ABSTRACT
Health Technology Assessment (HTA) is a multidisciplinary tool to inform healthcare decision-making. HTA has been implemented in high-income countries (HIC) for several decades but has only recently seen a growing investment in low- and middle-income countries. A scoping review was undertaken to define and compare the role of HTA in least developed and lower middle-income countries (LLMIC). MEDLINE and EMBASE databases were searched from January 2015 to August 2021. A matrix comprising categories on HTA objectives, methods, geographies, and partnerships was used for data extraction and synthesis to present our findings. The review identified 50 relevant articles. The matrix was populated and sub-divided into further categories as appropriate. We highlight topical aspects of HTA, including initiatives to overcome well-documented challenges around data and capacity development, and identify gaps in the research for consideration. Those areas we found to be under-studied or under-utilized included disinvestment, early HTA/implementation, system-level interventions, and cross-sectoral partnerships. We consider broad practical implications for decision-makers and researchers aiming to achieve greater interconnectedness between HTA and health systems and generate recommendations that LLMIC can use for HTA implementation. Whilst HIC may have led the way, LLMIC are increasingly beginning to develop HTA processes to assist in their healthcare decision-making. This review provides a forward-looking model that LLMIC can point to as a reference for their own implementation. We hope this can be seen as timely and useful contributions to optimize the impact of HTA in an era of investment and expansion and to encourage debate and implementation.
1 Background
1.1 What is HTA
Healthcare resources are finite in every setting and, irrespective of the financing and organization of a country’s healthcare system, decisions on what interventions to cover, and under what circumstances, have to be made; ideally, in an evidence-based and equitable way[Citation1]. Health Technology Assessment (HTA) is a multidisciplinary tool used to inform decision-making in healthcare systems. Definitions of HTA have evolved over time but a common theme is the systematic process of evaluation to inform decisions. The World Health Organization (WHO) defines HTA as ‘the systematic evaluation of properties, effects and/or impacts of health technologies and interventions. It covers both the direct, intended consequences of technologies and interventions and their indirect, unintended consequences. The approach is used to inform policy and decision-making in health care, especially on how best to allocate limited funds to health interventions and technologies’ (WHO 2019). More recently, an international joint task group co-led and convened by the International Network of Agencies for Health Technology Assessment (INAHTA) and Health Technology Assessment international (HTAi) has proposed a revised definition of HTA: ‘A multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle. The purpose is to inform decision-making in order to promote an equitable, efficient and high-quality health system’ [Citation2]. Whilst this definition refers to the evaluation of a health technology, it also brings in the value to the health system as a whole – with perhaps more potential for traction at the policy level. We also refer to Glassman’s et al definition of HTA as ‘locally relevant, fair and evidence-informed processes’ [Citation3] used to inform health benefit plans or essential medicines lists recognizing that existing priority setting mechanisms, particularly in low- and middle-income countries (LMIC), are often not – if at all – referred to as HTA.
Typically, the term ‘HTA’ refers to individual studies on a specific technology. However, increasingly, HTA is used to refer to a more systematic process at the systems level to inform priority setting and decision-making, i.e. as a tool for priority setting with its explicit consideration of costs and benefits [Citation4]. We thus distinguish between HTA at the system level as a tool or process for priority setting, and as an assessment and/or appraisal of an individual technology (or technologies). In this review, we are interested in the former definition and use a systems level framework for the literature search.
1.2 Emergence of HTA
Technology assessment first emerged from the public sector in the United States (US). Health technologies in the US were evaluated at the request of Congress in the 1970s and from this came the term ‘Health Technology Assessment’ [Citation5], now used internationally. The adoption of this term gained popularity in wealthier countries that prioritized the evaluation and improvement of healthcare in the face of rising costs and the adoption of unproven technologies with the 1990s seeing an era of institutionalization of HTA in Europe and elsewhere. The first national agency was established in 1987, namely the Swedish Council on Technology Assessment in Health Care (SBU). The Canadian Agency for Drugs and Technologies in Health (CADTH), a rebranding of the Canadian Coordinating Office for Health Technology Assessment (CCOHTA) was formed in 1989. Australia was the first country in the world to introduce a cost component to HTA decisions in the early 1990s [Citation6]. The United Kingdom’s National Institute for Health and Care Excellence (NICE) followed later in 1999 to reduce variation in the availability and quality of treatments and care in the UK’s National Health Service (NHS)Footnote1; and the Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) in Germany in 2004 (O’Donnell et al., 2009) see . Many countries around the world now have well-established HTA processes at the national level.Footnote2
Figure 1. Emergence of HTA.
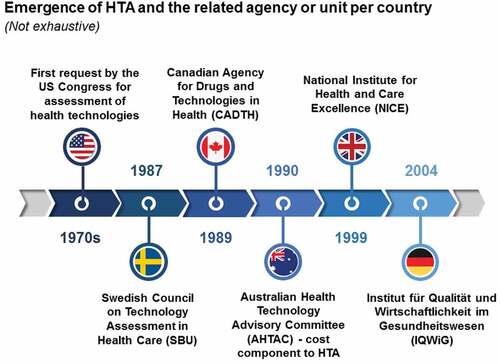
Decision-making bodies use HTA to support a myriad of health decisions including the roll-out of broad public health programs, priority setting by identifying interventions that produce the greatest health gain at the lowest cost to improve resource allocation, decisions to publicly subsidize medical services and medicines, and developing clinical guidelines (WHO 2019).
There is, however, necessarily much diversity in the role and application of HTA given the heterogeneity of culture, history, politics, health‐care financing, and the underlying rationale or purpose that all have had important effects on the setup and functioning of these processes [Citation7]. As demands for efficiency and value in healthcare increase, HTA is increasingly requested at different levels of the health system: by local health authorities, hospitals, and other healthcare organizations. Countries continue to improve transparency, accountability, and share knowledge and good practice in HTA, with many international networks and relationships existing among countries and their HTA bodies. For instance, the European Network for HTA (EUNetHTA) supports collaboration between European HTA organizations to bring value at the European, national, and regional levelFootnote3; and the International Decision Support Initiative (iDSI) is a global network of health, policy, and economic expertise, supporting LMIC countries to make better decisions about how much public money to spend on healthcare and how to make that money go further.Footnote4
1.3 How can HTA support Universal Health Coverage
Globally, a growing commitment to universal health coverage (UHC) is promoting the role and institutionalization of HTA [Citation8–10] in LMIC. UHC aims to provide effective health services to populations, without financial hardship (WHO 2015). The institutionalization of HTA is seen as pivotal to supporting UHC as a means of improving allocation of finite resources and the maximization of health. As a country’s Gross Domestic Product (GDP) per capita increases, they are expected to transition from aid and to take more responsibility for the strategic planning of their investments [Citation3,Citation11]. Priority setting in health is a necessary response to inform decision-makers in a budget constrained environment in those countries transitioning from donor support to a greater reliance on domestic resources with a consequent increased risk of financing gaps in the social sectors [Citation11]. HTA has been used in India to inform national clinical guidelines and quality standards to improve quality-of-care delivery while in Sub-Saharan Africa, countries including Ghana, Tanzania and Zambia are seeking to use HTA for in the design of their health benefit packages and other purchasing decisions [Citation12–14]. In Thailand, a country at the forefront of UHC, HTA has been used to inform decision-making for over a decade, notably to define the benefits package and the National List of Essential Medicines (NLEM) [Citation15]. Whilst each county has unique goals and challenges in relation to HTA and their health systems, observing or collaborating with other countries has helped to establish and improve HTA. WHO has supported regional and national HTA capacity building for countries moving toward UHC by encouraging information sharing and exchange (WHO, 2021).Footnote5 For instance, the WHO Eastern Mediterranean, South East Asia and Western Pacific regions all have inter-country meetings aimed at supporting the technical development of national HTA programs. These meetings provide a network for HTA stakeholders to share knowledge and develop capacity. Leveraging expertise and resources across the global movement for priority setting in health, especially now to support countries in their COVID-19 recovery, is paramount.
