ABSTRACT
Cancer is a leading cause of mortality, with 10 million deaths in 2020. With the number of people impacted by cancer projected to increase, a better-integrated cancer care is needed. Evidence suggests that Hospital-Based Cancer Registries (HBCRs) that collect administrative and clinical data could improve integrated and equitable evidence-based care. However, the state and HBCR’s role in the delivery of integrated cancer care for improved health outcomes, particularly in low- and middle-income countries (LMICs), is poorly understood and is assessed in this scoping review. A systematic search was conducted in April 2020. Thirty articles were included. This review found that while HBCRs have been implemented in several countries, few studies have evaluated the quality and effectiveness of registries, especially in LMICs. HBCRs in LMICs function more as data collection tools than information systems to influence clinical care decisions and monitoring, missing the opportunity to guide cancer care priorities and policies.
1. Introduction
More than 10 million people died in 2020 due to cancer (WHO, Citation2021). The global burden of cancer continues to increase and is projected to result in over 29 million cancer cases and over 16 million deaths per year by 2040 (GLOBOCAN, Citation2018). Reducing premature mortality due to non-communicable disease by one-third by 2030 will only be possible by strengthening the current health systems and reducing the evidence-to-practice gap (SDG 3.4, 3.8) (UN, Citation2015). Given the projected increase in the cancer burden globally, there is a need for effective integrated cancer care across the pathway, from diagnosis to treatment and follow-up, which is accompanied by appropriate supportive care. This necessitates effective recording and monitoring processes to strengthen appropriate supportive care. Cancer registries systematically collect, manage, and report cancer patient data. There are two main types of cancer registries: population-based cancer registries (PBCR) and hospital-based cancer registries (HBCR). Population-based cancer registries collect data on all new cases within a population and provide statistics on the occurrence of cancer within that population. HBCRs record information on cancer patients at a particular hospital. These collect crucial administrative and clinical information on patients, including the patient’s treatment regimen and its outcome (IRAC Citationn.d.; Bray et al., Citation2014).
Existing literature highlights the importance of information systems such as Electronic Health Records and provides valuable lessons from both utilisation and provision perspectives of such information systems for improving equitable care (Berg et al., Citation2022; Paul et al., Citation2012; Tulu et al., Citation2016; Weeger & Gewald, Citation2015). The role of information systems such as HBCRs in this process has gained attention in the past decade, especially in some high-income countries. HBCRs can potentially help support a better understanding of healthcare systems, the process of cancer care, the clinical endpoints and patient outcomes of care (Noda-Narita et al., Citation2021; Pandey et al., Citation2019; Ruiz & Facio, Citation2004). A lack of engagement with a uniform functional disease surveillance system leads to the under-representation of vulnerable regions and populations, resulting in ineffective policies addressing this disease burden (Bakouny et al., Citation2020). Accumulating evidence suggests thatHBCRs that collect administrative and routine clinical data can potentially improve the provision of integrated evidence-based and equitable care (Chen et al., Citation2018; Mohammadzadeh et al., Citation2017). However, very little is known about the role of HBCRs in managing cancer care at hospital-, regional-, or country-level cancer care, particularly in low- and middle-income countries (LMIC).
It is essential to focus on LMICs as they report 57% of new cancer cases and 65% of global cancer deaths Torre et al., Citation2015. The incidence-to-mortality ratio in these settings is high due to screening at the latter stages of cancer and a lack of monitoring of cancer care. Recent evidence further suggests that cancer care, from screening to follow-up care, has been significantly disrupted due to the COVID-19 pandemic in several countries (Jazieh et al., Citation2020). Despite the physiological, psychological and economic impacts of cancer on patients and their families, little evidence exists on the role of HBCRs in managing cancer care in LMICs. This review aims to understand the current state and the role of HBCRs in delivering integrated cancer care in resource-restrained LMICs. It is vital to address the heterogeneity in the type of data collection (manual vs electronic), staff requirements, training of personnel and cost implications. Given the absence of research in LMICs, we also included studies from HIC to determine if the evidence from HICs can inform any solutions to improving integrated cancer care in LMICs. Besides, comparing evidence between LMIC and HIC contexts might help us identify research and policy gaps.
2. Methods
A scoping literature review (Arksey & O’Malley, Citation2005; Munn et al., Citation2018) was conducted through systematic identification and analysis of all relevant literature. This review consists of five stages − 1) identification and formulation of the research questions; 2) identification and selection of relevant studies; 3) data extraction, 4) charting and analysis of data, and 5) summarising and reporting the results.
2.1. Search terms
Systematic searches were conducted using MeSH (Medical Subject Headings) terms and relevant keywords to identify all studies related to HBCRs. Search terms and synonyms (hospital OR hospital-based) AND (Cancer OR tumor OR tumour) AND (registry OR registries) OR (hospital-based cancer registry) were searched in Medline using PubMed (Title/Abstract) and ProQuest that included 17 databases (Abstract) in April 2020. Studies were limited to the English language. In addition, reference lists of included studies and Google Scholar were searched for any grey literature, relevant policy documents, and reports. These were then considered for inclusion if relevant.
2.2. Inclusion criteria
Studies that reported any data on HBCRs and health systems, cancer care pathways, or clinical cancer guidelines were included. All primary studies, including reports accessible in English, irrespective of study design, were included in the scoping review to gain insight into the existing literature on HBCRs and how they relate to cancer care and/or patient outcomes. PRISMA guidelines for scoping reviews (Tricco et al., Citation2018) were followed. As this is a scoping review, the quality of individual articles was not assessed. However, following PRISMA guidelines, the type of data source and context has been included (Tricco et al., Citation2018).
2.3. Study selection
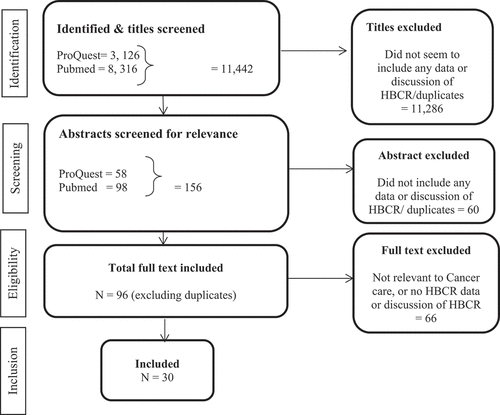
The searches by ST identified 11,442 citations. Of these, 11286 were excluded after title screening because they did not refer to data inclusion, discuss HBCRs, or were duplicate studies. Scanning of the remaining abstracts identified 156 studies that were deemed relevant. Further detailed scrutiny and discussion among co-authors excluded 60 abstracts as they did not meet the inclusion criteria. ST and AG reviewed full texts of 96 potentially eligible studies, and 66 articles were excluded because the studies did not discuss any aspect of HBCR data in cancer care or data systems. However, HBCR was either mentioned in their abstract or was referred to in the paper. Thirty articles were considered relevant and included in this scoping review. below shows the literature screening flowchart.
