ABSTRACT
Introduction
Chloroquine (CQ) is the drug of choice for treating uncomplicated Plasmodium vivax (P. vivax) malaria in India. The knowledge about the exact burden of CQ resistance in P. vivax in India is scarce. Therefore, this systematic review aimed to assess the prevalence of CQ resistance in reported P. vivax cases from India.
Methods
PubMed, EMBASE, and Web of Science, were searched using the search string: ‘Malaria AND vivax AND chloroquine AND (resistance OR resistant) AND India’. We systematically reviewed in-vivo and in-vitro drug efficacy studies that investigated the CQ efficacy of P. vivax malaria between January 1995 and December 2022. Those studies where patients were followed up for at least 28 days after initiation of treatment were included.
Results
We identified 12 eligible CQ therapeutic efficacy studies involving 2470 patients, Of these 2329 patients were assessed by in-vivo therapeutic efficacy methods and the remaining 141 were assessed by in-vitro methods. CQ resistance was found in 25/1787 (1.39%) patients from in-vivo and in 11/141 (7.8%) patients from in-vitro drug efficacy studies.
Conclusion
Based on the available studies, the prevalence of CQ resistance in P. vivax was found to be relatively lower in India. However, continued surveillance and monitoring are crucial to identify the emergence of CQ resistance.
Introduction
Plasmodium vivax malaria is the second most prevalent cause of malaria globally [Citation1]. An estimated 4.9 million P. vivax malaria cases were reported globally in 2021 [Citation2]. In 2021, India accounted for 79% of estimated cases and about 83% of all malaria deaths in the WHO South-East Asia Region; about 40% of all cases in the region were due to P. vivax [Citation2]. Unlike P. falciparum malaria, P. vivax produces dormant liver stages (hypnozoites), which cause infection to relapse weeks to months after the original attack [Citation3]. In India, Chloroquine (CQ) (25 mg/kg) over 3 days is the drug of choice for P. vivax malaria. Primaquine (PQ) (0.25 mg/kg for 14 days) is added to CQ to target the hypnozoites and achieve a radical cure. The combination is highly effective against active infection and in preventing relapses.
In 1989, the first CQ-resistant P. vivax was reported, nearly 30 years after the discovery of CQ-resistant P. falciparum [Citation4,Citation5]. India’s first CQ-resistant P. vivax (CQR P. vivax) case was reported in 1995 [Citation6]. So far, P. falciparum malaria is considered more severe than P. vivax malaria and was prioritized by national and international malaria elimination policymakers. However, P. vivax malaria is increasingly being reported as the cause of severe malaria in several countries across the world, particularly in regions where CQ resistance is common [Citation7]. Detecting and monitoring antimalarial drug efficacy in P. vivax is challenging due to the difficulty in interpreting therapeutic efficacy study results and the inability to reliably distinguish between relapse, recrudescence, and reinfection [Citation8]. Delay in the detection of CQ resistance could further lead to a significant public health problem in an endemic country like India. Therefore, it is essential to recognize the prevalence of CQ resistance in P. vivax malaria cases in India to revise the treatment guidelines and develop and implement effective malaria elimination methods. This systematic review article summarizes CQ resistance in P. vivax in India.
Methods
The present study adhered to the Preferred Reporting Items for Systematic Reviews (PRISMA). Furthermore, this systematic review protocol was registered in PROSPERO (ID: CRD42023445274).
Data source and search strategies
We searched PubMed, EMBASE, and Web of Science databases by the following search string: ‘Malaria AND vivax AND chloroquine AND (resistance OR resistant) AND India’.
Eligibility of the studies
Inclusion criteria
All studies published in English between January 1995 and December 2022, which investigated the efficacy of CQ in P. vivax malaria in the Indian subcontinent, were screened. All articles that assessed either in-vivo (with a minimum of 28 days follow-up) therapeutic efficacy and in-vitro CQ drug efficacy in P. vivax cases in India were included.
Exclusion criteria
Reviews, case reports, conference abstracts, registered protocols for clinical trials, letters to the editor, and personal opinions were excluded.