1.4 Why this review
In 2015, WHO undertook a surveyFootnote6 to assess the status of HTA globally in response to the World Health Assembly Resolution (WHA 67.23) [Citation9]. Since then, there has been an increase in the published literature of HTA in LMIC, including global surveys on specific topics such as the impact of HTA [Citation16] and the use of deliberative processes [Citation17], regional reviews [Citation18] and several individual country-level assessments and roadmaps [Citation19,Citation20].
Unlike these studies that are specific to a topic or issue, the aim of this paper is to provide a comprehensive overview of the HTA landscape in least developed to lower middle-income countries (LLMIC).Footnote7 Our focus is on the available literature pertaining to LLMIC in keeping with the Journal’s particular emphasis on diseases impacting on the poorest regions of the world. Taking WHO 2015 survey as our baseline, we have reviewed the most recent literature to capture how LLMIC are now using HTA (or not) in terms of methods and objectives, at what level, funding, and partnerships. We exclude upper-middle-income countries (UMIC) and HIC which are generally at a more advanced stage of HTA development.Footnote8 This is important in being able to share more relevant insights and lessons in an era of investment and expansion of HTA, in particular, for LLMIC, through better understanding of HTA’s role in delivering health outcomes and value for money at the health system level. Ultimately, we envisage this can help to generate a forward-looking model that these countries can use to implement HTA.
2. Methods
A search strategy was undertaken following best practice guidelines and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-Scr) guidance [Citation21]. The rationale for a scoping review being the best methodology for this paper is because it is to provide an overview and mapping of the emerging evidence on HTA in LLMIC at a systems level in order to identify and inform the potential role of HTA in these countries that are very new to these processes. This is also to develop a better understanding of the nature and distribution of this emerging literature (where being produced, by whom etc.) rather than a more precise research question as would befit a systematic review [Citation22,Citation23]. Authors’ existing knowledge and researchFootnote9 was drawn upon to produce a matrix to facilitate data extraction. We searched the literature for country- and region-specific papers to populate the matrix by categories (and sub-categories) as follows:
HTA (Applied or Methodological)
Objective and Methods (Budget and resource allocation, UHC, research and development, technical assessment, policy impact)
Technology type
Funding (Stakeholders, Agencies, Policy-makers)
Geographies (Regional, LMIC, individual country)
Scope of Analysis (local, national)
Partnerships (Referencing other HTA bodies or countries, private health care, public sector).
We have used this matrix to analyze our findings, revealing themes, commonalities, and differences in the role of HTA across countries.
2.1 Search strategy
The search was conducted on the 20 August 2021 using MEDLINE and EMBASE databases to identify publications between 1 January 2015 – the year of publication of the WHO HTA surveyFootnote10 – and August 2021. The search strategy comprised terms relating to ‘health technology assessment’ and LLMIC (Supplementary Material Table 1). Inclusion criteria were HTA in LLMIC countries as identified from the list of countries classified by The World Bank according to their level of income and the United Nation’s definition of LDC (Supplementary Material Table 2). Reference was made to gray literature but we did not search HTA agencies’ websites given we were excluding HTA reports of specific technologies. Exclusion criteria were articles focusing on HTA in UMIC and HIC; technical assessments or reports including economic evaluations; articles not in English. The screening and selection of studies were performed by two independent reviewers (FM and GC). We extracted details for each article on study design, whether the first and last author is from LMIC or HIC, and the data to populate the matrix (JB, AF, HF, EG, NS, and WS).Footnote11
3. Results
3.1 Articles retrieved
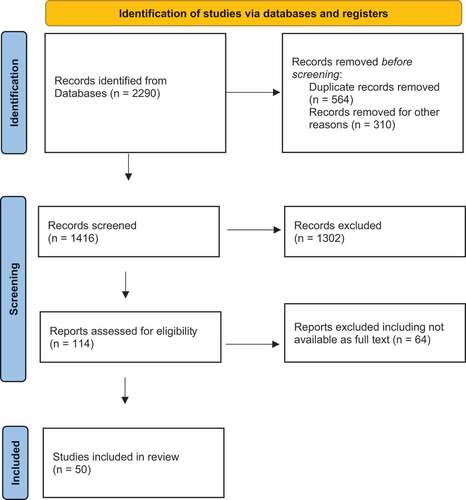
We retrieved 2,290 records and included 50 studies (). The search strategy was used to populate a matrix containing the categories and subcategories as described above (Supplementary Material – Matrix). This provides the details of all articles included. We have used this matrix to synthesis the data and present our findings.
3.2 Characteristics of included articles
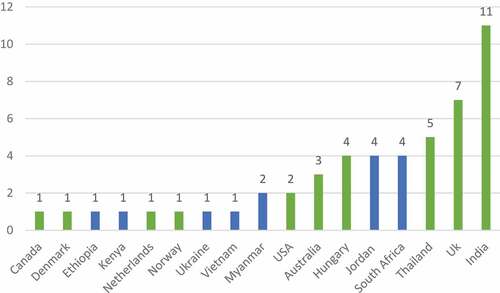
We extracted first and last authors’ country of their research institution and study type to describe characteristics of the article. Collaboration in authorship across LMIC and HIC has increased local LMIC capacity in health research [Citation24–26]. Where an author was affiliated with more than one institution, we chose the institution in a LLMIC as their country of representation. Of the 50 papers, most first authors (n = 23) were from LMICs, 9 were from UMIC and 18 were from HIC. Most first authors were from India (n = 11 papers), followed by the UK (n = 7) - see . The highest number of last authors () were from HIC (n = 21) followed by UMIC (n = 20) and LMIC (n = 9). The proportion of LMIC authorship across all papers ranged from 10% to a 100% (Supplementary Materials – Matrix).
Figure 3. First authors by country.
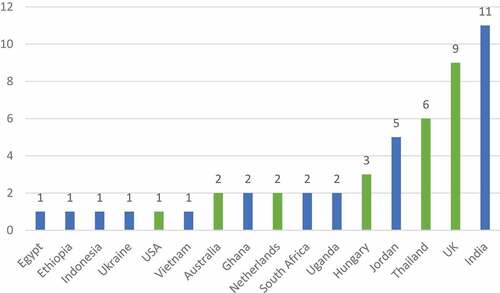
We highlight here in blue the LLMIC countries of last authorship, namely Ethiopia, India, Jordan, Kenya, Myanmar, South Africa, Ukraine, and Vietnam.