2.4. Data extraction
ST conducted the search and screened the titles/abstracts using inclusion and exclusion criteria. The final inclusion list was consolidated through discussions by the research team. ST extracted data on all included studies using a standard form developed and piloted using the SPIDER framework (Cook et al., Citation2012), and AG double-checked this. For each study (where applicable), the data extraction form included author(s), year of publication, journal, title, setting, source origin/country of origin, study population (age, type of cancer and/or sample size if applicable), the phenomenon of interest/aim, study design, key findings, and evaluation of outcomes and research type.
2.5. Data analysis
A narrative synthesis summarising the available data and identifying patterns across studies was deemed appropriate due to heterogeneity in the study designs for this scoping review (Popay et al., Citation2006). Using our review questions, we identified relevant phenomena by organising our studies into groups. First, for each included study, a descriptive summary of data was produced (Appendix 1). This process helped to identify and map emerging themes that were relevant to the objectives of this review. During the analysis phase, several discussions were held within the research team to reflect on the data and be iterative in the analysis to balance the data and emergent themes. Four questions guided the analysis of each article: What data are collected by the (HBCR) at a local, regional, or national level and for what purpose? How are the HBCR data used within cancer care? How is the quality of HBCR data evaluated/determined? Have any barriers or facilitators in the use of HBCRs in cancer care been identified? The final set of key concepts deemed relevant for identifying the gap in the literature on HBCRs was reviewed and agreed upon by all members of the research team. The countries were categorised into HIC and LMIC in accordance with the Development Assistance Committee (DAC) list of official development assistance (ODA) recipients based on Gross National Income (GNI) per capita as published by the World Bank at the time of the study.
3. Results
The 30 included articles, which were published between 1991 and 2020, included systematic reviews, quantitative studies, mixed-methods studies, and commentaries. The studies differed in their focus, methods, location, and sampling. The included HIC primary studies were conducted in Australia (3), Denmark (1), Estonia (1), Germany (1), the USA (6), Japan (4), The Netherlands (1), and Singapore (1) and the LMICs were conducted in India (2), Iran (1), Nepal (1), Nigeria (2), and Pakistan (1). One primary study included multiple African countries, and four were reviews of studies from various countries. There were 17 quantitative studies, three mixed-methods studies, three literature reviews, and seven narrative review/overview studies.
Three key themes are presented in the narrative synthesis: the nature of HBCR data collected, the use of HBCR data, and the quality of HBCR data (see ). For the purpose of this paper, where the number of studies is not mentioned, “a few” indicates <6, “several”/”some” indicates 6–15, and “many” indicates 16–30 studies. All papers, regardless of their design, are referred to as ”study” or ”studies”. Most of the studies included in this review focused on HICs. Only seven studies focused on LMICs, and two global reviews had LMICs in their studies. The findings are structured into three sections (3.1–3.3), based on three key themes that present the state of HBCRs in HICs and how they compare to the needs and limitations of current systems highlighted by studies in LMICs to examine whether the current HIC systems provide guidance or solutions.
Table 1. Study characteristics.
3.1. Nature of data collected by HBCRs
The first theme highlights the heterogeneity in the range of variables, level of data collection, and the method of data collection, which are discussed in detail below.
3.1.1. Range of variables
Most of the HIC studies (N = 20) in this review demonstrated that HBCRs typically collect (or intend to collect) a range of data, including demographics and details of cancer care across the care pathway. Cancer-related information typically starts with details on the date and type of diagnosis and prognosis, treatment course, treatment response, adverse events, and discharge date. A smaller proportion of HBCRs collected detailed biomarker information (Coory et al., Citation2009; Mohammadzadeh et al., Citation2017). Only a few studies followed up with patients for additional information, including cancer recurrence and additional treatment and mortality data.
Only three out of eight LMIC studies highlighted that data variables collected by these registries are consistent with HBCRs in HICs. These studies collected comparable socio-demographic variables and clinical data, including diagnosis and tumour information, treatment, and care (Jedy-Agba et al., Citation2012; Mohammadzadeh et al., Citation2017; Shrestha et al., Citation2019). Mohammadzadeh et al. (Citation2017) indicated that the design and performance of the HBCRs vary widely in different countries.
To improve the design, feasibility, and use of HBCRs in LMICs, Valsecchi and Steliarova-Foucher (Citation2008) argued that those responsible for HBCRs should ensure the collection of essential data (such as “patients” demographics, diagnosis, treatment, and outcome).
3.1.2. Level and design of data collection
Coory et al. (Citation2009) suggested that in order to provide feedback about all patients, not just those in larger academic hospitals with the most developed data systems, a sustainable data system such as the HBCR that captures information on prognostic factors at the time of initial diagnosis and information on the management of the disease progression is required. Only four HIC studies discussed limitations and highlighted that data collected by these registries are often not longitudinal, and there are gaps in data linkage across the care pathways as patients, after discharge from an HBCR hospital, could end up receiving follow-up care in another hospital (Bickell & Chassin, Citation2000; Higashi et al., Citation2014; Lang et al., Citation2003; Penberthy et al., Citation2003). None of the LMIC studies focused on research design. One LMIC study argued that establishing and sustaining HBCRs in urban and rural regions is crucial for producing cancer registrations with good population reach in LMICs (Curado, Citation2019).
3.1.3. Method and tools of data collection
Eleven of the included HIC studies discussed the tools of data collection. HBCR data can be paper-based, electronic, or a combination of both. Eleven of the included HIC studies addressed the method of data collection. Our review also found that these studies argued the need for electronic and comprehensive data that can be easily accessed (Anema et al., Citation2013; Hendren et al., Citation2014; Piccirillo et al., Citation2004; Roder et al., Citation2018; Shiki et al., Citation2008; Stangl et al., Citation2020; Voith von Voithenberg et al., Citation2019). A combination of manual and computerised cancer registry data was also considered to be the optimum solution for a web-based cancer registry where technological limitations may not allow for complete digitisation of the cancer registry (Huang et al., Citation2010).
Four LMIC studies (Jedy-Agba et al., Citation2012; Mohammadzadeh et al., Citation2017; Shrestha et al., Citation2019; Valsecchi & Steliarova-Foucher, Citation2008) suggested existing methods of data collection, such as paper forms or lack of digitisation of registry, could be a barrier for comprehensive coverage and quality control of the collected data.