Study selection
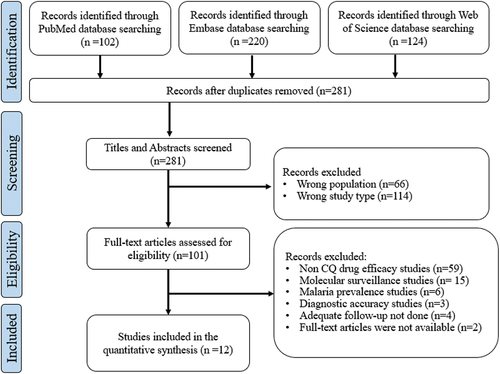
Records acquired from the bibliographic searches were uploaded into Rayyan’s online software [Citation9]. After the removal of duplicates, the titles and abstracts of the remaining records were independently screened for potential inclusion by two reviewers. A third reviewer was consulted in case of a conflict. Afterwards, suitable full-text copies of articles were retrieved and assessed for eligibility. Finally, all excluded studies were listed, along with the reasons for their exclusions ().
Data extraction and management
We systematically extracted study characteristics and CQ treatment failure data from the articles: study site/region, study period, study designs, sample size, clinical setting, CQ regimen (dose, frequency, duration, and supervision of treatment), concurrent administration of primaquine, and outcome characteristics (treatment failure [TF], early treatment failure [ETF], late clinical failure [LCF], and late parasitological failure [LPF]). The data extraction was carried out independently by two reviewers and the third reviewer resolved the discrepancies.
Definitions of treatment failures
In-vivo treatment failures were divided into the following early treatment failure (ETF), late clinical failure (LCF), and late parasitological failure (LPF) as per WHO standard definitions (supplementary Table S2) [Citation10]. Resistance criteria followed by each study were categorized in .
Table 1. Classification of treatment failures in-vivo efficacy studies.
In-vitro CQ-resistance
It was assessed using endpoints defined by the study for schizont maturation assay ().
Table 2. Classification of drug resistance criteria for in-vitro studies.
Risk of bias assessment
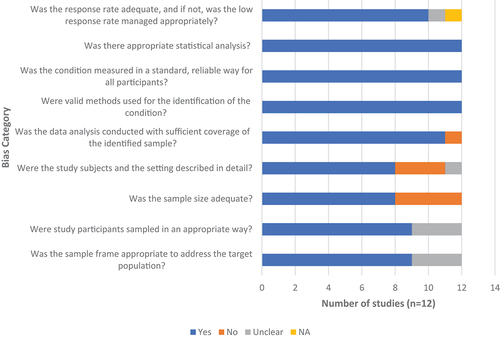
The quality assessment was carried out independently by two reviewers using Joanna Briggs Institute’s (JBI) critical appraisal checklist [Citation26]. Any reviewer disagreements were settled through consensus or discussion with a third reviewer. While performing risk of bias (RoB), each item was categorized into one of four choices: yes, no, unclear, or not applicable. One point is assigned to a ‘yes’ response, and the RoB score is the sum of the nine items, ranging from zero to nine, with a higher score indicating a lower RoB. In this study, the level of RoB of included studies was operationally categorized into low (RoB score of ‘7–9’), moderate (RoB score of ‘4–6’), and high (RoB score of ‘0–3’). A score of nine represents ‘completely low RoB’.
Data analysis
We calculated the pooled proportion of in-vivo treatment failures (TF) per protocol with the number of patients with TF between day 3 and day 28, with the denominator being the number of patients that have finished follow-up. We estimated the in-vitro CQ-resistant strains per protocol with the denominator being the number of patients that were assessed for schizont maturation assay.