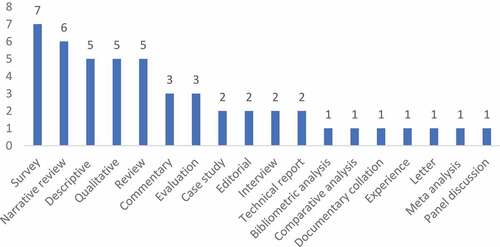
The most common study designs were surveys, descriptive, narrative, and qualitative articles - see . About half the articles (n = 23) were related to methodological research. Examples of methodological research included adapting economic evaluations to accommodate demand and supply constraints of health services in LMIC [Citation27]; and how multi-criteria decision analysis (MCDA) can improve upon existing economic evaluation methods by adding dimensions of broader social value but the challenges it represents for HTA in LMIC [Citation28,Citation29].Footnote12
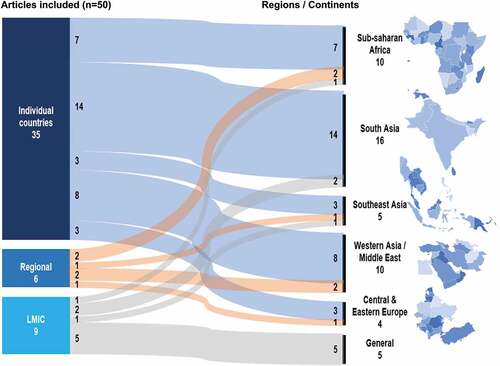
3.2 Geographic distribution
We reported articles referring to LMIC, regions (such as Sub-Saharan Africa), and individual countries -see . Additionally, we classified the scope of HTA analysis as local and national. Local scope of analysis refers to HTA at a city or township level, and national scope of analysis refers to studies at a systems level. Nine articles referred to LMIC generally [Citation17,Citation27,Citation30–36] whereas six articles discussed HTA regionally: South-East Asia [Citation37], Eastern Europe [Citation38], Middle East [Citation29,Citation39], Sub-Saharan Africa [Citation18], and East Africa [Citation40]. Many (n = 35) articles referred to specific countries [Citation18–20,Citation28,Citation31,Citation36,Citation39,Citation41–67]. The categories are not mutually exclusive.
3.3 The different roles of HTA
Information produced by HTA was found to underpin many different uses at different levels of the healthcare system: national and state (e.g. India), and local e.g. hospital-based HTA (Jordan). Employing headings defined by O’Brien et al. [Citation32] of ‘priority setting’, ‘purchasing’ and ‘quality improvement’, we grouped HTA activities as shown in . Examples of HTA activities under priority setting were the most common [Citation18,Citation30,Citation31,Citation34,Citation36,Citation38,Citation39,Citation42,Citation44,Citation47,Citation49,Citation52,Citation61]; followed by purchasing [Citation41,Citation51,Citation61,Citation66,Citation67]. Quality improvement activities, as defined by O’Brien et al., had only minimal mention [Citation29,Citation52]. In making policy-makers and payers better aware of how they can use HTA, O’Brien et al. use these headings to illustrate different practical applications. We note there was also little mention of disinvestment where evidence from HTA could be used to identify and exclude ineffective health innovations causing ethical and social concerns within the health system in order to promote efficiency and equity gains [Citation68].
Figure 7. Roles of HTACitation12.
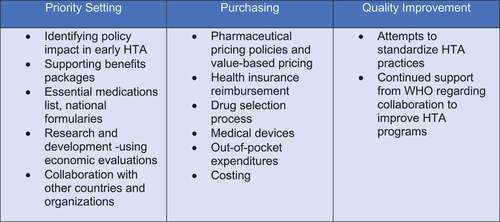
Most country articles indicated that the criteria for HTA encompassed economic evaluation (value of a technology) and budget impact (affordability). Given the limited resources, decision-makers need to consider affordability alongside assessments of value, and this will depend how HTA and budget responsibilities align in any given country.Footnote13 With the recent introduction of some innovative and curative – but very high cost – medicines (e.g. for Hepatitis C) affordability issues may outweigh value considerations even in HIC. It might be helpful to countries without formal HTA agencies to use evidence-informed deliberative processes [Citation17,Citation29] to trade-off health gains and other values, for example, equity,Footnote14 and from what perspective, against affordability [as a function of treatment costs and population size, it is not seen as ‘value’ [Citation69]] in addition to methodological reference cases such as the iDSI guidance [Citation70].
3.3 Type of technology
Pharmaceuticals were the most frequently mentioned type of technology [Citation19,Citation29,Citation39–41,Citation51,Citation57,Citation63,Citation66,Citation67]. Only a few articles mentioned other types of technologies, including medical devices [Citation57] and community-based interventions [Citation44,Citation57]; and despite the WHO 2015 survey indicating that population-wide interventions have a greater focus in LMIC with greater potential impact, and generally being more equitable, this was also found lacking. Reflecting the lack of HTA activities under ‘quality improvement’ above, there is potential for ‘meso’ (e.g. clinical practice guidelines to manage patient care pathways within a healthcare system) and ‘macro’ (e.g. efficiency, organization, and strengthening of the healthcare system) level interventions to have a greater role [Citation71].Footnote15
3.4 Partnerships
The importance of HTA partnerships is paramount and was a common theme in many articles. Three types of partnerships emerged from the matrix: international resources referring to support capacity development and the sharing of ideas between LLMIC, and with other countries and international organizations; collaborations with universities; and with Ministries of Health and other governmental bodies, including medical research councils (). Regarding the latter, LLMIC governments have traditionally not allocated sufficient resources into health research [Citation19,Citation30,Citation36] and with the absence of HTA-specific units within governments, this makes it even more challenging to allocate funding for HTA [Citation30]. The limited availability of budgets to fund HTA, coupled with other barriers such as lack of capacity, and explicit HTA policies and guidelines limit HTA absorption by national governments [Citation36,Citation54]. Although the use of HTA from a private payer’s perspective was discussed [Citation29] there was limited mention of the private sector as a service provider, with government institutions expected to be the main users of HTA [Citation72]. The latest WHO survey [Citation73] similarly found that the private sector was the least-represented stakeholder across the different stages in HTA.
Figure 8. PartnershipsCitation15.

4. Future directions
4.1 Applications of HTA in LLMIC
LLMIC are more likely to use HTA in its more familiar roles of determining coverage of healthcare provision, pricing, and priority setting. We identify a couple of gaps in the literature for consideration regarding other potential roles of HTA in LLMIC. Arguably, both these activities discussed below have been exacerbated by the Covid-19 pandemic given the need to better spend health dollars in the face of economic contraction further compounding existing pressures on healthcare budgets; and where developers need to rapidly evaluate and optimize or adapt existing technologies [Citation74].
Firstly, disinvestment has been defined as ‘the process of (partially or completely) withdrawing health resources from any existing healthcare practices, procedures, technologies, or pharmaceuticals that are deemed to deliver little or no health gain for their cost, and thus are not efficient health resource allocations’ [Citation75]. Although there has been a lot of research in this field [Citation76,Citation77], disinvestment is still being done in an ad hoc manner by HTA bodies, not least because disinvesting in a technology that is in current usage or in clinical practice is challenging, with the ‘stopping’ of doing something already in the system less easy to do compared with ‘taking up’ a technology [Citation78]. The application of disinvestment or health technology reassessment (HTR) have been highlighted in previous reviews [Citation77,Citation78]. As HTA is mostly used for new investments and single technology appraisals, it has been found to be more challenging to apply as a disinvestment framework [Citation79]. A scoping review on disinvestment (under review) [Citation80] identifies HTA/HTR and PBMA as the most common methods used to assess potential candidates for disinvestment, with most disinvestment initiatives implemented in HIC and UMIC. Given the current climate, if countries are unable to spend more, they need to spend better. Priority setting processes are needed to ensure that investments in health continue to strive for improving overall population health. With an estimated less than $4 out of every $100 USD in public funds being spent on a health maximizing technology in LMIC [Citation81], there is much scope for LMIC to lead on this.