Bickell and Chassin (Citation2000) investigated the accuracy of cancer registry data by comparing their data with data collected from numerous other sources for a breast cancer quality improvement project. They found that data from cancer registries provided accurate measures for hospital-based surgical treatments but not for outpatient therapies based on radiation, chemo, and hormones. They argue that developing shared information systems and methods could allow affiliated outpatient practices to provide relevant data to the cancer registries (Bickell & Chassin, Citation2000).
3.2. Use of HBCR data
Several authors discussed the current use of HBCR data. Many studies in this review highlighted that despite some limitations and the issues of missing or incomplete data, HBCRs hold the potential as an efficient data system to help improve patient care (Higashi et al., Citation2014; Mohammadzadeh et al., Citation2017). While some HBCRs have focused on monitoring trends, others have used the data to study variations in care, adherence to guidelines and planning of services, as discussed below.
3.2.1. Monitoring trends
Eleven HIC studies discussed how HBCRs are vital for monitoring the prevalence of cancer. These studies argued that the monitoring of trends was beneficial when planning to improve cancer care (Higashi et al., Citation2014; Mohammadzadeh et al., Citation2017; Opstelten et al., Citation2017; Penberthy et al., Citation2003; Roder et al., Citation2018; Ruiz & Facio, Citation2004; Ruseckaite et al., Citation2016; Sobue, Citation2008). Studies suggest that the most common use (or intended use) of HBCR data was to map cancer trends in a specific population. The majority of the studies highlighted that HBCR data were being used at a local level to identify trends and prevalence of certain cancers and to evaluate the service delivery at a specific hospital or region (Coory et al., Citation2009; Opstelten et al., Citation2017; Ruseckaite et al., Citation2016).
A few studies indicated that only those HBCRs that were part of a regional or national registry programme collected data in a comparable and timely format so that the central registry could examine regional or national trends in the prevalence of certain cancers (Anema et al., Citation2013; Roder et al., Citation2018; Voith von Voithenberg et al., Citation2019).
Some LMIC studies also agreed that registries primarily function as data collection tools to map the prevalence and trends of various cancers on a smaller scale (Al-Haddad et al., Citation2015; Aziz et al., Citation2003; Curado, Citation2019; Jedy-Agba et al., Citation2012; Mohammadzadeh et al., Citation2017; Nair et al., Citation2017; Shrestha et al., Citation2019). For example, in India, considering the population size, the National Cancer Registry Programme only has 29 HBCRs (including all Regional Cancer Centres), including 103 hospitals. One of the LMIC studies also viewed HBCR data as a complementary source of information to population-based cancer registries (PBCR) (Curado, Citation2019) that can contribute to monitoring cancer trends.
3.2.2. Understanding variation in quality of cancer care
Fourteen studies recognised that HBCR could be a useful tool for clinical management, quality assurance, evaluating outcomes and follow-up of patients with cancer (Anema et al., Citation2013; Coory et al., Citation2009; Hendren et al., Citation2014; Higashi et al., Citation2014; Iachina et al., Citation2017; Malin et al., Citation2002; Mohammadzadeh et al., Citation2017; Opstelten et al., Citation2017; Roder et al., Citation2018; Ruiz & Facio, Citation2004; Ruseckaite et al., Citation2016; Stangl et al., Citation2020; Valsecchi & Steliarova-Foucher, Citation2008; Young, Citation1991).
Using HBCR data, Roder et al. (Citation2018) confirmed that 5-year disease-specific survival rate in Australia for vulval cancer in selected HBCRs was comparable to 5-year relative survivals reported for Australia overall. The study found that treatments (surgery and radiotherapy) were not different based on geographic measures of remoteness and socioeconomic status, suggesting equity in service delivery. This study confirmed that HBCRs could fill an evidence gap when clinical data are lacking in population-based registries and highlighted the value of HBCRs in evaluating the quality of service and survival outcomes in local settings (Roder et al., Citation2018).
3.2.3. Improving the process of care
Four studies have focused on how HBCRs can be useful for examining the process of care (Ruiz & Facio, Citation2004; Young, Citation1991). Higashi et al. (Citation2014) argued that registry data could be used to improve care but with the caveat that this may not provide a definitive conclusion on the quality of care. For example, Iachina et al. (Citation2017) through analysis of lung cancer registry data, were able to identify delays in treatment, especially when a patient is transferred between hospitals across the care pathway from diagnosis and confirmation of the diagnosis to treatment. Transfer patients waited longer for referral and treatment after the diagnosis than no-transfer patients and had a lower likelihood of being diagnosed and treated within the acceptable time thresholds described in the care pathway. It was also shown that HBCR data that links tumour registry data to quality-improvement data could help in assessing and improving the quality of cancer care and monitoring the impact of care quality improvement initiatives (Hendren et al., Citation2014; Higashi et al., Citation2014; Iachina et al., Citation2017; Opstelten et al., Citation2017). None of the LMIC studies focused on such linkages.
A few LMIC studies have argued that establishing and sustaining HBCRs in urban and rural regions is crucial for producing cancer registrations with good population reach in LMICs (Curado, Citation2019; Mohammadzadeh et al., Citation2017). For example, as the HBCRs in India cover only 3% of the population, primarily located in urban areas, the data available for the prevalence of cancers are not representative (Nair et al., Citation2017).
3.2.4. Understanding adherence to guidelines
Nine HIC studies focused on how indicators derived from HBCR databases allowed monitoring of the diagnostic and therapeutic pathways of cancer patients (Coory et al., Citation2009; Hendren et al., Citation2014; Higashi et al., Citation2014; Iachina et al., Citation2017; Malin et al., Citation2002; Opstelten et al., Citation2017; Ruseckaite et al., Citation2016; Voith von Voithenberg et al., Citation2019; Young, Citation1991). These suggested that the indicators can be measured repeatedly over time, be retrospectively linked to the administrative databases, and provide information on the most up-to-date clinical practice at a population level, allowing stakeholders to have feedback on the adherence to clinical practice guidelines. The combination of demographic and treatment information collected by HBCRs can be valuable in monitoring and evaluating the adherence to guidelines based on patient’s characteristics (Hendren et al., Citation2014; Higashi et al., Citation2014; Iachina et al., Citation2017; Malin et al., Citation2002; Opstelten et al., Citation2017; Ruseckaite et al., Citation2016; Young, Citation1991).
Coory et al. (Citation2009) found that 119 guideline items (49%) could be measured using hospital registry data compared to only 8 of 243 guideline items (3.3%) using a population-based registry.
It is also advised to use the collected data to inform the development of treatment protocols and guidelines for patient management across their care pathway from the day of symptom presentation to recovery, which is not currently practised in LMICs. None of the LMIC studies in this review reported on the use of HBCR data to study adherence to guidelines.
