ABSTRACT
FLA-related conditions are a rare medical occurrence. Despite their rarity, they are considered a public health concern for two reasons: the absence of a regular treatment regimen in the case of central nervous system infections and the fast progression of the symptoms leading to fatal outcomes. A total of 358 articles were retrieved from different databases (91 from PubMed, 26 from NCBI, 138 from Academia, 102 from Science Direct, and one from IJMED). 7 (46.6%) clinical cases came from Egypt, 2 (13.3%) cases of FLA infection came from Nigeria, 3 (20%) cases came from the Gambia, and 1 (6.6%) case was reported from African countries like Algeria, Tunisia, South Africa, and Zambia. Medical conditions caused by free-living amoeba are considered significant public health concerns. These ubiquitous organisms can cause both fatal and debilitating health conditions. Immediate diagnosis of cases and proper hygienic practices are necessary to provide direct medical intervention. They may be the key to reducing the morbidity and mortality rates from FLA-acquired infections. Although several government-led initiatives have been implemented to mitigate a plethora of parasitic diseases, the case of FLA-related conditions in African countries has yet to be realized.
Introduction
Free-living amoebae (FLA) are ubiquitous organisms considered emerging zoonotic pathogens. FLAs invade the central nervous system (CNS), resulting in fatal meningitis-like conditions [Citation1]. For this reason, the occurrence of FLAs in different environmental matrices has been identified as a public health risk for transmission [Citation2]. Recent studies focusing on epidemiological cases involving FLA-related infections have been indispensable estimates for determining the extent of morbidity cases in some parts of the world [Citation3,Citation4]. Although existing literature is available on epidemiological data on FLAs, most of these studies focus on isolation cases in the environment rather than human cases [Citation5–7]. There is a vacuity of information in the case of data that focuses on the human morbidity-mortality issues from FLAs.
The World Health Organization (WHO) has named four important genera under this group considered to be pathogenic: Naegleria, which causes primary amoebic meningoencephalitis (PAM), Acanthamoeba causing granulomatous amoebic encephalitis (GAE), Balamuthia causing Balamuthia amoebic encephalitis (BAE), and Sappinia which causes Sappinia amoebic encephalitis (SAE). Acanthamoeba spp., however, is known to cause non-fatal Acanthamoeba keratitis (AK) infection [Citation8]. The challenge for health practitioners in the case of FLA infection is the inability to provide a fast definitive diagnosis due to the rapid progression of the symptoms upon the onset of the disorder, and clinical features presented by FLA-caused meningitis are the same compared with bacterial and viral CNS infection. The Centers for Disease Control and Prevention (CDC) has declared that conditions from FLA-related infections as rare diseases, with only 34 documented cases from the last ten years (2010 to 2019) [Citation9]. FLAs exist as free-living forms in the environment; it is highly pathogenic once it infects mammalian hosts. Despite its public health concerns: surveillance and epidemiologic report of cases have gone behind over the years in several countries [Citation10,Citation11].
The continent of Africa has seen significant economic progress over the last century [Citation12]. Along with its progressive trend include rapid industrialization of its countries. This economic shift has led to several environmental concerns, such as pollution from factories set up by foreign investors, which, in turn, gives rise to health hazards [Citation13]. Among these hazards include parasitic organisms that the population and visiting tourists may contract while visiting the continent. Africa has a tropical climate, and the continuous thermal pollution of its natural lakes and environment paves the way for the emergence of zoonotic pathogens; among them are FLAs. Although current literature is available on parasitic organisms present on the continent, they focus on helminthic and selected protozoans only [Citation14]. Also, the existing studies would only account for potential transmission, symptomatology, and treatment protocols of FLA-related conditions, and the exact number of documented cases is essentially not discussed [Citation15]. This study aims to provide insight into the status of FLA infection in both human and animal reservoirs in Africa between 2010 to 2020.