Secondly, for countries with more constraints on capacity, HTA may be made more relevant by greater explicit consideration of organizational issues [Citation79]. Although implementation is not strictly considered an integral part of the HTA process, providing evidence about the expected impact of a technology on health system structure, processes and resources might be valuable to inform the construct and recommendations of an HTA or develop an implementation plan [Citation79]. Yet, this aspect of HTA is – not unexpectedly – found to be lacking [Citation82,Citation83]. In the UK, the Medical Research Council (MRC) recently updated their guidance on the development and evaluation of complex interventions [Citation84]. The MRC recommend that implementation be considered alongside economic evaluation throughout the development and evaluation of a health technology. The National Institute for Health Research’s (NIHR) Health Technology Assessment (HTA) programme is the largest funder of health research in the UK but a recent review found that methods to incorporate implementation within economic evaluation were typically inconsistently applied and that no generally accepted guidance or methodology is currently available [Citation85]. To date, implementation is not recommended as a core component of a health technology evaluation by NICE. Further research will be required to address the challenge of incorporating implementation within economic evaluation in HIC and LMIC.
Rather than maintaining an emphasis on user-focused HTA i.e. where the mainstay consists of a synthesis of clinical evidence and economic evaluation comparing available technologies, we consider the application of ‘early HTA’ where system constraints are addressed whilst the technology is still under development [Citation74] i.e. encouraging engagement with stakeholders at an early stage to identify additional costs and non-health outcomes, and crucially, to use these insights and data to inform the development and implementation of health technologies. Indeed, early HTA has been promoted as a tool to support LMIC’s need for a way to choose more of the ‘right’ new products and less of the ‘wrong’ products [Citation86]. For example, the adaption of COVID testsFootnote16 to context could be seen as what the Economic and Social Committee of the United Nations describe as ‘frugal’ innovation – taking an existing technology and making it less expensive and more accessible – or ‘hybrid’ innovation – repurposing an existing technology (United Nations ESC 2016) [Citation74]. By drawing on a broad range of multidisciplinary methods, early HTA aims to inform the developers of the technology about a wider range of questions including how the technology should be designed, used, and implemented. We suggest that early HTAFootnote17 can offer scientific, transparent, and systematic methods such as epidemiological analysis, qualitative methods (e.g. expert elicitation, patient interviews) or quantitative (e.g. modeling to understand behavior in complex systems, or alternative service delivery practices) to gain a thorough understanding of the human factors, infrastructure, and healthcare organization of the context where the technology is to be deployed [Citation74]. Furthermore, this should also facilitate greater integration and communication among HTA and delivery systems’ stakeholders as evidence-generating methods gather and synthesize many perspectives.
4.2 LLMIC use of types of technologies in HTA
HTA activities under ‘quality improvement’, for example, treatment and referral pathways, and education and training of the health workforce [Citation32] were found to be lacking. Indeed, the more recent definition of HTA explicitly links the goals of HTA with that of the health systems’ objectives of efficiency, equity and quality [Citation68]. Although the conventional focus of HTA on technologies that are marginal or incremental to the system is still relevant, a refocus to such process or systems-wide interventions may result in improved methods to deliver an existing technology, or help develop innovative ways of overcoming barriers and challenges in adoption behavior or infrastructure [Citation74]. As Garrido et al. state [Citation87], ‘although health products and health care services have been its preponderant focus to date, HTA should develop to increase its focus on the ‘technologies applied to health care’ (i.e. the regulatory and policy measures for managing and organizing health care systems) and on policies in non-health care sectors”. It may be that many governments do not yet demand this approach of HTA but given the poor infrastructure in many LMIC ‘macro HTA aimed at developing performance in the healthcare system may be of greater importance in this context than in HICs where HTA has had a more traditional micro HTA role of appraisal of single/related technologies’ [Citation71]. Whilst the focus of HTA on technologies that are marginal or incremental to the system remains relevant and the convention, a refocus to process or system-wide interventions may result in an improvement in methods used to deliver an existing technology, or help develop innovative ways of overcoming barriers and challenges in adoption behavior or infrastructure [Citation88]. This would not be without its methodological challenges and Thomas and Chalkidou [Citation89] discuss these as well as the benefits of applying economic evaluation at the macro level. Garrido et al.’s [Citation90] recommendation of over a decade ago that countries embarking on HTA should not consider establishing separate agencies for HTA, quality development, performance measurement, and health services development but should rather combine these functions and goals into a common knowledge strategy for evidence-informed decision-making on health care and the health system would seem to still stand and speak to this refocus.
4.3 LLMIC and partnerships in HTA
The evident lack of partnerships found with the private sector is an important omission where the presence of private health providers is significant as a prominent provider of health service delivery [Citation91]. Yet, policymakers struggle to identify the role of the private sector in their UHC objectives with WHO recently publishing a strategy report addressing a critical health system governance gap for the effective engagement of the private health service delivery sector in this context.Footnote18 The inclusion of the private sector could also be strategically important to provide funding [Citation30,Citation46] and with the potential to increase transparency [Citation34]. For example, Chalkidou et al. propose a market-driven, value-based advance commitment to ‘crowd-in’ private sector funding for health research and development in LMIC [Citation92].
The pandemic has forged greater collaborative working across the healthcare system and beyond in many countries. For example, in Jordan, private–public partnerships have played an essential role in building capacity in HTA. The private sector has provided training to the Ministry of Health (MoH) and has also brought in regional experts to support the undertaking of HTA on their behalf. During the pandemic, these private–public partnerships came to the fore. Patients, regardless of their insurance status and whether they were admitted to a private or public hospital, were covered by the MoH. Such a response can help pave and accelerate the way to UHC [Citation93]. Cross-sectoral partnerships, and the inclusion, co-ordination, and integration of governmental, private and NGO sectors require the responsible oversight of state authorities to shape evidence-based policy. For instance, in Nepal, though no formal HTA body presides over health policy decisions, there are numerous partnerships between foreign universities, INGOs and NGOs that has led to success of projects that promote sustainable development goals. Furthermore, the collaboration noted with the health research council in Nepal (and India) is a way of directly aligning HTA with the research and data, the latter being a well-known challenge in LMIC that often lack comprehensive and reliable local health and economic data but we acknowledge that this challenge not limited to LMIC [Citation94]. Another example of a cross-sectoral partnership is that of Thanzi la Onse (Health of All) operation in southern and east Africa where universities (York, Kings College, Malawi universities), the Ministry of Health of Malawi, the Medical Research Council Uganda Research Unit, Center for Global Development, and East, Central and Southern Africa Research Community have partnered with funding from the UK Research and Innovation’s Global Challenges Research Fund for priority setting within health benefit packages.
Some regional HTA networks [Citation72,Citation95] bring together countries with different income levels, e.g. the Association of Southeast Asian Nations (ASEAN) brings together 10 countries (Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand and Vietnam) supporting the region’s continuous effort to build and strengthen evidence-informed decision-making. This approach could be replicated in other regional groups e.g. African Union or ‘sub’ regional e.g. West Africa (West African Health Organization).Footnote19 For example, the Africa Centers for Disease Control and Prevention (Africa CDC), within the African Union, is working to incubate a health economics unit,Footnote20, Footnote21 that will support and guide member states on priority setting practices in each of the Africa CDC five strategic pillars (disease intelligence and surveillance; preparedness and response; information systems; laboratory networks and systems; national public health institutes and public health research) [Citation96]. This will strengthen the Africa CDC health economics unit support to member states in undertaking HTA.