Results
We initially identified a total of 446 studies, of which 165 duplicates were excluded prior to the title and abstract screening. The remaining 281 studies were screened and assessed for eligibility. After screening 269 articles were excluded () and the remaining 12 studies were included and evaluated for the efficacy of CQ against P. vivax. Of these studies, nine were in-vivo therapeutic efficacy studies [Citation11,Citation12,Citation14,Citation17–19,Citation21–23]; two were assessed by in-vitro methods [Citation24,Citation25]; and the remaining one was assessed by both in-vivo and in-vitro methods [Citation20]. In this systematic review, we observed 1.39% of in-vivo treatment failures and 7.8% were CQ-resistant strains.
In-vivo efficacy studies
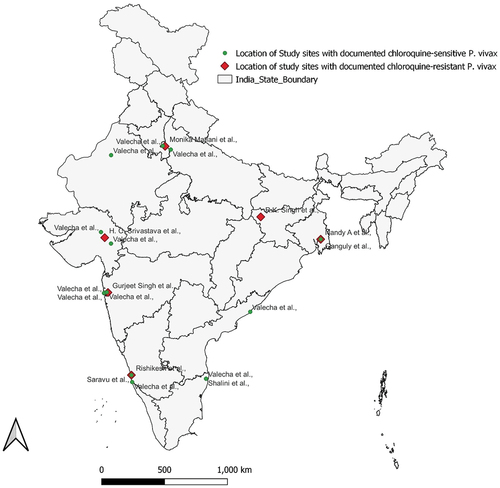
The current systematic review has assessed pooled CQ efficacy in 2329 patients from the different endemic regions of India ( and ). Of the 2329 patients included in the analysis, 1843 (79.1%) were treated with chloroquine alone, 286 (12.27%) were treated with chloroquine plus early primaquine commencing before day 3, and 200 (8.58%) received primaquine after day 3. Of these, a total of 1921 patients were followed up for 28 days and the remaining 408 patients were followed up for 42 days (). A total of 502 patients were lost to follow-up, and 40 were withdrawn from the study. A total of 1787 patients completed their follow-up visits and were included in the final assessment; 25 (1.39%) cases were found to be clinical treatment failures for CQ. Among these, 18 (72%) were late parasitological failures, six (24%) cases were late clinical failures, and the remaining 1 (4%) was early treatment failure ( and ).
Figure 3. Flow chart of the patient enrollment and follow-up in CQ drug efficacy studies.
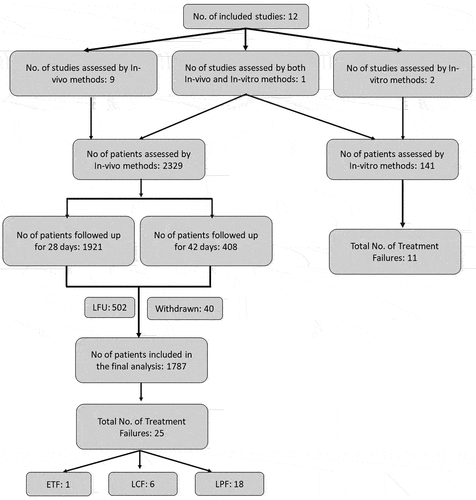
Table 3. Methodological characteristics of included studies.
Table 4. Summary of CQ treatment failures in Plasmodium vivax malaria infections in India.
In-vitro drug efficacy studies
A total number of 141 patients were enrolled and CQ efficacy was assessed by schizont maturation assay (SMA) in 3 studies. All the samples were processed for schizont maturation assay within 4 hours of the sample collection. Of these 11 (7.8%) were found to be CQ-resistant strains ().
Risk of bias assessment
The methodological quality assessment of included prevalence studies for the possibility of bias in its design, conduct, and analysis has been performed and mentioned in . In total, 9 studies had a RoB score of ‘7–9’ and 3 had a RoB score of ‘4–6’. In our study, we have found that RoB is low to moderate and no study has shown a high RoB (supplementary table S3).
