 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Climate change may increase the risk of dengue and yellow fever transmission by urban and sylvatic mosquito vectors. Previous research primarily focused on Aedes aegypti and Aedes albopictus. However, dengue and yellow fever have a complex transmission cycle involving sylvatic vectors. Our aim was to analyze how the distribution of areas favorable to both urban and sylvatic vectors could be modified as a consequence of climate change. We projected, to future scenarios, baseline distribution models already published for these vectors based on the favorability function, and mapped the areas where mosquitoes’ favorability could increase, decrease or remain stable in the near (2041–2060) and distant (2061–2080) future. Favorable areas for the presence of dengue and yellow fever vectors show little differences in the future compared to the baseline models, with changes being perceptible only at regional scales. The model projections predict dengue vectors expanding in West and Central Africa and in South-East Asia, reaching Borneo. Yellow fever vectors could spread in West and Central Africa and in the Amazon. In some locations of Europe, the models suggest a reestablishment of Ae. aegypti, while Ae. albopictus will continue to find new favorable areas. The results underline the need to focus more on vectors Ae. vittatus, Ae. luteocephalus and Ae. africanus in West and Central sub-Saharan Africa, especially Cameroon, Central Africa Republic, and northern Democratic Republic of Congo; and underscore the importance of enhancing entomological monitoring in areas where populations of often overlooked vectors may thrive as a result of climate changes.
Introduction
The extent of occurrence of both dengue and yellow fever, two of the most important arboviral diseases affecting humans worldwide [Citation1,Citation2], has been changing due to intensive agriculture, irrigation, deforestation, population movements, rapid unplanned urbanization, and phenomenal increases in international travel and trade [Citation3]. The mosquito species Aedes aegypti and Ae. albopictus are considered to be main vectors of these diseases, and so the shifting trend of both species’ ranges is of public health concern [Citation4]. However, these mosquitoes are not the only species transmitting dengue and yellow fever, as some sylvatic species are also involved in viral spillover between non-human primates and humans [Citation5].
Continued globalization of trade contributes to increased risks related to the establishment of a range of vectors [Citation6]. Haines [Citation7] and others in the 1980s suggested that global warming could become a driving factor for the future spread of mosquito-borne diseases. The incidence and distribution of these diseases are often conditioned by the abundance and distribution of their vectors [Citation8,Citation9]. Numerous modeling studies have predicted range changes for Ae. aegypti and Ae. albopictus in response to climate change [Citation10–12]. Aedes mosquito vectors are currently expanding their distributions to more temperate climates across all continents where they now occur [Citation13–15], also favored by the globalization of trade through increased international travel and shipping. Many ports and airports are easy entry points for mosquito species such as Ae. aegypti and Ae. albopictus. For example, Ae. albopictus was introduced in Europe by the transport of tyres on ferries [Citation16]. Predicting which might be the most favorable points of entry in the future could be useful for surveillance efforts. Recent research has suggested that, on average, in the last 50 years of the 20th century, the environmental conditions became around 1.5% more suitable for Ae. aegypti per decade globally, which could increase to 3.2–4.4% per decade by 2050 [Citation10]. Climate changes could even be forcing vectors to develop adaptation mechanisms facilitating infection spread [Citation17] (e.g. high temperatures alter the activity of vectors and their biting rate; floods alter the aquatic environment suitable for breeding, increasing exposure to vector bites; droughts increase the population of dipteran vectors [Citation18]). However, up to now, the effects of climate change on sylvatic vectors have not been taken into account.
Areas prone to sylvatic arboviral transmission to humans from non-human primates may be larger than estimated [Citation14,Citation19]. Anthropogenic disturbance in forests often allows the interaction of different mosquito communities with varying habitat preferences at ecotonal forest edges [Citation20]. So, any attempt to make forecasting of dengue and yellow fever transmission risk in geographic contexts involving tropical areas should take both urban and sylvatic vectors into account. These species include Ae. africanus, Ae. luteocephalus, Ae. vittatus for dengue and yellow fever; Sabethes chloropterus, Haemagogus leucocelaenus and Hg. janthinomys for yellow fever and Ae. niveus for dengue [Citation21].
In this context, the objectives of this research are to answer the following questions: (1) Which mosquito species could be implicated in future increases in the risk of transmission for dengue and yellow fever due to climate change?, and (2) Where would these increases most likely occur?
Methods
Baseline vector models
In order to analyze the effect of climate change on dengue and yellow fever vector distributions, we took into account the favorability models for these species published for the period 2001–2017 [Citation14,Citation19], henceforth referred to as the baseline models. These models were based on the favorability concept, which permits model combination through fuzzy logic operations [Citation22]. This capacity allowed Aliaga-Samanez and colleagues [Citation14,Citation19] to integrate information on vectors, reservoir species and environmental variables in the geographic analysis of disease transmission risk. Vector favorability models represent the degree of environmental favorability for vector species to occur on a grid of 18,874 hexagons of 7,774-km2 covering the whole World. Favorability is defined as the degree to which local conditions allow a higher or lower local probability than expected by chance (see below, Equationequation (1)(1)
(1) ). This chance probability is defined as the total prevalence of the analyzed event [Citation22]. Here, for each vector species, the total prevalence is the number of hexagons with presences divided by the number of hexagons in the study area. The baseline models considered Ae. aegypti and Ae. albopictus, and known sylvatic vector species (Ae. africanus, Ae. luteocephalus, Ae. niveus, Ae. vittatus, Sabethes chloropterus, Haemagogus leucocelaenus and Hg. janthinomys) (see Table S1).
