Abstract
Introduction: Dengue has emerged as a major public health problem in Sri Lanka. Vector control at community level is a frequent and widespread strategy for dengue control. The aim of the study was to assess Aedes mosquito breeding sites and the prevention practices of community members in a heavily urbanized part of Colombo.
Methods: A cross-sectional entomological survey was conducted from April to June 2013 in 1469 premises located in a subdistrict of the City of Colombo. Types of breeding sites and, where found, their infestation with larvae or pupae were recorded. Furthermore, a questionnaire was administered to the occupants of these premises to record current practices of dengue vector control.
Results: The surveyed premises consisted of 1341 residential premises and 110 non-residential premises (11 schools, 99 work or public sites), 5 open lands, and 13 non-specified. In these 1469 premises, 15447 potential breeding sites suitable to host larvae of pupae were found; of these sites18.0% contained water. Among the 2775 potential breeding sites that contained water, 452 (16.3%) were positive for larvae and/or pupae. Schools were associated with the proportionally highest number of breeding sites; 85 out of 133 (63.9%) breeding sites were positive for larvae and/or pupae in schools compared with 338 out of 2288 (14.8%) in residential premises. The odds ratio (OR) for schools and work or public sites for being infested with larvae and/or pupae was 2.77 (95% CI 1.58, 4.86), when compared to residential premises. Occupants of 80.8% of the residential premises, 54.5% of the schools and 67.7% of the work or public sites reported using preventive measures. The main prevention practices were coverage of containers and elimination of mosquito breeding places. Occupants of residential premises were much more likely to practice preventive measures than were those of non-residential premises (OR 2.23; 1.49, 3.36).
Conclusion: Schools and working sites were associated with the highest numbers of breeding sites and lacked preventive measures for vector control. In addition to pursuing vector control measures at residential level, public health strategies should be expanded in schools and work places.
Introduction
Dengue is a vector borne disease that has emerged in the last two decades as a major public health problem in many countries of the tropics and subtropics. Around 2.5 billion people are estimated to live in dengue-affected countries and there are 390 million dengue infections per year.Citation1–3 The Asia Pacific Region bears a particularly high disease burden. It was estimated in 2008, in the latest strategic plan, that 1.8 billion people residing in the Asia pacific region were at risk of dengue infection.Citation4,5
Dengue is caused by a virus of the family flaviviridae with four serotypes, and transmitted by Aedes (Ae.) mosquitoes, mainly Ae. aegypti and Ae. albopictus.Citation2,6 While Ae. albopictus is usually more prevalent in rural and semi-urban areas,Citation7 Ae. aegypti is particularly well adapted to urban areas because of its propensity for breeding in man-made containers and its strong anthropophilic biting behavior being enhanced in densely populated areas.Citation2,7,8 Both species are characterized by diurnal feeding and a short flying range, estimated as usually less than 25 m, but potentially up to 400 m, from their emergence sites in urban environments.Citation7,9
Given the absence of specific antiviral therapy, vector control measures remain the mainstay of the prevention and control of dengue.Citation6,10 Big strides have been made in developing a dengue vaccine.Citation11 The first dengue vaccine – Dengvaxia – was licensed in December 2015 in Mexico, followed by four other countries to date. However, Dengvaxia has not been filed for licensure in Sri Lanka yet, and is unlikely to be introduced in the next few years.Citation12,13
No single vector control intervention has proven effective or sustainable, hence there is a growing consensus that multiple, integrated, and synergistic interventions are needed. Even when dengue vaccines become widely available, vector control activities need to continue for several reasons. Dengvaxia has only moderate efficacy and will only be administered to a restricted age group above nine years.Citation14 Furthermore, Aedes populations need to be kept at bay because they transmit other viruses too, such as chikungunya, zika, and yellow fever. Participatory community involvement plays an additional key role in vector control programs.Citation15–17 In a large parallel group cluster randomized controlled trial in Nicaragua, community mobilization was shown to add effectiveness to dengue vector control.Citation18
In Sri Lanka dengue was made legally reportable in 1996.Citation19 Over the past two decades, dengue outbreaks have increased in magnitude and frequency, with over 40,000 cases reported in 2012 and 2014.Citation20 The highest disease incidence is found in urban areas, especially in the district of Colombo, the most densely populated part of the country.Citation20 To address this growing problem, vector control activities have been intensified. Vector control programs in Sri Lanka consist mainly of source reduction and thermal fogging. In recent years, collective approaches including environmental management of breeding sites, assessment of the influence of climatic factors (through climatic forecast data) on disease and vector, mapping for outbreak prediction, and raising awareness and improvement of case management have increasingly been employed.Citation6,21 Furthermore, health education campaigns have been conducted that include community-level education and participation. Despite such measures, dengue does not appear to have abated. It is important to assess the impact of these measures on breeding sites at community level in order to better target and adapt vector control strategies in the future.