Discussion
Despite the dwindling burden of malaria in India, P. vivax malaria continues to be a major burden in this country [Citation23]. The elimination efforts can be further dampened by the emergence and spread of drug resistance [Citation27]. Continuous surveillance with therapeutic efficacy studies are essential to overcome this hurdle [Citation27]. In the past, ineffective surveillance methods led to severe global public health concerns by delaying the detection and containment of CQ-resistant P. falciparum [Citation3]. In order to avoid the repetition of history, it is necessary to understand the threat posed by CQ-resistant P. vivax and to allocate more resources for the development of standardized and validated methods for the detection and containment of CQ-resistant P. vivax [Citation3]. In this study, we investigated the pooled prevalence of CQ resistance in P. vivax in India through a systematic review of published literature between 1995 to 2022. CQ is the first-line treatment for P. vivax malaria in India but the exact burden of CQR P. vivax in India is unclear. Since India has already declared its aim to eliminate malaria by 2030 [Citation28], the detection and monitoring of CQR P. vivax are crucial. In this systematic review, CQ therapeutic efficacy was assessed by in vivo efficacy studies (n = 1787) and ex-vivo (n = 141) studies from different malaria-endemic regions in India. The pooled prevalence of clinical treatment failures and in-vitro CQ-resistant strains were 1.39% and 7.8%, respectively. In this study, we have observed the pooled prevalence of LPFs, LCFs, and ETFs to be 1.01%, 0.33%, and 0.06%, respectively.
Except for one study that has reported a high prevalence of CQ resistance, most studies reported a low prevalence of resistance [Citation14,Citation19,Citation21]. This study by Singh et al., from South Bihar reported 22.7% treatment failures. Of these treatment failures, 66.67% were LPFs and the remaining 33.33% were LCFs [Citation11]. While these are important findings, and it is possible that South Bihar has higher resistance, it must be noted that these results were not reproduced by any other studies from the same region. Additionally, the possibility of mixed infections were not ruled out. The poor response could have been due to poor CQ absorption in malnourished patients as well. Another study conducted by Kulkarni et al. [Citation28] similarly reported a high prevalence of treatment failures (51.7%) from Mumbai. However, the study’s sample size was very small (n = 29) and the duration of follow-up was very short (less than 7 days). The study was excluded because the follow-up period of 28 days required to assess late treatment failure was not observed in this study. In addition, there were 6 case reports/case series (Number of cases = 7) reporting resistance/treatment failures from different parts of India which were not included in the quantitative synthesis [Citation6,Citation29–33] (supplementary document ).
WHO guidelines recommend a combination of chloroquine for three days followed by a 14-day course of primaquine (0.25–0.5 mg/kg/day) as the treatment of choice for P. vivax. Primaquine is usually started with chloroquine as it has activity against both blood and liver stages. This makes it extremely useful in preventing relapse originating from the dormant liver stages of the parasites [Citation34–36]. Primaquine may have some activity against the chloroquine-resistant strains as well. In our study, 1843 (79.1%) were treated with chloroquine alone till day 28, 286 (12.27%) were treated with chloroquine plus early primaquine commencing before day 3, and 200 (8.58%) received primaquine after day 3 (). A total of 25 CQ-resistant cases were reported among the patients who received CQ monotherapy. However, the group treated with the combination had shown better susceptibility than the group treated with monotherapy.
Within 28 days of receiving chloroquine treatment, recurrent parasitemia does not always indicate chloroquine resistance. It might be due to incomplete treatment, wrong dosage, and poor absorption of the drug. Therefore the definitive diagnosis of CQ-resistance requires documentation of adequate drug exposure at the time of recurrence. It is crucial to confirm the growth of parasites in concentrations of drugs above the minimum inhibitory concentration [Citation35]. However, elaborate laboratory set-up is required to carry out this pharmacokinetic analysis, which is often extremely difficult in resource-limited settings. In our analysis, only one study assessed the plasma drug concentrations for P. vivax in clinical isolates.
Furthermore, there is a high risk of getting new infections during the follow-up due to the fact that most of the studies were carried out in malaria-endemic areas. The risk of infection is proportional to the follow-up duration and the transmission intensity. Genetically homologous and heterologous infections can be distinguished by using molecular genotyping techniques. However, it cannot differentiate between the recrudescence and relapse with a homologous strain. The combination of blood-stage schizonticidal action and post-treatment prophylaxis suppressing early relapse or reinfection determines the effectiveness of chloroquine in P. vivax.
