patient journey mapping tool for embedding quality in healthcare service reform. Journal of Decision Systems, 25(1), 354–368. https://doi.org/10.1080/12460125.2016.1187394
- Medina, L. A., Kremer, G. E. O., & Wysk, R. A. (2012). Supporting medical device development: A standard product design process model. Journal of Engineering Design, 24(2), 1–37. https://doi.org/10.1080/09544828.2012.676635
- Naikar, N. (2017). Cognitive work analysis: An influential legacy extending beyond human factors and engineering. Applied Ergonomics, 59, 528–540. https://doi.org/10.1016/j.apergo.2016.06.001
- Norman, D. A., & Stappers, P. J. (2015). DesignX: Complex Sociotechnical Systems. She Ji, 1(2), 83–106. https://doi.org/10.1016/j.sheji.2016.01.002
- Ocampo, J. U., & Kaminski, P. C. (2019). Medical device development, from technical design to integrated product development. Journal of Medical Engineering & Technology, 43(5), 287–304. https://doi.org/10.1080/03091902.2019.1653393
- Ogrodnik, P. (2019). Medical device design (2nd ed.). Elsevier.
- Peters, D. H. (2014). The application of systems thinking in health: Why use systems thinking?. Health and Quality of Life Outcomes, 12(1), 1–6. https://doi.org/10.1186/1478-4505-12-51_old
- Pietzsch, J. B., Shluzas, L. A., Paté-Cornell, M. E., Yock, P. G., & Linehan, J. H. (2009). Stage-gate process for the development of medical devices. Journal of Medical Devices, Transactions of the ASME, 3(2). https://doi.org/10.1115/1.3148836
- Privitera, M. B. (2017). Designing Industrial Design In The Highly Regulated Medical Device Development Process. Defining our valuable contribution towards usability. Design Journal, 20(sup1), S2190–S2206. https://doi.org/10.1080/14606925.2017.1352735.
- Privitera, M. B., Southee, D., & Evans, M. (2015). Collaborative design processes in medical device development. The Value of Design Research - European Academy of Design Conference, 11 (Paris: Paris Descartes University), 1–12. https://doi.org/10.13140/RG.2.1.2711.4967
- REGULATION (EU) 2017/745. (2017). https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R0745&from=NL
- Sanders, L. (2008). ON MODELINGAn evolving map of design practice and design research. Interactions, 15(6), 13–17. https://doi.org/10.1145/1409040.1409043
- Sanders, E. B.-N., & Stappers, P. J. (2008). Co-creation and the new landscapes of design. CoDesign, 4(1), 5–18. https://doi.org/10.1080/15710880701875068
- Santos, A. L., & Wauben, L. S. G. L. (2014). Systems design perspective of healthcare provision in humanitarian aid. FormAkademisk - Forskningstidsskrift for Design Og Designdidaktikk, 7(3), 1–19. https://doi.org/10.7577/formakademisk.790
- Sevaldson, B. (2010). Discussions & movements in design research A systems approach to practice research in design. Form Akademisk, 3(1), 8–35. https://doi.org/10.7577/formakademisk.137
- Shah, S. G. S., & Robinson, I. (2007). Benefits of and barriers to involving users in medical device technology development and evaluation. International Journal of Technology Assessment in Health Care, 23(1), 131–137. https://doi.org/10.1017/S0266462307051677
- Stappers, P., Sleeswijk Visser, F., Keller, A.I. (2014). The role of prototypes and frameworks for structuring explorations by research through design. In P. Rodgers & J. Yee (Eds.), The Routledge Companion to Design Research, 1(1), (Routledge: Taylor & Francis), pp. 163–174. https://www.routledge.com/The-Routledge-Companion-to-Design-Research/Rodgers-Yee/p/book/9781138310247.
- Yock, P. G., Brinton, T. J., & Zenios, S. A. (2011). Teaching biomedical technology innovation as a discipline. Science Translational Medicine, 3(92). https://doi.org/10.1126/scitranslmed.3002222
- Yock, P. G., Zenios, S. A., Makower, J., Brinton, T. J., Kumar, U. N., Watkins, J., Denend, L., Krummel, T., & Kurihara, C. Q. (2015). Biodesign: The Process of Innovating Medical Technologie (2nd ed.). Cambridge University Press.
- Zimmerman, J., Stolterman, E., & Forlizzi, J. (2010). An analysis and critique of research through design: Towards a formalization of a research approach. DIS 2010 - Proceedings of the 8th ACM Conference on Designing Interactive Systems, 310–319. https://doi.org/10.1145/1858171.1858228