The aims of this study were twofold: first to assess the primary sites of mosquito breeding, measured by the presence of suitable and positive containers for larvae and pupae; second to discuss whether the practices for vector control carried out by the community were associated with lower number of positive breeding sites.
Methods
Study period and setting
A cross-sectional survey took place from April to June 2013, at the beginning of the southwestern monsoon season, in an administrative subdistrict (called a ‘ward’) of the City of Colombo. This administrative division, under the jurisdiction of the ‘Colombo Municipal Council’, is the largest and most densely populated area in the country with a population of 561,314 habitants in 2012.Citation22 The selected Ward 33 has a population of 21,326Citation22 and is highly dengue endemic.Citation5 This study was facilitated by partners from the DengueTools project.Citation23
Selection of premises
The Ward 33 is subdivided into 19 census blocks (Fig. ). A proportional random sample from each block was selected through the housing units enumerated in the National Census of 2012.Citation22 The aim was to cover 50% of premises in Ward 33 with survey teams investigating every other premise. In all 48.3% (1469/3044) of census-enumerated premises were investigated. Blinding was not done and allocation of surveyed premises was not concealed. Because blocks 1 and 6 had too few useable premises, only the remaining 17 blocks were included in the survey.
Research methods
Entomological baseline survey
We conducted an entomological survey using a standardized questionnaire. The questionnaire had three sections; the sections were named as follows: section 1 “Basic data”, section 2 “Inspection of breeding sites” and section 3 “Practices regarding dengue mosquito prevention”. Section 1 collected basic information about the premise (household assessment number, type of premise, status of the land tenure), and the number of adults and children living in the household. In Section 2, trained inspectors recorded all breeding places that were potentially suitable for collecting water, both inside and outside the premises. For all breeding sites that contained water, systematic sampling for larvae and pupae was conducted according to the methods outlined in Sri Lanka’s Practical Manual and Guidelines for Dengue Vector Surveillance.Citation21 Premises were subdivided into five sub-areas to assess the presence of breeding sites: both outdoor (ground, roofing areas) and indoor (living area, toilet and bathroom, and cooking and washing area). The survey instrument specified a list of 38 specified container types that might provide potential breeding sites (e.g. flower pots, roof gutter, vases, pots and pans, barrels). If a container type did not fit into any of these 38 specified categories, it was classified as ‘others’. In addition, breeding sites were arranged into three categories: containers without water, containers with water, and containers positive for larvae and/or pupae, see examples in Figure . Section 3 of the survey recorded the different preventive measures used by the occupants of the premises. In residential premises, household members were interviewed, in schools the school principal or his/her delegate, and in working sites senior personnel with authority. An open-ended question was asked: ‘What do you do to prevent mosquito breeding and biting?’ and the responses were classified by the interviewers into four categories: (1) keeping all water containers covered and protected from mosquitoes; (2) regularly eliminating mosquito breeding sites; (3) employing window screens; and (4) using personal protection measures against mosquito bites during daytime. If participants gave more than one answer to the question, it was recorded as an additional response from the participant. Therefore, the frequency of responses on this section is higher than the number of premises. Information on the use of different types of repellents and bed nets during day and night was also collected from occupants of residential premises.