Methods
Literature search strategy
The literature search was done in PubMed, NCBI, Academia, ScienceDirect, and Google Scholar databases. The search terms used were: ‘Free-Living Amoeba’, ‘Primary Amoebic Meningoencephalitis’, ‘Granulomatous Amoebic Encephalitis’, ‘Balamuthia amoebic encephalitis’, ‘Sappinia amoebic encephalitis’, ‘Acanthamoeba’, ‘Balamuthia’, ‘Naegleria’, ‘Sappina’, ‘Africa’, ‘Nigeria’, ‘Ethiopia’, ‘Democratic Republic of Congo’, ‘Egypt’, ‘South Africa’, ‘Tanzania’, ‘Kenya’, ‘Uganda’, ‘Algeria’, ‘Sudan’, ‘Morocco’, ‘Mozambique’, ‘Ghana’, ‘Angola’, ‘Somalia’, ‘Ivory Coast’, ‘Madagascar’, ‘Cameron’, ‘Burkina Faso’, ‘Niger’, ‘Malawi’, ‘Zambia’, ‘Mali’, ‘Senegal’, ‘Zimbabwe’, ‘Chad’, ‘Tunisia’, ‘Guinea’, ‘Rwanda’, ‘Benin’, ‘Burundi’, ‘South Sudan’, ‘Eritrea’, ‘Sierra Leone’, ‘Togo’, ‘Libya’, ‘Central African Republic’, ‘Mauritania’, ‘Republic of Congo’, ‘Liberia’, ‘Namibia’, ‘Botswana’, ‘Lesotho’, ‘Gambia’, ‘Gabon’, ‘Guinea-Bissau’, ‘Mauritius’, ‘Equatorial Guinea’, ‘Eswatini’, ‘Djibouti’, ‘Réunion’, ‘Comoros’, ‘Western Sahara’, ‘Cape Verde’, ‘Mayotte’, ‘São Tomé and Principe’, ‘Seychelles’, and ‘Saint Helena’, ‘Ascension’, and ‘Tristan da Cunha’. Titles and abstracts were screened using inclusion and exclusion criteria. Inclusion criteria consisted of (a) studies on the prevalence of FLA infection in humans and animals, (b) case reports, (c) retrospective studies, (d) prevalence studies conducted in different African countries on human and non-human infections, (e) studies conducted within the last ten years, and (f) although articles with full-text are of focus, articles without full-text were included provided that it includes statistical data on the prevalence of any of the FLAs were described in the abstract as this addressed the primary objective of the study. Exclusion criteria consisted of: 1. Studies were performed outside of the African continent, 2. Review papers, 3. Methods evaluation, 4. Modelling/simulation studies, 5. non-English language articles, 6. Studies conducted beyond ten years, and 7. Abstracts with incomplete data. This systematic review followed preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [Citation16].
Eligibility criteria
Studies published in English that report the prevalence of FLA infection in the African region are included in the study. Studies of the prevalence of pathogenic organisms not considered to be of FLA origin, effects of socio-behavioral patterns causing infection, reviews, and mini-reviews concerning FLA infection are excluded. Data from the collected studies, such as year of study, study site, sources or types of samples, clinical manifestations, isolated FLA, genotypes, prevalence, methods used for identification, title, and references, were extracted.