4.4 Investing in HTA for sustainability and impact
Finally, understanding how we might quantify the costs and benefits of investing in HTA is important for both policy makers and donors. If we think priority setting should be better informed by evidence and the use of tools such as HTA, then this necessarily requires institutional change to establish a sustainable system. The global expansion of HTA and an increasing investment in these processes at the systems level in LMIC has generated greater interest from policy makers about the value and return on investment (ROI) of HTA. Consequently, we have to consider the opportunity costs which are needed to establish and maintain HTA systems [Citation97]. Although countries where HTA has been institutionalized spend relatively little as a proportion of total health spending (estimates range from 0.01 to 1% of total public spending) [Citation98,Citation99] HTA competes with other health priorities in the context of scarce resources.Footnote22 The Organization for Economic Co-operation and Development (OECD) [Citation100] use a regression-based analysis to assess the effects of policies and institutions, including HTA, on public health spending and life expectancy. The approach computed an HTA score based on countries’ responses to questions about their structure and capacity for HTA, whether cost-effectiveness and affordability were taken into account, and how HTA information was used. Whilst this can only be assumed to be an association (rather than a causal relationship), HTA’s use in providing evidence related to new technologies was likely to both magnify life expectancy gains but also that of spending on health care.
We commend and encourage HTA impact evaluations, not just for accountability purposes but crucially, for learning, especially for those countries beginning to develop HTA processes to assist in their healthcare decision-making. A lack of funding was identified as a key reason for not investing in HTA according to both WHO HTA surveys with others including the OECD [Citation100] and Loblova et al. [Citation101] highlighting the upfront costs of establishing HTA systems that will potentially discourage LMIC from investing in these processes and agencies. Understanding the impact and value for money of HTA is pivotal to governmental buy-in. There have been many evaluations of HTA that could be categorized as impact frameworks [Citation100,Citation102–108] or country-level evaluations [Citation109–115]. Yet, there remains a paucity of studies that quantify the impact of HTA in terms of net health gains and return on investment at a country or systems level. Even in countries where HTA is well established, this type of evidence is limited given the methodological and data challenges in linking change in clinical practice and assessing impacts further downstream in the health system. A recent INAHTA report [Citation16] defines HTA impact assessment to be an evaluation of the uptake and the effects of an HTA report, distinguishing between the impact of an HTA report and the impact of the agency. The global expansion of HTA, its variable implementation, the lack of quantified evidence on health outcomes, along with an increasing investment in these processes at the systems level in LMIC means that a lack of longer-term impact assessment may undermine its importance and value.
4.5 Strengths and limitations
The main strength of this project was to have an authorship team with backgrounds in various disciplines and HTA experience in relevant countries including Nepal, India, Zambia, Indonesia, and Jordan (LLMIC), and Malaysia (UMIC). This project is a high-level review so it was particularly helpful to have a team with first-hand experience of HTA in relevant countries though we note that all coauthors work in HTA in some capacity, and this may bias toward HTA. HEHTA is fortunate to be able to tap into its multi-national team of colleagues, and networks of global HTA practitioners and external academic experts, enriching our knowledge base by bringing a greater global perspective to our research, teaching, and practice of HTA – and to some extent compensating for the limitations below.
Given the ‘newness’ of HTA in LLMIC, we did not aim to closely examine methods of assessment or value frameworks for appraisal, either in use or being developed. Rather, we aimed for a wider view by exploring the roles HTA can or could take in these contexts new to HTA. In line with keeping the review contained at a strategic level, we purposefully excluded technical reports on discrete applications of HTA. This means we may have missed detail on practical applications of HTA. For example, Indonesia has recently published an HTA to support disinvestment of adding cetuximab to chemotherapy for metastatic colorectal cancer from the Indonesian National Drug Formulary [Citation116]. We did not use critical appraisal tools with the selected articles but rather focussed on content and context that was relevant to our aims. This is in line with scoping review indications [Citation117].
Finally, we acknowledge the limitation of omitting non-English language articles. Five countries in Latin America fall into the LLMIC category (Bolivia, El Salvador, Haiti, Honduras, Nicaragua), of which Bolivia and El Salvador have an HTA presence according to WHO. With 21 French-speaking countries in Africa and the WHO 2021 survey listing half of those having an HTA presence, this is a notable shortcoming. We may have also missed important information in the gray literature, especially as professionals in this area may not have the means or capacity to publish open access research articles. However, it is unlikely many countries without institutionalized HTA would have extensive websites.
5. Conclusions and recommendations
Whilst HIC may have led the way, LLMIC are increasingly developing HTA processes to support healthcare decision-making. We provide insights into current uses of HTA in LMIC and highlight some under-studied or under-utilized aspects (macro HTA, disinvestment, early HTA/implementation, and cross-sectoral partnerships). We consider the following broad practical implications for decision-makers and researchers aiming to achieve greater interconnectedness between HTA and health systems, especially in LLMIC. Indeed, Garrido et al. [Citation90] recommend that ‘countries embarking on HTA should not consider establishing separate agencies for HTA, quality development, performance measurement, and health services development but should rather combine these functions and goals into a common knowledge strategy for evidence-informed decision-making on health care and the health system’.
Macro HTA: by broadening its scope to inform not only technology reimbursement decisions but also health system organization and service delivery, HTA may be made more impactful in LLMIC and a way to better integrate HTA within healthcare delivery systems.
Disinvestment: in a climate of economic pressure for all health systems, LLMIC could lead where other HTA bodies have been challenged in adopting systematic processes and methods.
Early HTA/implementation: Efforts to rationalize the use of resources are only valuable if recommendations are implemented in practice [Citation118]; it is only when those decisions result in practice change can better health be achieved.Footnote23 Using methods to adapt technology to context and generate data to bridge the divide between HTA recommendations and practice change, early HTA can support greater impact of HTA on health outcomes.
Cross-sectoral partnerships: WHO recognize UHC cannot be achieved without the private sector. It is essential to engage public and private sector – and we suggest the non-governmental sector too – in HTA, as partnerships to achieve better health.
This review provides a forward-looking model that LMIC can use for HTA implementation with the prospect of innovative HTA approaches. It may be that LLMIC can advance HTA in ways most relevant to them, in addition to the familiar role of HTA to determine coverage of healthcare services and medicines. We hope this is a timely and useful contribution to optimize the impact of HTA in an era of investment and expansion and to encourage debate and implementation.
Ethics
Ethics approval was not required because we did not collect primary data or work directly with participants.
Supplemental Material
Download MS Excel (25 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20477724.2022.2106108
Additional information
Funding
Notes
1 NICE’s first appraisal was to recommend healthy wisdom teeth should not be removed, estimated to save the NHS £5 m a year. The first clinical guideline was in mental health and was developed to cover the patient pathway from diagnosis to treatment. This had significant effect as there was then little in the way of guidance for mental health nationally or internationally. The Citizens’ Council was established providing a public perspective on ethical issues. The broad and inclusive nature of its various committees is a recognized strength of NICE. 20 years of NICE.
4 About Us | iDSI (idsihealth.org).
6 Second WHO HTA survey published December 2021.
7 We use this abbreviation referring to Least Development Counties (LDC), and Low-Income Countries and Lower Middle-Income Countries which are not LDC according to the OECD Development Assistance Committee list: https://www.oecd.org/dac/financing-sustainable-development/development-finance-standards/daclist.htm
8 We recognize some UMIC, for example, Thailand have progressed with HTA further than others.
9 Unpublished MSc Global Health dissertation (AF), supervised by JB and EG.
10 WHO HTA survey responses were received between 24 February 2015 and 31 August 2015.
11 Jordan reclassified as UMIC in 2022. Updates delayed regarding classifications according to DAC list on an exceptional basis in the light of the ongoing global pandemic.
12 Lists are non-exhaustive but highlight key data extracted.
13 For example, NICE’s guidance is mandatory yet it cannot suggest where the money should come from to fund the technologies it recommends. Other countries more explicitly consider budget impact as part of their HTA (Australia), and others are dominated by the budget (New Zealand).
14 Although some mention was made of other aspects, for example social, legal, ethical, equity and feasibility of implementation, there were notably less discussed (Teerawattananon, 2016). This was a similar finding as that in the WHO survey.