Data collection procedures
About 54 trained entomological assistants, divided into 15 groups of with 3–4 members, conducted the survey. The groups followed the standard operational protocol for the entomological survey tool.Citation24
Data entry procedure and cleaning
Data entry was done by trained personnel of the Ministry of Health’s Epidemiology Unit. Independent validation checks and cleaning procedures were performed.
Data analysis
Univariate logistic regressions were performed using STATA version 13.1 (STATA Corp., Texas, USA) to determine odds ratio (OR) and relationships between the presence of potential containers, the presence of Aedes larvae and pupae, and preventive action taken.Citation25 Statistical significance was set at p ≤ 0.05.
Ethical considerations
Ethical approval was obtained from Ethics Review Committee, Faculty of Medicine, University of Colombo, Sri Lanka (Reference number EC-12-04). Verbal consent was obtained from occupants of the premises prior to the survey and documented in Section 1 of the form. All data were anonymized.
Results
Surveyed premises
About 1469 premises were assessed during the entomological survey The different types of premises assessed were residential premises (91%), schools (0.7%), work or public sites (6.74%), and open lands (0.34%). Thirteen premises (0.88%) were not identified by type and were excluded from subsequent analysis (Table ). In each premise except on open land, a respondent was interviewed about vector control practices to a total of 1464 persons. All the respondents were adults, either residents of the residential premises or working at the non-residential premises. There were a total of 7186 persons living in the residential premises, 83.1% adults, and 16.5% children.
Table 1 Distribution of potential breeding sites (n = 15447) among the different types of premises
Mosquito breeding containers
About 15447 containers fulfilled the predetermined criteria of being potential breeding sites for Aedes mosquitoes. Of these, 18% contained water. Of the containers with water, 16.3% were positive for larvae and/or pupae (Table ).
About 11.9% residential premises, 66.7% schools, and 21.2% work or public sites had at least one container positive for larvae and or pupae. In terms of absolute numbers most containers positive for larvae and/or pupae were found in residential premises (338), followed by schools (85) and work places (27). However, the proportion of containers positive for larvae and/or pupae was lowest in residential premises (14.8%), followed by public work places (9.2%), and schools had the highest proportion of positive containers (63.9%) (Table ). In univariate analysis, the OR for non-residential premises being infested with larvae and/or pupae was 2.77 (95% CI 1.58, 4.86).
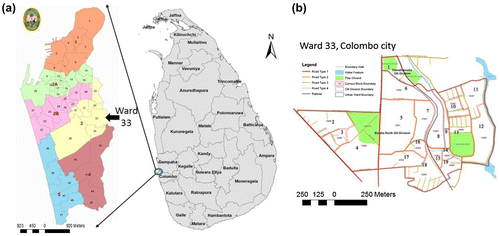
Of the five locations that were investigated in the premises, the outdoor grounds and roofing areas presented 1348 and 129 containers with water, respectively, and out of the total 1477, 22.6% containers with water were positive for larvae and/or pupae. The indoors living, toilet and bathroom, and cooking and washing areas housed 379, 653, and 266 containers with water, respectively. Out of the total 1299, 9.9% containers with water were positive for larvae and/or pupae (the previous numbers are not presented in tables or figures). Figure summarizes the main types of containers holding water (n = 2775) and the area where they were identified. The outdoor ground area had the highest number of positive breeding sites (Fig. ).
Figure 3 Main types of containers with water (n = 2775) within surveyed premises (top 10 containers).