Results
Article search results and description of qualified articles
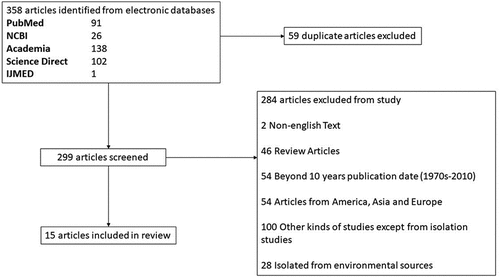
A total of 358 articles were retrieved from different databases (91 from PubMed, 26 from NCBI, 138 from Academia, 102 from Science Direct, and one from IJMED). Fifty-nine articles are duplicate titles and were removed from the study. A further 284 out of 299 articles were excluded from the review because they fall under the following exclusion criteria:
Non-English text (2)
Reviews (46)
Studies beyond ten years (54)
Non-African cases of FLA infection (54)
Other types of analyses (100)
Studies concerning the isolation of FLA from environmental sources (28)
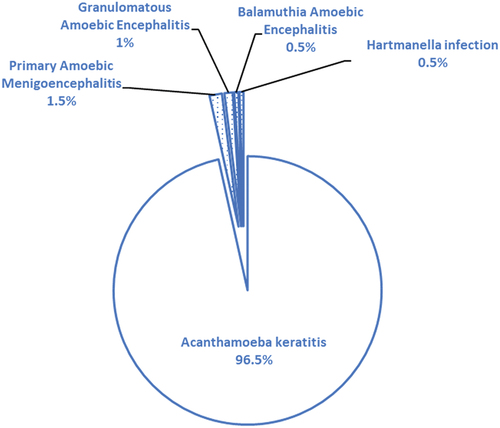
After the exclusion screening, 15 articles were included in this study (). Over the last decade, three (3) FLA-related conditions were documented in Africa. There is a total of 16 cases of reported FLA infections in Africa from 7 territories () (). Most of these cases are AK non-fatal conditions, totaling 194 (95.5%). Fatal cases from PAM and GAE account for only 1 (0.49%) case; respectively, three (3) subjects of fatal FLA-related conditions are documented from ruminants. At the same time, BAE, a rare FLA CNS infection, had 1 (0.49%) case in Africa (). There was a documented case of Hartmannella isolation in stool samples (1; 0.49%) (). The potential risk factors causing AK showed that unhygienic practices in storing contact lenses are the most probable cause of infection ().
Figure 2. Geographical distribution of FLA cases in Africa from 7 territories. Reports include 1 case report from South Africa, Zambia, and Tunisia while only 2–3 cases are reported from Nigeria and the Gambia. There are more than 5 cases reported from Egypt.
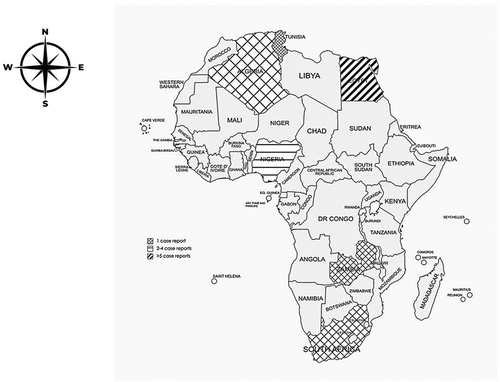
Table 1. Total number of FLA-related cases reported in the continent of Africa.
Table 2. Summary of fatal cases of FLA-related infections in Africa between 2010–2020.
Table 3. List of risk factors in acquiring Acanthamoeba keratitis in Africa.
Discussion
Management of fatal encephalitis infections in Africa
The main challenge in treating and managing patients with FLA-related meningitis infection is the variability of symptoms that most often mirrors symptomatology presented by bacterial or viral meningitis. This includes severe headache, fever, nuchal rigidity, and Kernig's sign [Citation22]. This alone poses great difficulty in establishing an early definitive diagnosis for an effective treatment regimen. Here, we observed that all three fatal cases present the same symptoms, although in varying degrees, and it may have been because a different type of FLA caused each case.
Nevertheless, it is essential to note that the case in Nigeria presented a patient who was afebrile during the time of hospital admission [Citation20]. This may have led clinicians to provide the patient’s wrong and delayed treatment regimen. Perhaps, the litmus test to differentiate a non-FLA caused meningitis from other forms is through the identification of the organism using microscopic methods, which will increase the chances of survival of the patient as compared to the case in India [Citation23], Mexico [Citation24], and the US [Citation25]. A timely CSF wet smear microscopy for the presence of microorganisms may be crucial for a successful diagnosis and choice of the proper treatment regimen, which was overlooked as presented in two African cases [Citation19,Citation20]. Although this may be true, a successful therapeutic regimen and survival of the patient will still depend on the manifestation of motile organisms in CSF wet mounts, thus resulting in the earliest diagnosis. In the case of the patient in Zambia, identification of motile trophozoites was observed on the 8th day on which the patient died [Citation18]; on the contrary, in the case of a 6-year-old boy in India, trophozoites were already observed on the first day after the onset of symptoms [Citation26]. These two cases present a potential argument on whether physiologic factors affect the immediate appearance of motile trophozoites in CSF fluid or whether it would be a matter of technical skill in the identification. Regardless of the reason, it needs to be placed in perspective for now. More importantly, patient survival from FLA-caused encephalitis is observed with a thorough history check on the patient’s activities before symptoms. This has been followed in the successful treatment of FLA-caused encephalitis [Citation23,Citation26]. The incidence of fatal encephalitis in African animal hosts further provides evidence of the ability of FLAs such as Acanthamoeba spp. and Naegleria spp. to cause the same deadly effects on non-human mammalian hosts [Citation17,Citation21]. There are a total of only three fatal human cases of encephalitis caused by FLAs documented in Africa in the last decade; this accounts for only 20% of the total FLA-related infections in the entire continent. Although this is the case, the mortality rate presented from the gathered literature is a dismal 100%. We, therefore, hypothesize that there are cases elsewhere in the continent that may not have been documented due to a lack of affirmative protocols. It should be taken into significant consideration that two of the cases presented in this review were only given correct diagnosis postmortem, which further required consent from relatives.