3.2.5. Planning and Commissioning of Services
HBCR data can guide resource allocation, cancer care policies and priorities, and investment in cancer prevention services. From the HIC studies within this review, it was evident that cancer registry data have been used in a wide variety of areas of cancer control, ranging from cancer epidemiology to primary and secondary prevention to healthcare planning and patient care. This benefited both the individual and the health systems.
Six studies from HICs in this review argued that HBCRs could indicate the demand for cancer care services, which is useful for health policy and planning. It has been noted that clinical cancer registries, which can be used to report on care pathways, may not cover smaller hospitals (Coory et al., Citation2009; Mohammadzadeh et al., Citation2017; Roder et al., Citation2018; Sobue, Citation2008; Stangl et al., Citation2020; Valsecchi & Steliarova-Foucher, Citation2008).
One LMIC study argued that the HBCR data has mainly been used to plan and evaluate cancer control programmes (Nair et al., Citation2017). Besides, the limited data collected did not support strengthening cancer care planning at an individual or hospital level. Three LMIC studies agreed that HBCRs could be viable tools for producing good-quality cancer registration data within LMICs to support cancer-control programmes (Curado, Citation2019; Jedy-Agba et al., Citation2012; Mohammadzadeh et al., Citation2017). A review of HBCRs in Africa argued that HBCRs could be used as tools to understand the demand for cancer care services, which is an important step in the efficient allocation of limited resources and in prioritising cancers that are more prevalent to invest appropriate cancer prevention services on (Jedy-Agba et al., Citation2012).
3.3. Quality of HBCR data
Consideration of the quality of HBCR data was prominent within the included studies. This was multi-faceted and included coverage, completeness, evaluation, and timeliness.
Whilst consensus existed on the potential role of HBCRs in achieving quality and equitable healthcare, there appears to be either limited development of HBCRs within central health systems or limited efforts to improve the efficacy of established HBCRs in LMIC studies.
3.3.1. Integrating data across the cancer care pathway
Three studies argued that registries that are limited to subsets of patients, as they often include data of cancer patients receiving advanced treatments rather than follow-up and screening or are not inclusive of all cancer patients and/or only include a specific cancer type. This highlights a missed opportunity for cancer case identification and clinical epidemiology of cancer (Huang et al., Citation2010; Stangl et al., Citation2020; Valsecchi & Steliarova-Foucher, Citation2008).
While five studies (including studies from LMICs) in this review indicated either the importance of and/or limitations in coverage of all cancer patients by certain HBCRs, only a few HIC studies discussed ways to improve coverage. One German study investigated the benefit of combining HBCR data with a breast cancer patient-centred registry (Stangl et al., Citation2020). Strategies for strengthening the inclusion of patient data in HBCRs included the identification of eligible patients through interdisciplinary tumour boards (groups of health care professionals/providers with different specialities that meet to discuss cancer cases and share knowledge), recruitment of dedicated professionals to improve coverage of data, standardise reminder algorithms to increase patient response rates, adoption of online questionnaires to allow patients to keep track of their own data, and improving the visibility of the registry by the distribution of information on the rationale of the study via self-aid groups or patient leaflets in order to widen the coverage of HBCRs (Huang et al., Citation2010; Stangl et al., Citation2020). A few LMIC studies highlight the lack of coverage of HBCRs both in terms of cancer patient identification at the population level and patients within their care pathway (Nair et al., Citation2017; Shrestha et al., Citation2019).
3.3.2. Strengthening of HBCR
The studies in this review suggested various methods and measures to strengthen the HBCRs (Hendren et al., Citation2014; Huang et al., Citation2010; Lang et al., Citation2003; Mohammadzadeh et al., Citation2017; Penberthy et al., Citation2003; Shiki et al., Citation2008). These include consistency checks by the registry software or linkage of different data sources, improvement of data quality through training of the registrars (Hendren et al., Citation2014), use of appropriate registration manuals and software, and audit and supervision of the registry processes (Shiki et al., Citation2008). They have also been highlighted as informing the use of unified modelling language (UML) checks, a hospital-level model for the HBCR registration process and quality control. UML coordination with hospital-level information system improved the completeness (Shiki et al., Citation2008) and registry programme (Mohammadzadeh et al., Citation2017). A combination of manual and computerised cancer registries for web-based cancer registries or using hospital discharge files data to supplement central cancer registry data have been advised to register complete data (Huang et al., Citation2010; Lang et al., Citation2003; Penberthy et al., Citation2003).
Where HBCRs were functional, they faced several challenges that posed obstacles to reaching their full potential in LMICs. These challenges included a lack of internationally recognised software for cancer registry and workforce issues such as inadequate training of data registry team relating to data entry, quality control, and statistical analysis (Jedy-Agba et al., Citation2012; Shrestha et al., Citation2019; Valsecchi & Steliarova-Foucher, Citation2008). Shrestha et al. (Citation2019) and Mohammadzadeh et al. (Citation2017) further suggested that some data quality control considerations, such as de-duplication (eliminating multiple registrations or reporting of the same patient) and cancer coding according to the International Classification of Diseases for Oncology exist. Even where the registration procedures were of high diagnostic validity and reporting timelines were comparable to each other and international standards, studies have reported evidence of incomplete data in Nigeria (Al-Haddad et al., Citation2015) and Nepal (Shrestha et al., Citation2019). Such incomplete data could result in lower cancer rates than the GLOBOCAN data. For example, in a study evaluating the completeness and comparability of registry data in Nigerian cancer registries, including four HBCRs, Al-Haddad et al. (Citation2015) found evidence of incompleteness (generally lower than the estimated rates from GLOBOCAN).
Similarly, incomplete data and a lack of follow-up were highlighted by an overview study focusing on 12 cancer registries in Nepal (Shrestha et al., Citation2019). Regular reviews of registry case-finding procedures are advised to improve completeness (Al-Haddad et al., Citation2015). Chatterjee et al. (Citation2016) advanced a similar argument and suggested that HBCRs should focus on accuracy and administrative quality through regular audits.
3.3.3. Concordance with other forms of data
Seven HIC studies in this review focused on the concordance of HBCRs with cancer care (Anema et al., Citation2013; Bickell & Chassin, Citation2000; Malin et al., Citation2002; Penberthy et al., Citation2003; Piccirillo et al., Citation2004; Voith von Voithenberg et al., Citation2019; Zhang et al., Citation2012). Lack of concordance is reported between hospital-based cancer registry data and the “clinicians” databases because of varying interpretations of clinical information, different registration timings, varying information sources, and human errors (Zhang et al., Citation2012). In this regard, Cancer Performance Indicator scores are found to be plausible (despite incomplete data registry and limited data access), where regional cancer centres collect most of the indicator data in a standardised manner (Anema et al., Citation2013). It has been highlighted that HBCRs should include thorough clinical details (e.g., dosage of chemotherapy drugs and the number of treatments) and information about “patients” comorbidities, which are necessary for valid measurement of the process and outcomes of care (Malin et al., Citation2002; Piccirillo et al., Citation2004).