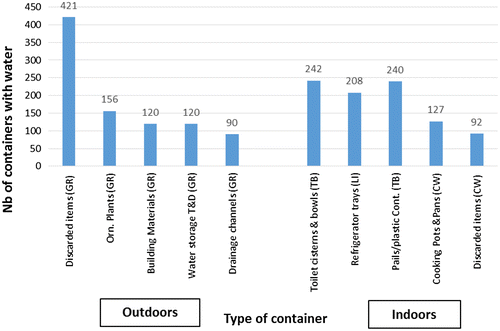
Figure 4 Main types of productive breeding sites positive for larvae and/or pupae (n = 452); (top 10 breeding sites).
The most common containers positive for larvae and/or pupae were identified as: discarded items (36.1%), ornamental ponds (11.5%), water tanks (5.8%), and flowerpots (4.4%), all in the outdoor ground areas. Despite there being 38 specified categories for containers, it was not possible to classify 34.3% positive breeding sites, 70.4% of these in indoor areas and 29.6% in outdoor areas. Figure summarizes the main types of positive breeding sites (n = 452), including the main ones that were not classified.
Vector control practices by community members
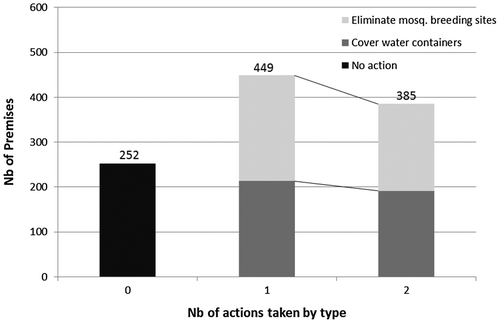
In 80.8% of residential premises, occupants indicated that they took measures to prevent mosquito breeding and biting. A total of 50 premises could not be assessed. In 45.5% of schools and 32.3% of work or public sites, respondents reported that no preventive measures were taken to prevent mosquito breeding. Being a respondent in a residential premise increased the odds of taking up vector control practices by a factor of 2.23 (1.49, 3.36).
The two most common responses to the open question ‘what do you do to prevent mosquito breeding and biting?’ were ‘keeping all water containers covered and protected from mosquitoes’ and ‘regularly eliminating mosquito-breeding sites’. These two actions were often reported as being performed together (Fig. ). Logistic regression performed for residential premises showed that taking at least one control measure had a preventive effect on the presence of potential breeding sites (OR = 0.47; 0.39–0.58), on the presence of containers with water (0.62; 0.54–0.72), and on the presence of containers positive for larvae and pupae (0.80; 0.61, 1.07), but not significantly for the latter.
Figure 5 Types and frequency of vector control actions taken against mosquito breeding in surveyed premises.
For prevention of mosquito bites, the use of (a) window screens and (b) personal protection measures, such as wearing long sleeved clothing, were reported by occupants in 11.3 and 10.6% of residential premises, respectively. For residential premises, logistic regression showed that the use of window screens did not provide a protective effect against positive breeding sites indoors (0.55, 0.13–2.34) not significant.
Among regularly used repellents, (a) coils and (b) electric fans were used by occupants of 268 (20%) and 201 (15%) residential premises, respectively. Occupants from 45.5% residential premises reported bed net use by adults. In addition, 33.4% of occupants of residential premises reported bed net use by children less than 12 years of age.
Discussion
This study helped to detect the prevalence of Aedes species and their breeding sites in the metropolitan Sri Lanka, thereby providing a tool to assist the development of more robust control strategies for dengue in the future. We documented that a substantial proportion of containers around residential premises and public places served as potential and actual breeding sites for Aedes mosquitoes.