Acanthamoeba keratitis in Africa
Acanthamoeba keratitis (AK) is considered the most common FLA-related infection and has been documented in several outbreaks in the past [Citation27–29]. In this study, AK accounts for a huge percentage of FLA-related infections in Africa and is more prevalent in people who wear contact lenses (CLW) [Citation30–32]. It is believed that it is acquired through unhygienic cleaning and storage of lenses [Citation33]. However, this debilitating eye infection is also observed in non-contact lens wearers (NCLW) [Citation34,Citation35]. Infection of AK in NCLW in Africa is mainly caused by exposure of the eye through washing with contaminated tap water [Citation36] or, to some extent, exposure to dust contaminated with Acanthamoeba infective cyst [Citation34]. Most AK cases in Africa involved CLW, but a small fraction of NCLW is identified as having the infection [Citation37]. To address the concerns in the growing number of AK cases in Africa, it is important to consider the potential causes and risk of infection, which can be grouped into either behavioral or non-behavioral. Risk factors considered behavioral include routine activities and practices like wearing contact lenses while sleeping, the continuous wearing of contact lenses for a week, and improper contact lens storage (). The potential infection can be avoided through behavior modification by establishing policy programs that promote good hygienic practices in handling contact lenses. On the other hand, non-behavioral causes involve CLW and NCLW having contact with environmental sources contaminated with Acanthamoeba spp., such as using tap water to clean contact lenses and engaging in water recreational activities while wearing them. Acanthamoeba spp. is considered ubiquitous in the environment and isolated in different water sources [Citation38–40]. Discouraging the use of tap water in cleaning contact lenses and observing proper water storage so as not to promote the growth of thermophilic FLAs are potential intervention methods that can be implemented to eliminate and avoid future infections. These intervention methods are important, especially with the emergence of non-Acanthamoeba-caused AK in the region, which is also the first report of human infection globally [Citation41].
Potential sources of FLA infection in Africa
The use of FLA-contaminated tap/stored water is considered the main reason for most AK cases in Africa. Groundwater is considered one of the major sources of freshwater in Africa for the purpose of drinking and domestic use [68]. Although there has been no direct evidence of FLAs causing disease by consumption of contaminated water, the possibility of contracting infection such as AK by using contaminated tap water to wash the face should be greatly considered as documented by some cases [69]. This enables FLAs to initiate infection upon contact with exposed membranes of the eye causing AK. In the case of fatal brain infections caused by FLAs, swimming in contaminated freshwaters such as rivers and lakes is the primary reason for infection. Although as argued, other forms of transmission routes may occur and are yet to be documented. Due to these reasons, it is important to consider the inclusion of survey studies of FLAs in different freshwater sources in Africa.