A few studies have suggested that common data guidelines on collecting biomarker data by registries and harmonising with clinical guidelines could lead to better data availability to the research community enabling large-scale real-world data analyses (Voith von Voithenberg et al., Citation2019). Bickell and Chassin (Citation2000) argue that hospitals that deal with fewer cancer cases and cannot afford to maintain an independent cancer registry could share regionally based “roving registers”. They suggest that cancer registries should be reviewed regularly to ensure adequate treatment data and that unverified data should not be used to measure the quality of care.
Our search did not find any LMIC study reporting on the concordance of HBCRs with other forms of data. While this lack of evidence could not be interpreted as a lack of practice, considering the technological limitations in data collection and management, the lack of concordance identified in the HIC setting could likely be evident in LMICs as well.
3.3.4. Timeliness
A few studies, including two reviews, demonstrated that the timeline for HBCR data was generally within 2–3 years or longer of initial cancer diagnosis (Malin et al., Citation2002; Mohammadzadeh et al., Citation2017; Voith von Voithenberg et al., Citation2019). Three studies describe that a 2-year duration between HBCR data collection and reporting appears to be common practice in many countries and even longer in some (similar to LMIC studies, Shrestha et al., Citation2019) (Malin et al., Citation2002; Mohammadzadeh et al., Citation2017; Voith von Voithenberg et al., Citation2019). These studies strongly advise that the duration between individual patient data registration by hospital registries and data availability from central registries needs to be shorter so that stakeholders and policymakers evaluating the quality of care can use accurate and timely data (Malin et al., Citation2002; Voith von Voithenberg et al., Citation2019).
4. Strengths and limitations
We do not claim this to be an exhaustive selection and grading of all of the relevant literature, as the searches were conducted in limited databases for studies in the English language and using limited search terms. There were only a small number of publications about HBCRs in LMICs, and these had a varying focus on topics, research design, and reporting. This made it difficult to make comparisons across studies. However, this is one of the few reviews about HBCRs and the first scoping review to focus on HBCRs in LMICs.
5. Discussion
Although the LMIC studies in this review are few in number, a majority of these studies indicated the presence of HBCRs across the country or at the regional level. This novel scoping review highlights the evidence that HBCRs have an essential role in improving cancer care pathways and patient outcomes in both HICs and LMICs. However, this is not currently being realised, and various barriers to achieving this were identified, particularly in the LMIC context. Due to improved technology, in the HIC context, a majority of HBCRs use electronic databases, whereas, in LMICs, there is still a heavy reliance on paper-based data collection (Parkin & Sanghvi, Citation1991; Purcell et al., Citation2020; Shrestha et al., Citation2019).
Given the limited research in LMICs on HBCRs and their lack of emphasis on high-quality data, in comparison to HIC studies, cancer policymakers must address this. This is crucial, as the review highlighted the impactful role that HBCRs could play in complementing population-based cancer registries to provide a national picture (Curado, Citation2019; Jedy-Agba et al., Citation2012; Mohammadzadeh et al., Citation2017). Besides, our review highlighted the need for longitudinal datasets to improve monitoring and quality of care in the LMIC context. Irrespective of the level of HBCR coverage in LMIC, all studies highlighted multiple challenges and barriers, such as the collection of data in paper format or a format that is not user-friendly, a lack of clear outcomes, and a lack of follow-up data, particularly following discharge of patients following cancer therapy. The absence of quality monitoring and maintenance and non-integration of HBCR data in health research, policy, and care were also highlighted as additional barriers. Existing literature on health information systems highlights the importance of tailoring information systems for specific healthcare contexts (Weeger & Gewald, Citation2015), both patients' and healthcare professionals' roles and acceptance of technology to fully realize their potential in providing equitable care (Mettler et al., Citation2014; Paul et al., Citation2012; Tulu et al., Citation2016).
The key focus of the current HBCRs is around the establishment and structure of the HBCR and the use of small-scale data to identify specific prevalence or testing. However, less attention has been paid to understanding the use of HBCRs within health systems and processes (e.g., training and workforce) or how they can help to achieve better and more equitable health outcomes. First, in instances where data was collected, most of the data were stored in paper format or in an electronic format that was not user-friendly. Secondly, LMIC HBCR data lack clear outcomes and do not include follow-up data. Thirdly, the data collected have not been used for improving patient outcomes through integrated care or higher-level policy. Besides these challenges, a wider question of the representation of data was evident. As HBCRs in LMICs tend to be based mostly in urban areas, they underrepresent rural and disadvantaged populations who are more likely to have barriers to accessing diagnosis and care. Due to poor screening, particularly in rural areas, people living in rural areas with cancer are likely to die without ever having a cancer screening. This has important implications for strengthening cancer care pathways for these populations, as the data do not sufficiently represent their challenges. There was agreement across the LMIC studies that whilst HBCRs can be essential sources of information for health planning and future research even in a resource-limited context, this is not currently being achieved (Al-Haddad et al., Citation2015; Aziz et al., Citation2003; Chatterjee et al., Citation2016; Curado, Citation2019; Jedy-Agba et al., Citation2012; Mohammadzadeh et al., Citation2017; Nair et al., Citation2017; Shrestha et al., Citation2019; Valsecchi & Steliarova-Foucher, Citation2008).
This scoping review highlights the importance of building on lessons learned from HICs. This includes collating HBCR data across cancer care pathways. Besides, the integration of HBCR data with tumour boards to evaluate the implementation of clinical practice guidelines could be proven beneficial. Finally, LMICs could focus on data quality and improve the outcome data to align the HBCR data with the provision of integrated care. This would allow a continuous cycle of measurement, communication, and action.
While some of the recommendations stemming from the HIC studies, such as training and skills development to ensure data quality, are feasible in the LMIC context, others might not be possible without sufficient resources to ensure that LMICs have the resources to keep up to date with technological advances. For instance, the implementation of well-tested digital registry tools, such as CANReg (Pardamean and Suparyanto Citation2017) or data quality tools such as “Abstract Plus” or “GenE” (Sergeons, 1996–2017) for ensuring validity and completeness and quality of data (Kim et al., Citation2010), necessitates accessible resources and infrastructure, such as electricity, reliable internet connectivity, and national-level digital network (Hernandez, Citation2018; Knight et al., Citation2019). Furthermore, data follow-up may not be possible without a structural change at the country level, as many LMICs lack national-level digital networks (Hernandez, Citation2018), or a unique patient identification number, which makes it an arduous task to follow-up in different hospitals. However, this scoping review offers enough support for the potential role and importance of HBCRs in improving cancer care and burden in LMICs for this to be an area of continued importance to research and policy.