Although residential premises had the highest absolute number of breeding sites, mainly because residential premises accounted for the vast majority of the surveyed premises, the proportion of containers positive for either larvae or pupae was found to be far higher in non-residential premises, particularly in schools. More than half of the schools had at least one container positive for either larvae or pupae. This is one of the key findings of our study and has important public health implications. Since school-aged children are a particularly vulnerable age group for dengue,Citation26–28 our findings call for more enhanced vector control measures at schools. A plausible reason for the high prevalence of breeding sites in schools and public work places is the lack of identifiable ‘ownership’ in the sense that it may be that no one is clearly assigned the responsibility for vector control at public places. Our survey showed that people operating in schools and public work places did not voluntarily implement vector control measures. In contrast, we found a high level of knowledge and implementation of simple vector control practices at the household level. In a recent randomized controlled trial in Nicaragua and Mexico involving more than 18,000 households, it was found that community mobilization improves the effectiveness of conventional dengue control programs.Citation18 A study in Laos found that proactive health education through appropriate mass media and community clean-up campaigns can strengthen and encourage community participation, particularly in addressing the problem of mosquito larvae in overlooked places, such as participants’ own homes.Citation29 Twinning of social participation and environmental management for improved dengue vector control was shown to be feasible and significantly reduced vector densities also in Mexico.Citation30 Because our survey was only cross-sectional, without a control group, we could present the current situation only in an urban Colombo subdistrict where extensive community health education and awareness of dengue has been promoted over the past decade. The overall container index (16.3%) was relatively low compared to figures reported in other studies of the vector population in similar settings.Citation31,32 To the best of our knowledge, there were no chemical control measures undertaken in residential premises during the time of the survey.
This study made a substantial effort to obtain detailed information on 38 different types of containers. However, containers were often not specified and the category ‘others’ ended up being the most important breeding sites for Aedes mosquitoes (70.4% in indoor areas and 29.6% in outdoor areas). It is important to identify theses containers to develop effective control strategies in the study site.Citation16,28,33 This survey confirmed that Aedes larvae and pupae were not concentrated, but dispersed among many containers.Citation34
The main containers in the outdoor ground areas (discarded items and receptacles, building materials, and flowerpots) stood out as the most potential breeding sites for larvae and/or pupae. Ornamental plants and water storage tanks were also represented in large numbers, consistent with previous findings.Citation16,35–37 Hence, community education should be targeted at addressing the importance of emptying, eliminating, or covering such containers. As for indoors, we found that household items and old appliances, such as refrigerator trays, were significant containers. The wide variety of potential and actual breeding containers underpins that no single intervention can be sufficient to control Aedes mosquitoes.
We also looked at the community measures taken beyond breeding sites. The use of window screens was reported in only 11.3% of residential premises. Coils and fans to deter mosquito biting were used regularly only by 20.0 and 14.9% of the residential premises, respectively, and this finding is consistent with other studies in the region.Citation38,39 Hardly any of the respondents used personal protective measures such as repellents to prevent mosquito biting. The use of bed nets, however, was a common practice in the community which is consistent with the data reported in a previous study.Citation38 Because Aedes mosquitoes are mainly day-biters, the use of bed nets is overall of limited value, but the World Health Organization still recommends their use, in particular for persons sleeping during the day-time. Under specific circumstances, the use of bed nets together with other preventive measures and vector control practices is recognized as a potential component of epidemic mitigation.Citation2,15,21
The study focused on the main breeding sites of larvae and/or pupae of the dengue vectors. Larvae and pupae of Aedes mosquitoes can provide information on Aedes infestation levels at immature stages and the effectiveness of a given vector control intervention; however they cannot provide exact information on the adult mosquito population.Citation2,33