In our analysis, we have observed that most of the studies (n = 7; 70%) have followed WHO standard definitions for treatment responses in therapeutic efficacy studies. Of these 5 studies have followed the WHO guidelines of 2009 [Citation18–21,Citation23], one followed guidelines of 2002&2003 [Citation14], and the other study has followed guidelines of 1986 [Citation12]. However, few studies have followed different criteria (). Moreover, the included studies were conducted in different endemic regions and populations, which further leads to a high degree of variability. All three in-vitro studies have assessed CQ drug efficacy by schizont maturation assay using different 50% inhibitory concentrations (IC50) to determine CQ resistance [Citation20,Citation24,Citation25]. Hence, the included studies have shown a high degree of variability with respect to the patient population and outcome definitions.
World Health Organization (WHO) recommends routine surveillance for early detection of resistance or the emergence of resistant parasites to guide and help in the progress made toward malaria control and elimination [Citation10]. According to national guidelines, the drug policy is changed for the area/block PHC reporting 10% or more total treatment failure (ETF + LCF) to the tested drug, i.e. the currently used antimalarials [Citation37]. The currently available data show a low prevalence of resistance to CQ in P.vivax cases in India except in a few regions. There is a need for routine surveillance activities in these regions to guide policy changes.
Limitations
Most studies included in the SR were of a small sample size. Most studies did not rule out mixed infections. Clinical failures could not be attributed to resistance as they were often not corroborated with microbiological studies. Also, clinical treatment failures may have been due to incomplete treatment duration, incorrect dosage, or poor absorption. Among in-vitro drug efficacy studies, authors did not mention about the chloroquine response profiles of isolates, the initial parasitemia stage, and compound incubation durations. Another limitation of our study is that we were not able to perform a meta-analysis because of the heterogeneity of the studies.
Conclusions
Evidence from a small number of studies with a moderate risk of bias suggests that the prevalence of CQ resistance in P.vivax cases in India might be low compared to other parts of South East Asia. There is a need for quality randomized clinical trials with appropriate sample size, adequate follow-up time, and without concomitant PQ use to understand the exact burden of CQ resistance in India.
Authors’ contributions
VTN, NG, and KS conceptualized the review. VTN and NG extracted the data and VTN wrote the first draft of the report. NG and KS reviewed the data and revised the final report. All the authors participated in the manuscript review. All the authors approved the final draft of the article.
Supplementary Materials.docx
Download MS Word (23.8 KB)PRISMA_2020_checklist.docx
Download MS Word (32.6 KB)Acknowledgements
Dr. Kavitha Saravu is thankful to the Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal for the DA Prasanna Endowment Seed Funding Scheme award. Mr. Vishnu Teja Nallapati is thankful to the Manipal Academy of Higher Education, Manipal for Dr. TMA Pai Ph.D. Scholarship. The contribution of Ms. Nayak Prathiksha Prakash and Ms. B Raksha Shetty in the preparation of the Map is greatly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20477724.2023.2285179
Additional information
Funding
References
- Mahgoub H, Gasim GI, Musa IR, et al. Severe Plasmodium vivax malaria among sudanese children at New Halfa Hospital, Eastern Sudan. Parasites Vectors. 2012;5(1):154. doi: 10.1186/1756-3305-5-154
- World malaria report. 2021 [Internet]. [cited 2023 Jan 27]. Available from: https://www.who.int/publications-detail-redirect/9789240040496
- Price RN, von Seidlein L, Valecha N, et al. Global extent of CQ-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014 Sep 8;14(10):982–991. doi: 10.1016/S1473-3099(14)70855-2
- Rieckmann KH, Davis DR, Hutton DC. PLASMODIUM VIVAX RESISTANCE to CQ? Lancet. 1989 Nov 18;334(8673):1183–1184. doi: 10.1016/S0140-6736(89)91792-3
- Baird JK, Basri H, Purnomo BM, et al. Resistance to CQ by Plasmodium vivax in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1991 May 1;44(5):547–552. doi: 10.4269/ajtmh.1991.44.547
- Garg M, Gopinathan N, Bodhe P, et al. Vivax malaria resistant to CQ: case reports from Bombay. Trans R Soc Trop Med Hyg. 1995 Nov 1;89(6):656–657. doi: 10.1016/0035-9203(95)90432-8
- Price RN, Auburn S, Marfurt J, et al. Phenotypic and genotypic characterization of drug-resistant Plasmodium vivax. Trends Parasitol. 2012 Nov;28(11):522–529. doi: 10.1016/j.pt.2012.08.005
- World Health Organization. Control and elimination of Plasmodium vivax malaria: a technical brief [Internet]. World Health Org. 2015. https://www.who.int/publications-detail-redirect/9789241509244
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan — a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4
- World Health Organization. Methods for surveillance of antimalarial drug efficacy. World Heal Organisation. 2009;85 https://www.who.int/docs/default-source/documents/publications/gmp/methods-for-surveillance-of-antimalarial-drug-efficacy.pdf?sfvrsn=29076702_2.