Non-anthropogenic impacts of free-living amoebae infections in Africa
Here, we presented from the gathered literature two fatal cases of FLA-related infections in ruminants [Citation17,Citation21]. Interestingly, these cases were caused by two different species of FLA, causing fatal health outcomes in humans. The mechanism of infection is believed to be due when ruminants drink in freshwater sources enabling FLAs to access the olfactory route. Livestock parasitosis has been considered a vital livestock husbandry problem that may reduce animal productivity and quality [Citation42]. Although several African countries are now industrialized, many territories are still considered highly agricultural and dependent on livestock for livelihood [Citation43,Citation44]. While there has been an established surveillance monitoring for known parasitic organisms in humans, such as those belonging to taxonomic group order Kinetoplastida and phylum Apicomplexa [Citation45], helminths [Citation46–48], and other types of parasitic organisms [Citation47,Citation49,Citation50]; there appears to be none for FLAs in both human and animal reservoir in the continent. Therefore, it is imperative to consider including FLAs in surveillance studies of natural freshwater sources and mortality cases in humans and livestock. The two separate cases involving cattle and ruminants in this study may potentially elucidate new transmission routes of FLAs, isolation of new pathogenic species from non-human hosts, and establishment of new protocols in veterinary medicine for the management of future cases. The current data for FLA occurrence in different environmental matrices in Africa is considered fragmented and are yet to be explored. This translates further to a low turnout of cases from the continent compared to other territories with an established surveillance and monitoring system [Citation3,Citation32,Citation51].
Future perspectives of FLA research in Africa
The continent of Africa is an amalgam of 59 countries, each having various ethnic, geographic, and cultural differences [Citation52]. The continent is home to the great lakes, considered vital sources of fresh water and food for its population [Citation53]. Although several studies concerning these aquatic matrices have been conducted [Citation54,Citation55], a survey on the biodiversity of FLAs in great African lakes has yet to be realized. It has been postulated that aquatic matrices are an essential point source for the initial infection of FLAs, as several studies have argued [Citation2,Citation5,Citation40,Citation56,Citation57]. The importance of environmental surveys for FLA has been proven vital not only for identifying sources of infection, as with the Zambian adult who swam in the Kafue River [Citation18] but also for identifying new pathogenic species, as it was with the case in Thailand [Citation58]. This evidence and other studies conducted on freshwater matrices worldwide have provided factual data on the biodiversity of FLAs in different aquatic sources [Citation58–62]. It is crucial to consider investigating the vast natural rivers and lakes found on the continent for the possibility of identifying new species of FLAs.
Opportunities to further enhance the identification of FLAs using modern methods may need to be implemented in several African health facilities. Although 9 out of 15 (60%) gathered cases in this study have confirmed the presence of FLA DNA from the samples, only one proceeded with sequencing for a definitive identification [Citation30]. Modernizing molecular diagnostic methods for pathogen identification is essential to health care delivery. As argued by studies, several African countries are not only faced with a high burden of various infectious and noninfectious diseases. Still, they are also challenged in terms of resources [Citation63]. The use of molecular methods not only increases the accuracy of diagnosis but also speeds up the delivery of proper therapeutic intervention for patients suffering from FLA-related fatal infections. As we stated in our previous work, the lapses on the part of healthcare diagnostics directly translate to two possible outcomes: underreporting and misdiagnosis of cases [Citation3].
Conclusions
Regardless of the disease, epidemiological data are essential for implementing public health programs to safeguard the population’s health and safety. The continent of Africa, along with the countries within it, are considered hotspots for development not only economically but also in the aspect of health. Although several government-led initiatives have been implemented to mitigate a plethora of parasitic diseases, the case of FLA-related infections should become a subject of investigations. As indicated in this review, managing fatal FLA-related conditions in the continent has been unsuccessful, considering the three deaths from the human cases reported. In the case of AK infections, the growing number of NCLW cases reflects hygiene and potential contamination of water sources used for daily use. Finally, the non/human cases of FLA infection open a new door of opportunities to investigate further the potential negative health impacts of FLA in livestock in several countries in Africa and around the world.
Acknowledgment
The authors would like to thank the Department of Medical Technology, the University of Santo Tomas, and the Department of Medical Technology, Far Eastern University, for providing technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17(2):413–433.
- Milanez GD, Masangkay FR, Scheid P, et al. Acanthamoeba species isolated from Philippine freshwater systems: epidemiological and molecular aspects. Parasitol Res. 2020;119:3755–3761.