15 Lists are non-exhaustive but highlight key data extracted.
16 Global partnership to make available 120 million affordable, quality COVID-19 rapid tests for low- and middle-income countries (who.int)
17 Early HTA is still an emerging field with limited guidance on methods but will differ according to the purpose and audience of HTA. It may not be easy or, in fact, desirable, to create guidance for all early HTA. Given that it is such a broad church, different approaches are suitable for different situations (including the amount of resources available to spend on HTA). The rigor required will depend on the needs of the audience for the HTA. For example, an exploratory analysis conducted privately for a technology developer to inform potential pricing policy may not require the same level of rigor as a decision on a potential screening programme for a national health provider. In all cases, we would encourage clarity of reporting of methods adopted and limitations of those methods.
18 Strategy Report: Engaging the private health service delivery sector through governance in mixed health systems. Geneva: World Health Organization; 2020. License: CC BY-NC-SA 3.0 IGO
19 WAHO | West African Health Organization (wahooas.org)
20 The Health Economics Unit (HEU) at the Africa Centers for Disease Control and Prevention (Africa CDC) was launched in collaboration with the Center for Global Development (CGD) to facilitate evidence-based priority-setting during the pandemic and beyond.
22 For example, NICE (and HTA in other countries, including Australia) does not help the health service to ’save money’. In fact, health spending continues to increase with investment in expensive new treatments according to NICE recommendations. Efficiency – which is the criteria the UK focuses on for it decision-making – is about value for money, spending on treatments that provide sufficient health returns.
23 To quote NICE’s December 2020 newsletter: ‘Over the last 20 years, NICE has established itself as a global leader in the development of evidence-based guidance for health and social care … but it is only by putting this advice into practice that it will make a difference to real people, to health outcomes and to equitable access to services’.
References
- Luz A, Santatiwongchai B, Pattanaphesaj J, et al. Identifying priority methodological issues in economic evaluation in low- and middle-income countries: finding the holy grail. http://gear4healthcom/gear. 2017.
- O’Rourke B, Oortwijn W, Schuller T. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care. 2020;36(1):1–4.
- Glassman A, Giedion U, Smith PC. What’s in, what’s out: designing benefits for universal health coverage. Washington DC: Center for Global Development; 2017.
- Grieve E, Hesselgreaves H, Wu O, et al. The Value of Health Technology Assessment: a mixed methods framework [version 1; not peer reviewed]. F1000res 2017, 6:2171 ( document) 10.7490/f1000research.1115169.1
- Stevens AJ, Longson C. At the center of health care policy making: the use of health technology assessment at NICE. Med Decis Mak. 2013;33(3):320–324.
- Hailey D. The history of health technology assessment in Australia. Int J Technol Assess Health Care. 2009;25(S1):61–67.
- O’Donnell JC, Pham SV, Pashos CL, et al. Health technology assessment: lessons Learned from around the world—an overview. Value Health. 2009;12:S1–S5.
- PAHO. Resolution CSP28.R9: health Technology Assessment and Incorporation into Health Systems. 2012.
- WHA. Resolution WHA67.3: health interventions and technology assessment in support of universal health coverage. 2014.
- WHO. Resolution SEA/RC66/R4: health intervention and technology assessment in support of universal health coverage. 2013.
- Piemonte C, Cattaneo O, Morris R, et al. Transition Finance: introducing a new concept. 2019. OECD Development Co-operation Working Papers, No. 54, OECD Publishing, Paris. https://doi.org/10.1787/2dad64fb-en.
- Hollingworth S, Gyansa-Lutterodt M, Dsane-Selby L, et al. Implementing health technology assessment in Ghana to support universal health coverage: building relationships that focus on people, policy, and process. Int J Technol Assess Health Care. 2020;36(1):8–11.
- Gad M, Lord J, Chalkidou K, et al. Supporting the development of evidence-informed policy options: an economic evaluation of hypertension management in Ghana. Value Health. 2020;23(2):171–179.
- iDSI. international Decision Support Initiative [ Available from: https://idsihealth.org/.
- Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. Lancet. 2017;389(10067):403–476.
- INAHTA. INAHTA impact assessment study 2020. Canada: The International Network of Agencies for HealthTechnology Assessment (INAHTA). Vol. 2020.
- Oortwijn W, van Oosterhout S, Kapiriri L. Application of evidence-informed deliberative processes in health technology assessment in low- and middle-income countries. Int J Technol Assess Health Care. 2020;36(4):440–444.
- Hollingworth S, Fenny AP, Yu S-Y, et al. Health technology assessment in sub-Saharan Africa: a descriptive analysis and narrative synthesis. Cost Eff Resour Allocation. 2021;19(1):39.
- Surgey G, Chalkidou K, Reuben W, et al. Introducing health technology assessment in Tanzania. Int J Technol Assess Health Care. 2020;36(2):80–86.
- Almomani E, Alabbadi I, Fasseeh A, et al. Implementation road map of health technology assessment in middle-income countries: the case of jordan. Value Health Reg Issues. 2021;25:126–134.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473.
- Anderson S, Allen P, Peckham S, et al. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Res Policy Syst. 2008;6(1):7.
- Aromataris E, Munn Z JBI manual for evidence synthesis. 2020.
- Schneider H, Maleka N. Patterns of authorship on community health workers in low-and-middle-income countries: an analysis of publications (2012–2016). BMJ Glob Health. 2018;3(3):e000797.
- English KM, Pourbohloul B. Increasing health policy and systems research capacity in low- and middle-income countries: results from a bibliometric analysis. Health Res Policy Syst. 2017;15(1):64.
- McGregor S, Henderson KJ, Kaldor JM. Capacity building in longitudinal HIV research. Lancet Global Health. 2015;3(1):e18–e9
- Vassall A, Mangham-Jefferies L, Gomez GB, et al. and supply constraints into economic evaluations in low-income and middle-income countries. Health Econ. 2016;25(S1):95–115.
- Watson M. Panel discussion on the application of MCDA tools. Cost Eff Resour Allocation. 2018;16(1):40.
- Fasseeh A, Karam R, Jameleddine M, et al. Implementation of health technology assessment in the middle East and North Africa: comparison between the current and preferred status. Front Pharmacol. 2020;11:15. Internet.
- Kim T, Sharma M, Teerawattananon Y, et al. Addressing challenges in health technology assessment institutionalization for furtherance of universal health coverage through south-south knowledge exchange: lessons from Bhutan, Kenya, Thailand, and Zambia. Value Health Reg Issues. 2021;24:187–192.
- MacQuilkan K, Baker P, Downey L, et al. Strengthening health technology assessment systems in the global south: a comparative analysis of the HTA journeys of China, India and South Africa. Glob Health Action. 2018;11(1):1527556.
- O’Brien N, Li R, Isaranuwatchai W, et al. How can we make better health decisions a best buy for all?: commentary based on discussions at iDSI roundtable on 2 (nd) May 2019 London, UK. Gates Open Res. 2019;3:1543.
- Li R, Hernandez-Villafuerte K, Towse A, et al. Mapping priority setting in health in 17 countries across Asia, Latin America, and sub-Saharan Africa. Health Syst Reform. 2016;2(1):71–83.
- Kumar MB, Taegtmeyer M, Madan J, et al. How do decision-makers use evidence in community health policy and financing decisions? A qualitative study and conceptual framework in four African countries. Health Policy Plan. 2020;35(7):799–809.
- Tantivess S, Chalkidou K, Tritasavit N, et al. Health technology assessment capacity development in low- and middle-income countries: experiences from the international units of HITAP and NICE. F1000Res. 2017;6:2119.