- Singh RK. Emergence of CQ-resistant vivax malaria in south Bihar (India). Trans R Soc Trop Med Hyg. 2000;94(3):327. doi: 10.1016/S0035-9203(00)90344-4
- Nandy A, Addy M, Maji AK, et al. Monitoring the CQ sensitivity of Plasmodium vivax from Calcutta and Orissa, India. Ann Trop Med Parasitol. 2003 Apr;97(3):215–220. doi: 10.1179/000349803235001868
- Bruce-Chwatt LJ, Black RH, Canfield CJ, et al. Chemotherapy of malaria. 2nd ed. Geneva: World Health Organization; 1986.
- Srivastava HC, Yadav RS, Joshi H, et al. Therapeutic responses of Plasmodium vivax and P. falciparum to CQ, in an area of western India where P. vivax predominates. Ann Trop Med Parasitol. 2008 Sep;102(6):471–480. doi: 10.1179/136485908X311759
- World Health Organization. (2002). Monitoring antimalarial drug resistance. Report of a WHO consultation. Geneva, Switzerland 3B5 December 2001. Document WHO/CDS/RBM/2002.39. Geneva: WHO.
- World Health Organization. (2003). Assessment and Monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Document WHO/HTM/RBM/2003.50. Geneva: WHO.
- Valecha N, Joshi H, Eapen A, et al. Therapeutic efficacy of CQ in Plasmodium vivax from areas with different epidemiological patterns in India and their pvdhfr gene mutation pattern. Trans R Soc Trop Med Hyg. 2006 Sep 1;100(9):831–837. doi: 10.1016/j.trstmh.2005.11.012
- Mishra N, Singh JPN, Srivastava B, et al. Monitoring antimalarial drug resistance in India via sentinel sites: outcomes and risk factors for treatment failure, 2009–2010. Bull World Health Organ. 2012 Dec 1;90(12):895–904. doi: 10.2471/BLT.12.109124
- Ganguly S, Saha P, Guha SK, et al. In vivo therapeutic efficacy of CQ alone or in combination with primaquine against vivax malaria in Kolkata, West Bengal, India, and polymorphism in pvmdr1 and pvcrt-o genes. Antimicrob Agents Chemother. 2013 Mar;57(3):1246–1251. doi: 10.1128/AAC.02050-12
- Shalini S, Chaudhuri S, Sutton PL, et al. CQ efficacy studies confirm drug susceptibility of Plasmodium vivax in Chennai, India. Malar J. 2014 Mar 31;13(1):129. doi: 10.1186/1475-2875-13-129
- Rishikesh K, Kamath A, Hande MH, et al. Therapeutic assessment of chloroquine–primaquine combined regimen in adult cohort of Plasmodium vivax malaria from a tertiary care hospital in southwestern India. Malar J. 2015 Aug 11;14(1):310. doi: 10.1186/s12936-015-0824-y
- Valecha N, Savargaonkar D, Srivastava B, et al. Comparison of the safety and efficacy of fixed-dose combination of arterolane maleate and piperaquine phosphate with CQ in acute, uncomplicated Plasmodium vivax malaria: a phase III, multicentric, open-label study. Malar J. 2016 Jan 27;15(1):42. doi: 10.1186/s12936-016-1084-1
- Saravu K, Kumar R, Ashok H, et al. Therapeutic assessment of CQ-Primaquine combined regimen in adult cohort of Plasmodium vivax malaria from primary care centres in Southwestern India. PLoS One. 2016 Jun 17;11(6):e0157666. doi: 10.1371/journal.pone.0157666