- Milanez GD, Masangkay FR, Martin IG, et al. Epidemiology of free-living amoebae in the Philippines: a review and update. Pathog Glob Health. 2022;116(6):331–340. DOI:10.1080/20477724.2022.2035626
- Gharpure R, Bliton J, Goodman A, et al. Epidemiology and clinical characteristics of primary amebic meningoencephalitis caused by Naegleria fowleri: a global review. Clin Infect Dis. 2021;73(1):19–27. DOI:10.1093/cid/ciaa520
- Fabros MRL, Diesta XRS, Oronan JA, et al. Current report on the prevalence of free-living amoebae (FLA) in natural hot springs: a systematic review. J Wat Health. 2021;19(4):563–574. DOI:10.2166/wh.2021.101
- Abdul Majid MA, Mahboob T, Mong BG, et al. Pathogenic waterborne free-living amoebae: an update from selected Southeast Asian countries. PLoS ONE. 2017;12(2):0169448. DOI:10.1371/journal.pone.0169448
- Chaúque BJM, dos Santos DL, Anvari D, et al. Prevalence of free-living amoebae in swimming pools and recreational waters, a systematic review and meta-analysis. Parasitol Res. 2022;121:3033–3050.
- Gompf SG, Garcia C. Lethal encounters: the evolving spectrum of amoebic meningoencephalitis. ID Cases. 2019;15:e00524.
- Centers for Disease Control and Prevention. National center for emerging and zoonotic infectious diseases (NCEZID), division of foodborne, waterborne and environmental diseases. 2020 [cited 2021 March 5]. Available from: https://www.cdc.gov/parasites/naegleria/general.html.
- Trabelsi H, Dendana F, Sellami H, et al. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol. 2012;60(6):399–405. DOI:10.1016/j.patbio.2012.03.002
- Rodriguez-Zaragosa S. Ecology of free-living amoebae. Crit Rev Microbiol. 2008;20:225–241.
- Ighobor K. African economies capture world attention. African renewal. 2012 [cited 2021 March 5]. Available from: https://www.un.org/africarenewal/magazine/august-2012/african-economies-capture-world-attention
- Ssali MW, Du J, Mensah IA, et al. Investigating the nexus among environmental pollution, economic growth, energy use, and foreign direct investment in 6 selected sub-Saharan African countries. Environ Sci Pollut Res. 2019;26:11245–11260.
- Karakavuk M, Aykur M, Ünver A, et al. Parasitic diseases that can infect travelers to Africa. Turkiye Parazitol Derg. 2018;42(2):154–160. DOI:10.5152/tpd.2018.5256
- Mallewa M, Wilmhurst JM. Overview of the effect and epidemiology of parasitic central nervous system infections in African children. Semin Pediatr Neurol. 2014;21(1):19–25.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. DOI:10.1371/journal.pmed.1000097
- Benterki MS, Ayachi A, Bennounce O, et al. Meningoencephalitis due to the amoeboflagellate Naegleria fowleri in ruminants in Algeria. Parasite. 2016;23:11.
- Chomba M, Mucheleng’Angga L, Fwoloshi S, et al. A case report: primary amoebic meningoencephalitis in a young Zambian adult. BMC Infect Dis. 2017;17:532.
- Beek van der Name, Tienen CV, de Haan JE, et al. Fatal Balamuthia mandrillaris meningoencephalitis in the Netherlands after travel to the Gambia. Emerg Infect Dis. 2015;21:896–898.
- Obaseki DE, Forae GD, Iyawe U, et al. A First reportable case of fatal granulomatous amoebic encephalitis in an immunocompetent Nigerian confirmed by molecular studies-polymerase chain reaction (PCR). Int J of Med Public Health. 2016;6:148–150.
- Gomma N, Atiba A, El-Habashi N, et al. Fatal encephalitis in cattle associated with Acanthamoeba infection in Egypt. Pakistani Vet J. 2016;36:114–117.
- Center for Disease Control and Prevention. Bacterial Meningitis. National center for immunization and respiratory diseases. 2019 [cited 2020 April 4]. Available from: https://www.cdc.gov/meningitis/bacterial.html
- Yadav D, Aneja S, Dutta R. Youngest survivor of Naegleria meningitis. Indian J Pediatr. 2013;80:253–254.
- Vargas-Zepeda J, Gómez-Alcalá AV, Vásquez-Morales JA, et al. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res. 2005;36:83–86.