The study had several limitations. The study had a cross-sectional design, and although provided a good baseline of potential breeding sites it could not infer causality between intervention measures and prevalence of breeding sites. The number of non-residential premises was small. Therefore, the conclusions reached about those must be interpreted with caution. The study points to types of premises with elevated risk because of relatively higher numbers of breeding sites; this finding will have to be corroborated with further investigations. The survey tool had specifications for 38 types of containers. However, because of the high diversity of the surveyed containers, it was difficult to classify all containers. Often containers that did not fit into our predetermined categories ended up being important breeding sites. This shows yet again how difficult vector control is at the community level, and may explain why so many community-based vector control measures have been ineffective.Citation6,16,38,40
Conclusion
Vector control practices, such as source reduction with community participation and appropriate handling of containers, are recognized as playing key roles in reducing mosquito populations in urban areas; and the relatively low container index in residential premises found in our study confirm this. When performed, the container management practices identified in the study were adequate for reducing the number potential containers and containers with water, but not significantly the number of breeding sites identified as containers positive for larvae or pupae. This study also provided a detailed information on the different types of containers that would be appropriately included and targeted in future vector surveillances programs. It is important to keep in mind that no single intervention will be sufficient to control the dengue vectors, and it is necessary to develop and promote integrated and synergistic interventions countrywide.Citation15
Schools and work or public sites were identified as carrying the highest risk for housing productive breeding sites, as well as manifesting shortcomings in preventive measures. These findings underpin the urgent need to educate not only occupants of residential premises, but also the senior management at schools and public work places. The association of construction sites with dengue outbreaks has been well documented.Citation37 Our findings have major implications for policy-makers, urban planners, and public health practitioners. Policies need to be in place to ensure that school management implements vector control measures at school level, both in classrooms and outdoor play areas. Unfortunately, health education for dengue control aimed at schools or public work places often lacks the necessary resources to maintain a regular program.Citation16,41 Our study underpins that health education together with strategies to ensure community participation are interventions that should be integrated into vector control programs. Resources should be allocated to enhance vector control activities in schools and work places.
Conflict of interest
The authors declare no conflict of interest.
Funding
DengueTools is funded under the Health theme of the Seventh Framework Programme of the European Community [grant agreement number 282589].
Acknowledgments
This research was facilitated by partners from the ‘DengueTools’ consortium. We wish to thank all Entomological Assistants for their contribution in the survey, D-3 MOH of Colombo Municipal Council for logistical support, and the Medical Research Institute for their technical assistance.
References
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010 Dec;8(12):S7–16.10.1038/nrmicro2460
- WHO. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization; 2009. 159 p.
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013 April;496(7446):504–7.10.1038/nature12060
- WHO-WPRO. The dengue strategic plan for the Asia Pacific Region 2008–2015. WHO. South-East Asia Region, Western Pacific Region. 2008;49.
- Tissera H, Amarasinghe A, De Silva AD, Kariyawasam P, Corbett KS, Katzelnick L, et al. Burden of dengue infection and disease in a pediatric cohort in urban Sri Lanka. Am J Trop Med Hyg. 2014 Jul 2;91(1):132–7.10.4269/ajtmh.13-0540
- Sirisena PD, Noordeen F. Evolution of dengue in Sri Lanka-changes in the virus, vector, and climate. Int J Infect Dis. 2013 Dec;11(19):6–12.
- Halstead SB. Dengue. Tropical medicine : science and practice. London / Hackensack, NJ: Imperial College Press / Distributed by World Scientific Publishing; 2008. xx, 485 p.
- WHO. Guidelines for dengue surveillance and mosquito control. 4th ed. Manila: World Health Organization, Regional Office for the Western Pacific; 2003. 105 p.
- WHO. The mosquito [Internet]. WHO. [cited 2015 Feb 12]. Available from: http://www.who.int/denguecontrol/mosquito/en/.
- Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next. Microbes Infect. 2010 Apr;12(4):272–9.10.1016/j.micinf.2009.12.011
- Wilder-Smith A. Dengue vaccines: dawning at last? The Lancet. 2014 Oct;384(9951):1327–9.10.1016/S0140-6736(14)61142-9
- Dengue Vaccine Initiative. Statement by the dengue vaccine initiative on philippines’ FDA regulatory approval of dengvaxia [Internet]. 2015 [cited 2016 Mar 16]. Available from: http://www.denguevaccines.org/sites/default/files/Statement%20by%20the%20Dengue%20Vaccine%20Initiative%20on%20Philippines’%20FDA%20Regulatory%20Approval%20of%20Dengvaxia.pdf.