- Singh G, Singh R, Urhehar AD. Simple molecular methods for early detection of CQ drug resistance in Plasmodium vivax and Plasmodium falciparum. J Clin Diagn Res JCDR. 2016 Jul;10(7):DC19–23. doi: 10.7860/JCDR/2016/18596.8154
- Matlani M, Kumar A, Singh V. Assessing the in vitro sensitivity with associated drug resistance polymorphisms in Plasmodium vivax clinical isolates from Delhi, India. Exp Parasitol. 2021 Jan 1;220:108047. doi: 10.1016/j.exppara.2020.108047
- Munn Z, Moola S, Lisy K, et al. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris E, and Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020 doi:10.46658/JBIMES-20-06.
- Ferreira MU, Nobrega de Sousa T, Rangel GW, et al. Monitoring Plasmodium vivax resistance to antimalarials: persisting challenges and future directions. Int J Parasitol Drugs Drug Resist. 2021 Apr;15:9–24. Epub 2020 Dec 5. PMID: 33360105; PMCID: PMC7770540. doi: 10.1016/j.ijpddr.2020.12.001.
- Kulkarni AV, Kasturi L, Amin A, et al. Therapy and drug resistance in malaria. Indian J Pediatr. 2000 Jan;67(1):33–35. doi: 10.1007/BF02802634
- Dua VK, Kar PK, Sharma VP. CQ resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996;1(6):816–819. doi: 10.1111/j.1365-3156.1996.tb00116.x
- Kshirsagar NA, Gogtay NJ, Rajgor D, et al. An unusual case of multidrug-resistant Plasmodium vivax malaria in Mumbai (Bombay), India. Ann Trop Med Parasitol. 2000 Mar;94(2):189–190. doi: 10.1080/00034983.2000.11813528
- Virdi VS, Goraya JS, Khadwal A, et al. Neonatal transfusion malaria requiring exchange transfusion. Ann Trop Paediatr. 2003 Sep;23(3):205–207. doi: 10.1179/027249303322296529
- Shah I. CQ resistant vivax malaria in an infant: a report from India. J Vector Borne Dis. [2008 Jun 1];45(2):176.
- Mohan K, Maithani MM. Congenital malaria due to CQ-resistant Plasmodium vivax: a case report. J Trop Pediatrics. 2010 Dec 1;56(6):454–455. doi: 10.1093/tropej/fmq025
- Pukrittayakamee S, Imwong M, Looareesuwan S, et al. Therapeutic responses to antimalarial and antibacterial drugs in vivax malaria. Acta Trop. 2004 Feb;89(3):351–356. PMID: 14744561. doi: 10.1016/j.actatropica.2003.10.012
- Kevin Baird J, Hoffman SL. Primaquine therapy for malaria. Clinl Infect Dis. 1 November 2004;39(9):1336–1345. doi: 10.1086/424663
- Baird JK, Leksana B, Masbar S, et al. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997 Jun;56(6):621–626. doi: 10.4269/ajtmh.1997.56.621. PMID: 9230792
- National Framework for Malaria Elimination (2016–2030). Delhi: National Vector Borne Disease Control Programme 2016. Available from: http://www.indiaenvironmentportal.org.in/files/file/National-framework-for-malaria-elimination-in-India-2016%E2%80%932030.pdf.