It is important to note that our review highlighted the relative absence of evaluation and integration studies, and the included papers from LMICs mainly focused on small-scale pilots or discussions of the potential held by HBCRs. LMIC settings demonstrate that inequalities in cancer care and screening are often delayed, and data structures are weaker or non-existent (LaVigne et al., Citation2017; Siddiqui & Zafar, Citation2018; WHO). Given the common reliance on HBCRs and electronic health records (EHRs) in HICs and the emergence, evaluation, and impact of AI and Big Data (Ben-Assuli & Padman, Citation2018; Cirillo et al., Citation2021; Kudyba & Perry, Citation2015; Muhsen et al., Citation2019), the lack of studies addressing the issue is surprising and warrants more attention.
While the themes address mostly data-related aspects, they fail to address the general health equity issues that are relevant to this paper. The health systems literature identifying the role of politics, economics, and policy as the primary determinants of population health is relevant to this study. These studies demonstrate that countries with higher public spending, including health and those with lower-income inequalities and poverty, have better overall health in general. Although the papers on HIC did not address these aspects explicitly, our research suggests that improvement in spending and addressing some of the inequality issues could be critical factors in improving HBCR performance and relevance.
6. Conclusion
HBCR and improved cancer information systems are critical aspects of any health system. An enhanced focus on this within research and practice focusing on strengthening health systems could help to achieve quality and equitable cancer care in LMICs.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Al-Haddad, B. J., Jedy-Agba, E., Oga, E., Ezeome, E. R., Obiorah, C. C., Okobia, M., Ogunbiyi, J. O., Ukah, C. O., Omonisi, A., Nwofor, A. M., Igbinoba, F., & Adebamowo, C. (2015). Comparability, diagnostic validity and completeness of Nigerian cancer registries. Cancer Epidemiology, 39(3), 456–464. https://doi.org/10.1016/j.canep.2015.03.010
- Anema, H. A., Kievit, J., Fischer, C., Steyerberg, E. W., & Klazinga, N. S. (2013). Influences of hospital information systems, indicator data collection and computation on reported Dutch hospital performance indicator scores. BMC Health Services Research, 13(1), 212. https://doi.org/10.1186/1472-6963-13-212
- Arksey, H., & O’Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. https://doi.org/10.1080/1364557032000119616
- Aziz, Z., Sana, S., Saeed, S., & Akram, M. (2003). Institution based tumor registry from Punjab: Five year data based analysis. Journal of the Pakistan Medical Association, 53(8), 350–353.
- Bakouny, Z., Hawley, J. E., Choueiri, T. K., Peters, S., Rini, B. I., Warner, J. L., & Painter, C. A. (2020). COVID-19 and cancer: Current challenges and perspectives. Cancer Cell, 38(5), 629–646. https://doi.org/10.1016/j.ccell.2020.09.018
- Ben-Assuli, O., & Padman, R. (2018). Analysing repeated hospital readmissions using data mining techniques. Health Syst (Basingstoke), 7(2), 120–134. https://doi.org/10.1080/20476965.2017.1390635
- Berg, K., Doktorchik, C., Quan, H., & Saini, V. (2022). Automating data collection methods in electronic health record systems: A Social Determinant of Health (SDOH) viewpoint. Health Systems, 1–9. https://doi.org/10.1080/20476965.2022.2075796
- Bickell, N. A., & Chassin, M. R. (2000). Determining the quality of breast cancer care: Do tumor registries measure up? Annals of Internal Medicine, 132(9), 705–710. https://doi.org/10.7326/0003-4819-132-9-200005020-00004
- Bray, F., Znaor, A., Cueva, P., Korir, A., Swaminathan, R., & International Agency for Research on Cancer. (2014). Planning and Developing Population-Based Cancer Registration in Low- or Middle-Income Settings. Lyon (FR): International Agency for Research on Cancer. PMID: 33502836.
- Chatterjee, S., Chattopadhyay, A., Senapati, S. N., Samanta, D. R., Elliott, L., Loomis, D., Mery, L., & Panigrahi, P. (2016). Cancer registration in India - current scenario and future perspectives. Asian Pacific Journal of Cancer Prevention: APJCP, 17(8), 3687–3696.
- Chen, J. -G., Chen, H. -Z., Zhu, J., Yang, Y. -L., Zhang, Y. -H., Huang, P. -X., Chen, Y. -S., Zhu, C. -Y., Yang, L. -P., Shen, K., Qiang, F. -L., & Wang, G. -R. (2018). Cancer survival in patients from a hospital-based cancer registry, China [Research Paper]. Journal of Cancer, 9(5), 851–860. https://doi.org/10.7150/jca.23039
- Cirillo, D., Núñez-Carpintero, I., & Valencia, A. (2021). Artificial intelligence in cancer research: Learning at different levels of data granularity. Molecular Oncology, 15(4), 817–829. https://doi.org/10.1002/1878-0261.12920
- Cooke, A. Smith, D, and Booth, A. (2012). Beyond PICO. Qual Health Res, 22(10), 1435–1443. https://doi.org/10.1177/1049732312452938
- Coory, M., Thompson, B., Baade, P., & Fritschi, L. (2009). Utility of routine data sources for feedback on the quality of cancer care: An assessment based on clinical practice guidelines. BMC Health Services Research, 9(1), 84. https://doi.org/10.1186/1472-6963-9-84
- Curado, M. P. (2019). Importance of hospital cancer registries in Africa. Ecancermedicalscience, 13, 948. https://doi.org/10.3332/ecancer.2019.948
- GLOBOCAN. (2018). GLOBOCAN 2018 Nepal (Fact Sheet). https://gco.iarc.fr/today/data/factsheets/populations/524-nepal-fact-sheets.pdf
- Hendren, S., McKeown, E., Morris, A. M., Wong, S. L., Oerline, M., Poe, L., Campbell, D. A., Jr., & Birkmeyer, N. J. (2014). Implementation of a hospital-based quality assessment program for rectal cancer. Journal of Oncology Practice / American Society of Clinical Oncology, 10(3), e120–129. https://doi.org/10.1200/jop.2014.001387
- Hernandez, K. A. R. T. (2018). Leaving no one behind in a digital world (K4D emerging issues report, issue.