- Asia-Pacific Dengue Prevention Board Meeting. Development of dengue vaccines. Status review and future considerations [Internet]. 2014 [cited 2016 Mar 16]. Available from: http://www.denguevaccines.org/sites/default/files/APDPBreport_Seoul%20Nov%202014_10315.pdf.
- Wilder-Smith A, Massad E. Age specific differences in efficacy and safety for the CYD-tetravalent dengue vaccine. Expert Rev Vaccines. 2016 Apr;15(4):437–41.10.1586/14760584.2016.1143366
- Achee NL, Gould F, Perkins TA, Reiner RC Jr, Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Neglected Trop Dis. 2015 May 7;9(5):e0003655.10.1371/journal.pntd.0003655
- Arunachalam N, Tana S, Espino F, Kittayapong P, Abeyewickreme W, Wai KT, et al. Eco-bio-social determinants of dengue vector breeding: a multicountry study in urban and periurban Asia. Bull World Health Organz. 2010 Mar;88(3):173–84.10.2471/BLT.00.000000
- Abeyewickreme W, Wickremasinghe AR, Karunatilake K, Sommerfeld J, Axel K. Community mobilization and household level waste management for dengue vector control in Gampaha district of Sri Lanka; an intervention study. Pathog Global Health. 2012 Dec;106(8):479–87.10.1179/2047773212Y.0000000060
- Andersson N, Nava-Aguilera E, Arosteguí J, Morales-Perez A, Suazo-Laguna H, Legorreta-Soberanis J, et al. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): cluster randomized controlled trial. BMJ. 2015 Jul;8(351):h3267.10.1136/bmj.h3267
- Tissera H, Ooi E, Gubler D, Tan Y, Logendra B, Wahala W, et al. New dengue virus type 1 genotype in Colombo, Sri Lanka. Emerg Infect Dis [Internet]. 2011 Nov [cited 2014 Oct 28];17(11). Available from: http://wwwnc.cdc.gov/eid/article/17/11/10-1893_article.htm.
- Thalagala N. Health system cost for dengue control and management in Colombo district, Sri Lanka in 2012. Colombo: Dengue Tool Surveillance Project, Epidemiology Unit, Ministry of Health; 2014. 42 p.
- Weerasinghe IS, Aryaprema S, Kusumawathie P, Perera D, Peiris L. Practical manual and guidelines for dengue vector surveillance. Sri Lanka: Medical Research Institute and National Dengue Coordination Unit; 2011. 138 p.
- Department of Census and Statistics. Census of population and housing [Internet]. Sri Lanka: Ministry of Finance and Planning; 2012. 274 p. Available from: http://www.statistics.gov.lk/PopHouSat/CPH2011/index.php?fileName=Activities/TentativelistofPublications.
- Wilder-Smith A, Renhorn K-E, Tissera H, Abu Bakar S, Alphey L, Kittayapong P, et al. DengueTools: innovative tools and strategies for the surveillance and control of dengue. Glob Health Action. 2012;5:1–9.
- Epidemiology Unit. Standard operational protocol for the entomological survey tool. Colombo: Ministry of Health; 2013.
- StataCorp. Stata statistical software. College Station, TX: StataCorp LP; 2013.
- Ooi E-E, Goh K-T, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006 Jun;12(6):887–93.10.3201/eid1206.051210
- Sabchareon A, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, et al. Dengue infection in children in Ratchaburi, Thailand: a cohort study. I. Epidemiology of symptomatic acute dengue infection in children, 2006–2009. PLoS Neglected Trop Dis [Internet]. 2012 Jul 31 [cited 2015 Sep 18];6(7):22–23. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3409110/.