- Seidel JS, Harmatz P, Visvesvara GS, et al. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306:346–348.
- Sharma A, Sharma A, Guleira S. Successful treatment of a case of primary amoebic meningoencephalitis: how important is history taking. Indian J Critic Care Med. 2015;19:126–127.
- Verani JR, Lorick SA, Yoder JS. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg Infect Dis. 2009;15:1236–1242.
- Johnston SP, Sriram R, Qvarnstrom Y, et al. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J Clin Microbiol. 2009;47:2040–2045.
- Yoder JS, Verani J, Heidman N, et al. The persistence of cases following a multistate outbreak. Ophthalmic Epidemiol. 2012;19(4):221–225. DOI:10.3109/09286586.2012.681336
- Dendana F, Sellami H, Trabelsi H, et al. Acanthamoeba T4 genotype associated with keratitis infections in Tunisia. Parasitol Res. 2013;112:401–405.
- Emina MO, Idu FK. Bacteria and parasites in contact lenses of asymptomatic wearers in Nigeria. J Optom. 2011;4(2):69–74.
- Hara T, Yagita K, Sugita Y. Pathogenic free-living amoebic encephalitis in Japan. Neuropathol. 2019;39(4):251–258.
- Maycock NJ, Jayaswal R. Update on Acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea. 2016;35:713–720.
- Syam PP, Narendran R, van der Hoek J. Persistent Acanthamoeba keratitis in a non-contact lens wearer following exposure to bird seed dust. Br J Ophthalmol. 2005;89:388–389.
- Garg P, Kalra P, Joseph J. Non-contact lens-related Acanthamoeba keratitis. Indian J Ophthalmol. 2017;65:1079–1086.
- Buerano CC, Trinidad AD, Fajardo LSN, et al. Isolation of Acanthamoeba genotype T4 from a non-contact lens wearer from the Philippines. Trop Med Health. 2014;42:145–147.
- El-Sayed NM, Younis MS, Elhamshary AM, et al. Acanthamoeba DNA can be directly amplified from corneal scrapings. Parasitol Res. 2014;113:3267–3272.
- Herrawy AA, Bhagat M, Mohammed A, et al. Acanthamoeba species in swimming pools of Cairo, Egypt. Iran j parasitol. 2014;9:194–201. PMID: 25848385.
- Hagosojos B, Masangkay F, Fernandez JB, et al. Molecular identification of Acanthamoeba sp. in Lake Buhi, Philippines. Ann Parasitol. 2020;66:111–114.
- Milanez G, Masangkay F, Hapan F, et al. Detection of Acanthamoeba spp. in two major water reservoirs in the Philippines. J Wat Health. 2020;18:118–126.
- Tolba MEM, Hussein EAM, Farrag HMM, et al. Allovahlkampfia spelaea causing keratitis in humans. PLoS Negl Trop Dis. 2016;10:e0004841.
- Grace D, Songe M, Nairobi KJT. Impact of neglected diseases on animal productivity and public health in Africa. Africa-OIE Regional; 2015 05 June 2020.
- Oluwatayo IB, Ojo AO. Is Africa’s dependence on agriculture the cause of poverty in the continent? An empirical review. J Dev Areas. 2016;50(1):93–102.
- Bjornlund V, Bjornlund H, Van Rooyen AF. Why agricultural production in sub-Saharan Africa remains low compared to the rest of the world – a historical perspective. Int J Wat Res Dev. 2020;36(1):20–53.
- MacGregor P, Nene V, Nisbet RER. Tackling protozoan parasites of cattle in sub-Saharan Africa. PLOS Pathog. 2021;17(10):e1009955.
- French MD, Evans D, Fleming FM, et al. Schistosomiasis in Africa: improving strategies for long-term and sustainable morbidity control. PLoS Negl Trop Dis. 2018;12(6):e0006484. DOI:10.1371/journal.pntd.0006484
- Westerhuis JB, Mank TG. Intestinal parasites in African asylum seekers: prevalence and risk factors. Ned Tijdschr Geneeskd. 2002;146(32):1497–1501.