- Babigumira JB, Jenny AM, Bartlein R, et al. Health technology assessment in low- and middle-income countries: a landscape assessment. J Pharm Health Serv Res. 2016;7(1):37–42.
- Sharma M, Teerawattananon Y, Dabak SV, et al. A landscape analysis of health technology assessment capacity in the association of South-East Asian nations region. Health Res Policy Syst. 2021;19(1):19.
- Kaló Z, Gheorghe A, Huic M, Kaló Z, Gheorghe A, Huic M, Csanádi M, Kristensen FB. HTA Implementation. Roadmap in Central and Eastern European Countries. Health Econ. 2016;25(S1):179–192.
- Al Rabayah AA, Al Froukh RF, Sawalha RD. A capacity-building programme in health technology assessment for hospital pharmacists in a low- to middle-income country. J Pharm Health Serv Res. 2018;9(3):275–280.
- Odoch WD, Dambisya Y, Peacocke E, et al. The role of government agencies and other actors in influencing access to medicines in three East African countries. Health Policy Plan. 2021;36(3):312–321.
- Wasir R, Irawati S, Makady A, et al. The implementation of HTA in medicine pricing and reimbursement policies in Indonesia: insights from multiple stakeholders. PLoS One. 2019;14(11):e0225626.
- Vo T. Health technology assessment in developing countries: a brief introduction for Vietnamese health-care policymakers. Asian J Pharm. 2018. Jan-March 2018 (Special Issue), S1-7.
- Csanádi M, Inotai A, Oleshchuk O, et al. Health technology assessment implementation in Ukraine: current status and future perspectives. Int J Technol Assess Health Care. 2019;35(5):393–400.
- Dabak SV, Teerawattananon Y, Win T. From design to evaluation: applications of health technology assessment in myanmar and lessons for low or lower middle-income countries. Int J Technol Assess Health Care. 2019;35(6):461–466.
- Zegeye EA, Reshad A, Bekele EA, et al. The state of health technology assessment in the ethiopian health sector: learning from recent policy initiatives. Value Health Reg Issues. 2018;16:61–65.
- Zegeye EA, Mbonigaba J, Kaye SB, et al. Economic evaluation in ethiopian healthcare sector decision making: perception, practice and barriers. Appl Health Econ Health Policy. 2017;15(1):33–43.
- Addo R, Goodall S, Hall J, et al. Assessing the capacity of Ghana to introduce health technology assessment: a systematic review of economic evaluations conducted in Ghana. Int J Technol Assess Health Care. 2020;36(5):500–507.
- Addo R, Hall J, Haas M, et al. The knowledge and attitude of Ghanaian decision-makers and researchers towards health technology assessment. Soc Sci Med. 2020;250:112889.
- Uzochukwu BSC, Okeke C, O’Brien N, et al. Health technology assessment and priority setting for universal health coverage: a qualitative study of stakeholders’ capacity, needs, policy areas of demand and perspectives in Nigeria. Global Health. 2020;16(1):58.
- Dabak SV, Pilasant S, Mehndiratta A, et al. Budgeting for a billion: applying health technology assessment (HTA) for universal health coverage in India. Health Res Policy Syst. 2018;16(1):115.
- Piniazhko O. Pharmaceutical system in Ukraine: implementation of external reference pricing, reimbursement programs and health technology assessment. Pharmacia. 2018;65(2):28-39.
- Jain S, Rajshekar K, Sohail A, et al. Department of health research-health technology assessment (DHR-HTA) database: national prospective register of studies under HTAIn. Indian J Med Res. 2018;148(3):258–261.
- Bakre MM. Suggestions to make health technology assessment in India an efficient process. Value Health Reg Issues. 2021;24:214–215.
- Sundararajan S, Pattanshetty S, Aatre KR, et al. Stakeholder perception of health technology assessment in industrial setting. Indian J Public Health Res Dev. 2019;10(4):309–312
- Mukherjee K. A SMART framework for HTA capability development: lessons from India. Health Policy Technol. 2020;9(1):42–44.
- Dwivedi R, Athe R, Pati S, et al. Mapping of health technology assessment (HTA) teaching and training initiatives: landscape for evidence-based policy decisions in India. J Family Med Prim Care. 2020;9(11):5458–5467.
- Prinja S, Jyani G, Gupta N, et al. Adapting health technology assessment for drugs, medical devices, and health programs: methodological considerations from the Indian experience. Expert Rev Pharmacoecon Outcomes Res. 2021;21(5):859–868.
- Prinja S, Downey LE, Gauba VK, et al. Health technology assessment for policy making in India: current scenario and way forward. Pharmacoecon Open. 2018;2(1):1–3.
- Downey LE, Dabak S, Eames J, et al. Building capacity for evidence-informed priority setting in the indian health system: an international collaborative experience. Health Policy open. 2020; 1: 100004.
- Downey LE, Mehndiratta A, Grover A, et al. Institutionalising health technology assessment: establishing the medical technology assessment board in India. BMJ Glob Health. 2017;2(2):e000259.
- Dang A, Dang D, Vallish BN. Importance of evidence-based health insurance reimbursement and health technology assessment for achieving universal health coverage and improved access to health in India. Value Health Reg Issues. 2021;24:24–30.
- Dang A, Vallish BN. Real world evidence: an Indian perspective. Perspect Clin Res. 2016;7(4):156–160.
- Singh D, Luz ACG, Rattanavipapong W, et al. Designing the free drugs list in Nepal: a balancing act between technical strengths and policy processes. MDM Policy Pract. 2017;2(1):2381468317691766.
- Gheorghe A, Gad M, Ismail SA, et al. Capacity for health economics research and practice in Jordan, Lebanon, the occupied Palestinian territories and Turkey: needs assessment and options for development. Health Res Policy Syst. 2020;18(1):99.
- Swami S, Srivastava T. Role of culture, values, and politics in the implementation of health technology assessment in india: a commentary. Value Health. 2020;23(1):39–42.
- Hammad EA. The use of economic evidence to inform drug pricing decisions in Jordan. Value Health. 2016;19(2):233–238.
- Alabbadi I, Almomani E, Alshazili M. Drug selection for formulary inclusion: an exploratory case study of oncology medicines in Jordan. Value Health Reg Issues. 2020;21:211–221.
- Mukherjee K. Integrating technology, innovation and policy: COVID-19 and HTA. Health Policy Technol. 2021;10(1):16–20.
- Hampson G, Henshall C, Towse A. ASSESSING VALUE, BUDGET IMPACT, AND AFFORDABILITY IN ASIA. Int J Technol Assess Health Care. 2017;33(2):315–322.
- Wilkinson T, Sculpher MJ, Claxton K, et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health. 2016;19(8):921–928.
- Towse A HTA’s macro role in health care systems https://www.slideshare.net/OHENews/macro-hta-presentation-ispor-beijing-towse-20142014
- Teerawattananon Y, Luz K, Yothasmutra C, et al. HISTORICAL DEVELOPMENT OF THE HTAsiaLINK NETWORK AND ITS KEY DETERMINANTS OF SUCCESS. Int J Technol Assess Health Care. 2018;34(3):260–266.
- World Health Organisation. WHO HTA Survey 2021 WHO, Geneva: WHO; 2021 [ Available from: https://www.who.int/teams/health-systems-governance-and-financing/economic-analysis/health-technology-assessment-and-benefit-package-design/survey-homepage.
- Bouttell J, Grieve E, Hawkins N The role of development-focused health technology assessment in optimising science, technology and innovation to achieve SDG3. Science, Technology and Innovation for Meeting Sustainable Development Goals: OUP; in press.