- Olano VA, Matiz MI, Lenhart A, Cabezas L, Vargas SL, Jaramillo JF, et al. Schools as potential risk sites for vector-borne disease transmission: Mosquito vectors in rural schools in two municipalities in Colombia. J Am Mosq Control Assoc. 2015 Sep;31(3):212–22.10.2987/moco-31-03-212-222.1
- Sayavong C, Chompikul J, Wongsawass S, Rattanapan C. Knowledge, attitudes and preventive behaviors related to dengue vector breeding control measures among adults in communities of Vientiane, capital of the Lao PDR. J Infect Public Health. 2015 Oct;8(5):466–73.10.1016/j.jiph.2015.03.005
- Caprara A, Lima JWDO, Peixoto ACR, Motta CMV, Nobre JMS, Sommerfeld J, et al. Entomological impact and social participation in dengue control: a cluster randomized trial in Fortaleza, Brazil. Trans R Soc Trop Med Hyg. 2015 Feb;109(2):99–105.10.1093/trstmh/tru187
- Alcalá L, Quintero J, González-Uribe C, Brochero H. Estimation of Aedes aegypti (L.) (Diptera: Culicidae) productivity in households and public spaces in a dengue endemic city in Colombia. Bioméd Rev Inst Nac Salud. 2015 Jun;35(2):258–68.
- Getachew D, Tekie H, Gebre-Michael T, Balkew M, Mesfin A. Breeding sites of Aedes aegypti: potential dengue vectors in dire Dawa, East Ethiopia. Interdiscip Perspect Infect Dis. 2015;2015:1–8.
- Gubler DJ, Kuno G. Dengue and dengue hemorrhagic fever [Internet]. Wallingford ; New York: CAB International; 1997. xi, 478 p. Available from: http://www.loc.gov/catdir/enhancements/fy0605/97013184-d.html.
- Alexander N, Lenhart AE, Romero-Vivas CME, Barbazan P, Morrison AC, Barrera R, et al. Sample sizes for identifying the key types of container occupied by dengue-vector pupae: the use of entropy in analyses of compositional data. Ann Trop Med Parasitol. 2006 Apr;100(Suppl 1):S5–16.10.1179/136485906X105471
- Chadee DD, Doon R, Severson DW. Surveillance of dengue fever cases using a novel Aedes aegypti population sampling method in Trinidad, West Indies: the cardinal points approach. Acta Trop. 2007 Oct;104(1):1–7.10.1016/j.actatropica.2007.06.006
- Burkot TR, Handzel T, Schmaedick MA, Tufa J, Roberts JM, Graves PM. Productivity of natural and artificial containers for Aedes polynesiensis and Aedes aegypti in four American Samoan villages. Med Vet Entomol. 2007 Mar;21(1):22–9.10.1111/mve.2007.21.issue-1
- Sampaio AMM, Kligerman DC, Júnior SF. Dengue, related to rubble and building construction in Brazil. Waste Manage. 2009 Nov;29(11):2867–73.10.1016/j.wasman.2009.06.017
- Gunasekara T, Velathanthiri V, Weerasekara M, Fernando S, Peelawattage M, Guruge D, et al. Knowledge, attitudes and practices regarding dengue fever in a suburban community in Sri Lanka. Galle Med J [Internet]. 2012 May 29 [cited 2015 Nov 19];17(1). Available from: http://www.sljol.info/index.php/GMJ/article/view/4355.
- Yboa BC, Labrague LJ. Dengue Knowledge and Preventive Practices among Rural Residents in Samar Province, Philippines. Am J Public Health Res. 2013;1(2):47–52.10.12691/ajphr-1-2-2
- Brusich M, Grieco J, Penney N, Tisgratog R, Ritthison W, Chareonviriyaphap T, et al. Targeting educational campaigns for prevention of malaria and dengue fever: an assessment in Thailand. Parasites Vectors. 2015 Jan 23;8(1):1–14.10.1186/s13071-015-0653-4
- Khun S, Manderson L. Community and school-based health education for dengue control in rural Cambodia: a process evaluation. PLoS Neglected Trop Dis. 2007;1(3):e143.10.1371/journal.pntd.0000143