- Higashi, T., Nakamura, F., Shibata, A., Emori, Y., & Nishimoto, H. (2014). The national database of hospital-based cancer registries: A nationwide infrastructure to support evidence-based cancer care and cancer control policy in Japan. Japanese Journal of Clinical Oncology, 44(1), 2–8. https://doi.org/10.1093/jjco/hyt013
- Huang, H., Sim, H. G., Chong, T. W., Yuen, J. S., Cheng, C. W., & Lau, W. K. (2010). Evaluation of data completeness of the prostate cancer registry after robotic radical prostatectomy. Annals of the Academy of Medicine, Singapore, 39(11), 848–853. https://doi.org/10.47102/annals-acadmedsg.V39N11p848
- Iachina, M., Jakobsen, E., Fallesen, A. K., & Green, A. (2017). Transfer between hospitals as a predictor of delay in diagnosis and treatment of patients with non-small cell lung cancer - a register based cohort-study. BMC Health Services Research, 17(1), 267. https://doi.org/10.1186/s12913-017-2230-3
- (IARC) International Agency for Research on Cancer. IARC-Technical Report-Sources of information for the population-based cancer registry. International Agency for Research on Cancer (IARC); [Google Scholar]
- Jazieh, A. R., Akbulut, H., Curigliano, G., Rogado, A., Alsharm, A. A., Razis, E. D., Mula-Hussain, L., Errihani, H., Khattak, A., Guzman, R. B. D., Mathias, C., Alkaiyat, M. O. F., Jradi, H., Rolfo, C., & Care, O. Impact of the COVID-19 pandemic on cancer care: A global collaborative study. (2020). JCO Global Oncol, 6(6), 1428–1438. b. o. t. I. R. N. o. C.-I. o. C. https://doi.org/10.1200/go.20.00351
- Jedy-Agba, E. E., Curado, M. P., Oga, E., Samaila, M. O., Ezeome, E. R., Obiorah, C., Erinomo, O. O., Ekanem, I. O., Uka, C., Mayun, A., Afolayan, E. A., Abiodun, P., Olasode, B. J., Omonisi, A., Otu, T., Osinubi, P., Dakum, P., Blattner, W., & Adebamowo, C. A. (2012). The role of hospital-based cancer registries in low and middle income countries-The Nigerian case study. Cancer Epidemiology, 36(5), 430–435. https://doi.org/10.1016/j.canep.2012.05.010
- Kim, H. J., Cho, J. H., Lyu, Y., Lee, S. H., Hwang, K. H., & Lee, M. S. (2010). Construction and validation of hospital-based cancer registry using various health records to detect patients with newly diagnosed cancer: Experience at Asan Medical Center. J Prev Med Public Health, 43(3), 257–264. https://doi.org/10.3961/jpmph.2010.43.3.257
- Knight, S. R., Ots, R., Maimbo, M., Drake, T. M., Fairfield, C. J., & Harrison, E. M. (2019). Systematic review of the use of big data to improve surgery in low- and middle-income countries. The British Journal of Surgery, 106(2), e62–72. https://doi.org/10.1002/bjs.11052
- Kudyba, S., & Perry, T. (2015). A data mining approach for estimating patient demand for mental health services. Health Systems, 4(1), 5–11. https://doi.org/10.1057/hs.2014.12
- Lang, K., Magi, M., & Aareleid, T. (2003). Study of completeness of registration at the Estonian cancer registry. European Journal of Cancer Prevention: The Official Journal of the European Cancer Prevention Organisation (ECP), 12(2), 153–156. https://doi.org/10.1097/00008469-200304000-00009
- LaVigne, A. W., Triedman, S. A., Randall, T. C., Trimble, E. L., & Viswanathan, A. N. (2017). Cervical cancer in low and middle income countries: Addressing barriers to radiotherapy delivery. Gynecol Oncol Rep, 22, 16–20. https://doi.org/10.1016/j.gore.2017.08.004
- Malin, J. L., Kahn, K. L., Adams, J., Kwan, L., Laouri, M., & Ganz, P. A. (2002). Validity of cancer registry data for measuring the quality of breast cancer care. Journal of the National Cancer Institute, 94(11), 835–844. https://doi.org/10.1093/jnci/94.11.835
- Mettler, T., Fitterer, R., Rohner, P., & Winter, R. (2014). Does a ’ ‘hospital’s IT architecture fit with its strategy? An approach to measure the alignment of health information technology. Health Systems, 3(1), 29–42. https://doi.org/10.1057/hs.2013.10
- Mohammadzadeh, Z., Ghazisaeedi, M., Nahvijou, A., Rostam Niakan Kalhori, S., Davoodi, S., & Zendehdel, K. (2017). Systematic review of Hospital Based Cancer Registries (HBCRs): necessary tool to improve quality of care in cancer patients. Asian Pacific Journal of Cancer Prevention: APJCP, 18(8), 2027–2033 doi:10.22034/APJCP.2017.18.8.2027.
- Muhsen, I. N., Jagasia, M., Toor, A. A., & Hashmi, S. K. (2019). Registries and artificial intelligence: Investing in the future of hematopoietic cell transplantation. Bone Marrow Transplantation, 54(3), 477–480. https://doi.org/10.1038/s41409-018-0327-x
- Munn, Z., Peters, M. D. J., Stern, C., Tufanaru, C., McArthur, A., & Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology, 18(1), 143. https://doi.org/10.1186/s12874-018-0611-x
- Nair, C. K., Mathew, A. P., & George, P. S. (2017). Lung cancer: Presentation and pattern of care in a cancer center in South India. Indian Journal of Cancer, 54(1), 164–168. https://doi.org/10.4103/ijc.IJC_56_17
- Noda-Narita, S., Kawachi, A., Okuyama, A., Sadachi, R., Hirakawa, A., Goto, Y., Yonemori, K. (2021). First-line treatment for lung cancer among Japanese older patients: A real-world analysis of hospital-based cancer registry data. PLos One, 16(9), e0257489. https://doi.org/10.1371/journal.pone.0257489
- Opstelten, J. L., de Wijkerslooth, L. R., Leenders, M., Bac, D. J., Brink, M. A., Loffeld, B. C., Meijnen-Bult, M. J., Minderhoud, I. M., Verhagen, M. A., van Oijen, M. G., & Siersema, P. D. (2017). Variation in palliative care of esophageal cancer in clinical practice: Factors associated with treatment decisions. Diseases of the Esophagus: Official Journal of the International Society for Diseases of the Esophagus I S D E, 30(2), 1–7. https://doi.org/10.1111/dote.12478
- Pandey, A., Raj, S., Madhawi, R., Devi, S., & Singh, R. K. (2019). Cancer trends in Eastern India: Retrospective hospital-based cancer registry data analysis. South Asian J Can, 8(4), 215–217. https://doi.org/10.4103/sajc.sajc_321_18
- Pardamean, B, and Suparyanto, T Pardamean, B., & Suparyanto T. ”Hospital-based cancer registry application,” (2017). International Conference on Information Management and Technology (ICIMTech), Special Region of Yogyakarta, Indonesia. 2017 International Conference on Information Management and Technology (ICIMTech) Special Region of Yogyakarta, Indonesia 2017 pp. 44–48. https://doi.org/10.1109/ICIMTech.2017.8273509
- Parkin, D. M., & Sanghvi, L. D. (1991). Cancer registration in developing countries. IARC Scientific Publications, 95 , 185–198.