- Liao CW, Fu CJ, Kao CY, et al. Prevalence of intestinal parasitic infections among school children in capital areas of the democratic republic of São Tomé and Príncipe, West Africa. Afr Health Sci. 2016;16(3):690–697. DOI:10.4314/ahs.v16i3.8
- Jaja IF, Ungeviwa PW. A 6-year retrospective report of livestock parasitic diseases in the Eastern Cape Province, South Africa. Open Vet J. 2022;12(2):204–211.
- Tawfeek GM, Bishara SA, Sarhan RM, et al. Genotypic, physiological, and biochemical characterization of potentially pathogenic Acanthamoeba isolated from the environment in Cairo, Egypt. Parasitol Res. 2016;115(5):1871–1881. DOI:10.1007/s00436-016-4927-3
- Texeira LH, Rocha S, Pinto RMF, et al. Prevalence of potentially pathogenic free-living amoebae from Acanthamoeba and Naegleria genera in non-hospital, public, internal environments from the city of Santos, Brazil. Braz J Infect Dis. 2009;13(6):395–397.
- WorldData. Africa. [cited 2022 September 29]. Available from: https://www.worlddata.info/africa/index.php
- Coulter GW, Allanson BR, Bruton MN, et al. Unique qualities and special problems of the African Great Lakes. Environ Biol Fish. 1986;17:161–183.
- Bootsma HA, Hecky RE. A comparative introduction to the biology and limnology of the African Great Lakes. J Great Lakes Res. 2003;29(2):3–18.
- Salzburger W, Bocxlaer BV, Cohen AS. Ecology and evolution of the African Great Lakes and their faunas. Ann Rev Eco Evo Sys. 2014;45:519–545.
- Gabriel S, Khan NA, Siddiqui R. Occurrence of free-living amoebae (Acanthamoeba, Balamuthia, Naegleria) in water samples in Peninsular Malaysia. J Wat Health. 2019;17(1):160–171.
- Hajissa K, Islam MA, Sanyang AM, et al. Prevalence of intestinal protozoan parasites among school children in Africa: a systematic review and meta-analysis. PLoS Neg Trop Dis. 2021;16(2):e0009971. DOI:10.1371/journal.pntd.0009971
- Putaporntip C, Kuamsab N, Nuprasert W, et al. Analysis of Acanthamoeba genotypes from public freshwater sources in Thailand reveals a new genotype, T23 Acanthamoeba bangkokensis sp. nov. 2021;11:17290. DOI:10.1038/s41598-021-96690-0
- Milanez G, Masangkay F, Somsak V, et al. Occurrence and the first report of Naegleria australiensis presence in a major lake in the Philippines. J Wat Health. 2019;17(4):647–653. DOI:10.2166/wh.2019.034
- Ghaderifar S, Najafpoor AA, Zarrinfar H, et al. Isolation and identification of Acanthamoeba from pond water of parks in a tropical and subtropical region in the Middle East, and its relation with physicochemical parameters. BMC Microbiol. 2018;18:139.
- Latifi A, Salami M, Kazemirad E, et al. Isolation and identification of free-living amoeba from the hot springs and beaches of the Caspian Sea. Parasite Epidemiol Control. 2020;11:e00194.
- Andalib S, Rahimi HM, Niyyati M, et al. Free-living amoebae in an oil refinery wastewater treatment facility. Sci Total Environ. 2022;839:156301.
- Okeke IN, Ihekweazu C. The importance of molecular diagnostics for infectious diseases in low-resource settings. Nat Rev Microbiol. 2021;19:547–548.
- Taher EE, Méabed EMH, Abdallah I, et al. Acanthamoeba keratitis in non-compliant soft contact lenses users: genotyping and risk factors, a study from Cairo, Egypt. J Infect Public Health. 2017;11:377–383.
- Saad MAH, Khalil HSM. Biofilm testing of microbiota: an essential step during corneal scrap examination in Egyptian acanthamoebic keratitis cases. Parasitol Int. 2018;67:556–564.
- Grün AL, Stemplewitz B, Scheid P. First report of an Acanthamoeba genotype T13 isolate as etiological agent of a keratitis in humans. Parasitol Res. 2014;113:2395–2400.