- Elshaug AG, Hiller JE, Tunis SR, et al. Challenges in Australian policy processes for disinvestment from existing, ineffective health care practices. Aust New Zealand Health Policy. 2007;4(1):23.
- Henshall C, Schuller T, Mardhani-Bayne L. Using health technology assessment to support optimal use of technologies in current practice: the challenge of “disinvestment”. Int J Technol Assess Health Care. 2012;28(3):203–210.
- Esandi ME, Gutiérrez-Ibarluzea I, Ibargoyen-Roteta N, et al. An evidence-based framework for identifying technologies of no or low-added value (NLVT). Int J Technol Assess Health Care. 2020;36(1):50–57.
- Esmail R, Hanson H, Holroyd-Leduc J, et al. Knowledge translation and health technology reassessment: identifying synergy. BMC Health Serv Res. 2018;18(1):674.
- Fronsdal KB, Facey K, Klemp M, et al. Health technology assessment to optimize health technology utilization: using implementation initiatives and monitoring processes. Int J Technol Assess Health Care. 2010;26(3):309–316.
- Farhana Kamaruzaman H, Grieve E, Wu O Disinvestment in healthcare: a scoping review of systematic reviews. 2022.
- CGD. [ cited 2020]. Available from: https://www.cgdev.org/blog/priority-setting-better-health-international-decision-support-initiative?utm_source=200428&utm_medium=cgd_email&utm_campaign=cgd_weekly.
- Cacciatore P, Specchia ML, Solinas MG, et al. The organizational domain in HTA reports: towards a technology-oriented assessment. Eur J Public Health. 2020;30(2):219–223.
- Lee A, Skött LS, Hansen HP. Organizational and patient-related assessments in HTAs: state of the art. Int J Technol Assess Health Care. 2009;25(4):530–536.
- Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ. 2021;374:n2061.
- Heggie R, Boyd K, Wu O. How has implementation been incorporated in health technology assessments in the United Kingdom? A systematic rapid review. Health Res Policy Syst. 2021;19(1):118.
- OHE TA. 2020. [ cited 2020]. Available from: https://www.ohe.org/news/unpacking-black-box-payer-policy-demand-side-approach-equitable-uptake-cost-effective-health-0.
- Towse A Unpacking the Black Box of Payer Policy: a Demand-Side Approach for Equitable Uptake of Cost-Effective Health Innovation. Office of Health Economics. 2020. Available from: https://www.ohe.org/news/unpacking-black-box-payer-policy-demand-side-approach-equitable-uptake-cost-effective-health-0
- Bouttell J, Grieve E, Hawkins N (2020) The role of development-focused health technology assessment in optimizing science, technology, and innovation to achieve sustainable development goal 3. In: Adenle AA, Chertow MR, Moors EHM and Pannell DJ (editors) Science, Technology, and Innovation for Sustainable Development Goals: Insights from Agriculture, Health, Environment, and Energy. Oxford University Press: New York, NY. ISBN 9780190949501
- Cylus J, Papanicolas I, Smith P. Health System Efficiency: how to make measurement matter for policy and management. London editor:WHO;2016.
- Velasco Garrido M, Gerhardus A, Rottingen JA, et al. Developing health technology assessment to address health care system needs. Health Policy. 2010;94(3):196–202.
- Bhattacharyya O, Khor S, McGahan A, et al. Innovative health service delivery models in low and middle income countries - what can we learn from the private sector? Health Res Policy Syst. 2010;8(1):24.
- Chalkidou K, Towse A, Silverman R, et al. Market-driven, value-based, advance commitment (MVAC): accelerating the development of a pathbreaking universal drug regimen to end TB. BMJ Glob Health. 2020;5(4):e002061.
- Al-Ajlouni R, Al Rabayah A. Will jordan be closer to UHC after the COVID-19 pandemic? J Glob Health. 2020;10(2):020360.
- OECD. Using routinely collected data to inform pharmaceutical policies. 2019.
- Gilardino RE, Mejía A, Guarín D, et al. Implementing Health technology assessments in latin america: looking at the past, mirroring the future. A perspective from the ISPOR health technology assessment roundtable in Latin America. Value Health Reg Issues. 2020;23:6–12.
- Devex. Africa CDC Q&A: africa CDC official on bringing African expertise to African emergencies | devex: devex
- Chang H-J. Institutions and economic development: theory, policy and history. J Institutional Econ. 2010;7(4):473–498
- Glassman A, Chalkidou K, 2012. ”Priority-Setting in Health: Building Institutions for Smarter Public Spending,” Working Papers id:5043, eSocialSciences.
- Glassman A, Chalkidou K, Giedion U, et al. Priority-setting institutions in health: recommendations from a center for global development working group. Glob Heart. 2012;7(1):13–34.
- Lea L. Which policies increase value for money in health care? Paris: OECD Publishing; 2018.
- Löblová O. What has health technology assessment ever done for us? J Health Serv Res Policy. 2017;23(2):134–136.
- Jacob R, McGregor M. Assessing the impact of health technology assessment. Int J Technol Assess Health Care. 1997;13(1):68–80.
- Buxton M, Hanney S. How can payback from health services research be assessed? J Health Ser Res. 1996;1(1):35–43.
- Davies L, Drummond M, Papanikolaou P. Prioritizing investments in health technology assessment. Can we assess potential value for money? Int J Technol Assess Health Care. 2000;16(1):73–91
- Wanke M An exploratory review of evaluations of health technology assessment agencies. 2006;Alberta Heritage Foundation for Medical Research (AHFMR) HTA Initiative #16.
- Lafortune L, Farand L, Mondou I, et al. Assessing the performance of health technology assessment organizations: a framework. Int J Technol Assess Health Care. 2008;24(1):76–86.
- Guthrie S, Hafner M, Bienkowska-Gibbs T, et al. Returns on research funded under the nihr health technology assessment (HTA) programme: economic analysis and case studies. Rand Health Q. 2016;5(4):5.
- INAHTA. INAHTA Impact Framework http://www.inahta.org/hta-tools-resources/briefs/#Impact [
- Schumacher I, Zechmeister I. ASSESSING THE IMPACT OF HEALTH TECHNOLOGY ASSESSMENT ON THE AUSTRIAN HEALTHCARE SYSTEM. Int J Technol Assess Health Care. 2012;29(1):84–91.
- de Sola-Morales O, Granados A. Health technology assessment in Catalonia: an overview of past and future perspectives. Int J Technol Assess Health Care. 2009;25(Suppl 1):88–93.
- Sigmund H, Kristensen FB. Does health technology assessment benefit health services and politics? Eur J Health Econ. 2002;3(1):54–58.
- Yazdizadeh B, Mohtasham F, Velayati A. Impact assessment of Iran’s health technology assessment programme. Health Res Policy Syst. 2018;16(1):15.
- Roza S, Junainah S, Izzuna MMG, et al. Health technology assessment in malaysia: past, present, and future. Int J Technol Assess Health Care. 2019;35(6):446–451.
- Lipska I, McAuslane N, Leufkens H, et al. A DECADE OF HEALTH TECHNOLOGY ASSESSMENT IN POLAND. Int J Technol Assess Health Care. 2017;33(3):350–357.
- Kao K-L, Huang L-Y, Wu Y-H, et al. Outcomes and impacts of 10-year HTA implementation in Taiwan. Int J Technol Assess Health Care. 2019;35(6):441–445.
- Kristin E, Endarti D, Khoe LC, et al. Economic evaluation of adding bevacizumab to chemotherapy for metastatic colorectal cancer (mCRC) patients in Indonesia. Asian Pac J Cancer Prev. 2021;22(6):1921–1926.
- Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143.
- Grol R, Kagay CR, Geppert JJ. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(1):39.