- Paul, R. J., Ezz, I., & Kuljis, J. (2012). Healthcare information systems: A patient-user perspective. Health Systems, 1(2), 85–95. https://doi.org/10.1057/hs.2012.17
- Penberthy, L., McClish, D., Pugh, A., Smith, W., Manning, C., & Retchin, S. (2003). Using hospital discharge files to enhance cancer surveillance. American Journal of Epidemiology, 158(1), 27–34. https://doi.org/10.1093/aje/kwg108
- Piccirillo, J. F., Tierney, R. M., Costas, I., Grove, L., & Spitznagel, E. L., Jr. (2004). Prognostic importance of comorbidity in a hospital-based cancer registry. Jama, 291(20), 2441–2447. https://doi.org/10.1001/jama.291.20.2441
- Popay, J., Roberts, H. M., Sowden, A. J., Petticrew, M., Arai, L., Rodgers, M., & Britten, N. (2006). Guidance on the conduct of narrative synthesis in systematic Reviews. A Product from the ESRC Methods Programme . Version 1.
- Purcell, L. N., Nip, E., Gallaher, J., Varela, C., Gondwe, Y., & Charles, A. (2020). Design and implementation of a hospital-based trauma surveillance registry in a resource-poor setting: A cost analysis study. Injury, 51(7), 1548–1553. https://doi.org/10.1016/j.injury.2020.04.044
- Roder, D., Davy, M., Selva-Nayagam, S., Paramasivam, S., Adams, J., Keefe, D., Olver, I., Miller, C., Buckley, E., Powell, K., Fusco, K., Buranyi-Trevarton, D., & Oehler, M. K. (2018). Using hospital registries in Australia to extend data availability on vulval cancer treatment and survival. BMC Cancer, 18(1), 858. https://doi.org/10.1186/s12885-018-4759-x
- Ruiz, A., & Facio, Á. (2004). Hospital-based cancer registry: A tool for patient care, management and quality. A focus on its use for quality assessment. Clinical & Translational Oncology, 6(2), 104–113. https://doi.org/10.1007/BF02710038
- Ruseckaite, R., Beckmann, K., O’Callaghan, M., Roder, D., Moretti, K., Millar, J., & Evans, S. (2016). A retrospective analysis of Victorian and South Australian clinical registries for prostate cancer: Trends in clinical presentation and management of the disease. BMC Cancer, 16(1), 607. https://doi.org/10.1186/s12885-016-2655-9
- Shiki, N., Ohno, Y., Fujii, A., Murata, T., & Matsumura, Y. (2008). Unified Modeling Language (UML) for hospital-based cancer registration processes. Asian Pacific Journal of Cancer Prevention: APJCP, 9(4), 789–796.
- Shrestha, G., Pradhananga, K. K., Mulmi, R., Subedi, K. P., & Siwakoti, B. (2019). Cancer registration in Nepal: Current status and way forward. JNMA; Journal of the Nepal Medical Association, 57(216), 144–148. https://doi.org/10.31729/jnma.4192
- Siddiqui, A. H., & Zafar, S. N. (2018). Global availability of cancer registry data. J Glob Oncol, 4(4), 1–3. https://doi.org/10.1200/jgo.18.00116
- Sobue, T. (2008). Current activities and future directions of the cancer registration system in Japan. International Journal of Clinical Oncology, 13(2), 97–101. https://doi.org/10.1007/s10147-008-0761-7
- Stangl, S., Haas, K., Eichner, F. A., Grau, A., Selig, U., Ludwig, T., Fehm, T., Stuber, T., Rashid, A., Kerscher, A., Bargou, R., Hermann, S., Arndt, V., Meyer, M., Wildner, M., Faller, H., Schrauder, M. G., Weigel, M., Schlembach, U., Heuschmann, P. U., & Wockel, A. (2020). Development and proof-of-concept of a multicenter, patient-centered cancer registry for breast cancer patients with metastatic disease—the “Breast cancer care for patients with metastatic disease” (BRE-4-MED) registry. Pilot and Feasibility Studies, 6(1), 11. https://doi.org/10.1186/s40814-019-0541-3
- Torre L A, Bray F, Siegel R L, Ferlay J, Lortet-Tieulent J and Jemal A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65(2), 87–108. 10.3322/caac.21262
- Tricco, A., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D., Horsley, T., Weeks, L., Hempel, S., Akl, E. A., Chang, C., McGowan, J., Stewart, L., Hartling, L., Aldcroft, A., Wilson, M. G., Garritty, C., Lewin, S., & Straus, S. E. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. https://doi.org/10.7326/M18-0850
- Tulu, B., Trapp, A. C., Strong, D. M., Johnson, S. A., Hoque, M., Trudel, J., & Garber, L. (2016). An analysis of patient portal utilisation: What can we learn about online patient behavior by examining portal click data? Health Systems, 5(1), 66–79. https://doi.org/10.1057/hs.2015.5
- UN. (2015). Transforming our world: The 2030 Agenda for Sustainable Development. U. Nations. http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E
- Valsecchi, M. G., & Steliarova-Foucher, E. (2008). Cancer registration in developing countries: Luxury or necessity? The Lancet Oncology, 9(2), 159–167. https://doi.org/10.1016/s1470-2045(08)70028-7
- Voith von Voithenberg, L., Crocetti, E., Martos, C., Dimitrova, N., Giusti, F., Randi, G., Rooney, R., Dyba, T., Bettio, M., & Negrao Carvalho, R. (2019). Cancer registries - guardians of breast cancer biomarker information: A systematic review. The International Journal of Biological Markers, 34(2), 194–199. https://doi.org/10.1177/1724600819836097
- Weeger, A., & Gewald, H. (2015). Acceptance and use of electronic medical records: An exploratory study of hospital ‘physicians’ salient beliefs about HIT systems. Health Systems, 4(1), 64–81. https://doi.org/10.1057/hs.2014.11
- WHO. WHO Guide to cancer early diagnosis. https://www.who.int/cancer/publications/cancer_early_diagnosis/en
- Young J. L. (1991).The hospital-based cancer registry. 95. IARC scientific publications.
- Zhang, M., Higashi, T., Nishimoto, H., Kinoshita, T., & Sobue, T. (2012). Concordance of hospital-based cancer registry data with a clinicians’ database for breast cancer. Journal of Evaluation in Clinical Practice, 18(2), 459–464. https://doi.org/10.1111/j.1365-2753.2010.01600.x