Forecasting the future distribution of vectors
In order to make forecasts for each of these species mentioned above, we recalculated favorability values (F) using the same equation as defined in the baseline models [Citation14,Citation19], replacing the values of climate variables in each hexagon according to predictions by the Intergovernmental Panel on Climate Change (IPCC) [Citation23] (see below for details). Those baseline models [Citation14,Citation19] are defined by the following equation:
where n1 and n0 are, respectively, the number of presences and absences considered as dependent variables for model training, and y is a linear combination of predictor variables. The y equations for every species can be seen in Tables S2 and S3. Values for the non-climatic variables (i.e. Human Concentration, Infrastructures, Livestock, Topography, Agriculture, Ecosystem Types) forming part of the model were assumed not to change in the future period considered, thereby isolating the effect of a changing climate (Table S4). To consider uncertainties in our forecasts according to a range of variation in climatic predictions, we used climatic variable values from different climate change scenarios based on IPCC reports [Citation24]. We used different Representative Concentration Pathways (RCPs) and atmosphere – ocean General Circulation Models (GCMs) over two time periods, namely to 2041–2060 (‘near future’) and to 2061–2080 (‘distant future’). RPCs represent different pathways of future emissions assuming different levels of greenhouse gas emissions and socio-economic development, leading to distinct climate outcomes and impacts. We considered two emissions scenarios: a stabilized emissions scenario RCP 4.5, and a high emissions scenario RCP 8.5. According to McSweeney et al. [Citation25] and Sanderson et al. [Citation26] we selected five GCMs that provided predictions available for the whole World and with the lowest detected biases in the available GCMs with respect to actual climate data (see Table S4) [Citation25,Citation26]: CESM1-CAM5, CNRM-CM5, FIO-ESM, GFDL-CM3, MPI-ESM-LR. The GCMs and RCP scenarios were taken from Climatologies at High resolution for the Earth Land Surface Areas (CHELSA) free high resolution climate data [Citation27–30]. The basal model for each vector species was therefore projected to a total of 10 GCM-RCP scenario combinations per future period. Finally, a climate change forecast consensus was calculated using average favorability values of the 10 projections for a given period. For every disease and time period, a projected model to the future was obtained by combining the corresponding single-vector consensual forecasts with each other using the fuzzy logic operator ‘fuzzy union’ [Citation31], which is equivalent to assigning the highest favorability value in each hexagonal unit.
Expected rates of change in favourability
We mapped the expected increment of favorability by calculating the difference between forecasted favorability values (i.e. for 2041–2060 or 2061–2080) and those in the baseline model (2001–2017). In order to quantify to what extent the current favorability (F0) is modified globally in the future forecasts (Ff), we calculated the fuzzy parameters (parameters defined in terms of membership in fuzzy sets) of increment and maintenance according to the equations [Citation23,Citation32]:
where I represents the global rate of increment in favorability, and M represents the global rate of maintenance of the original values. The factor c(Fx) is the cardinality of the Fx model or model projection – where favorability is treated as a fuzzy set [Citation31] – that is, the sum of all the hexagons’ favorability values (which, in turn, are treated as degrees of membership in the fuzzy set of hexagons favorable for the presence of vectors). The intersection between future (Ff) and present (F0) favorability values, which is needed to calculate M, is defined as followed:
Positive increment values (I) indicate a net increase in favorability, that is, a gain of favorable areas; whereas negative values mean a net loss of favorable areas. The maintenance values (M) indicate the degree of overlap between present and forecasted favorable areas.
Uncertainty in the forecasts
The local degree of uncertainty of forecasts resulting from a consensus based on averages (see above), was mapped by calculating the standard deviation, for each spatial unit, of the 10 possible favorability values projected for a given period (5 GCMs x 2 RCP scenarios). This standard deviation was interpreted as a measure of the reliability of forecasts in each hexagonal unit.
Results
Ae. aegypti and Ae. albopictus models projected into the future
Favorable areas for Ae. aegypti, according to the models projected into the future for the periods 2041–2060 and 2061–2080 show little differences compared to the 2001–2017 baseline, with changes being perceptible only at regional scales ( and S1). The models suggest that the areas currently favorable for the presence of Ae. aegypti are expected to remain that way from now to 2080 (maintenance index value, M ≥ 0.96). In the near future (2041–2060), there could be a slight global net loss of favorability (increment index value, I = −0.03); whereas a positive increase (I = 0.075) is expected in the distant future (2061–2080). This increase would affect some regions of the Amazon basin and of Central Africa where favorability values, currently being intermediate-low, would turn into intermediate-high ( and S1). Extensive areas of the United States, Europe, and Australia could experience a favorability increase of 0.01 to 0.03 (Fig. S1) although most parts of these regions would remain unfavorable for the presence of Ae. aegypti ().
Figure 1. Vector model future projections for the periods 2041–2060 and 2061–2080. Models of mosquitoes Ae. aegypti and Ae. albopictus, and of sylvatic species (Hg. janthinomys, Hg. leucocelaenus, Sa. chloropterus, Ae. luteocephalus, Ae. africanus, Ae. vittatus, Ae. niveus) for the current time (2001–2017) and average model projections into the future for the periods 2041–2060 and 2061–2080. Yellow triangles represent yellow fever vectors and red drops represent dengue vectors.
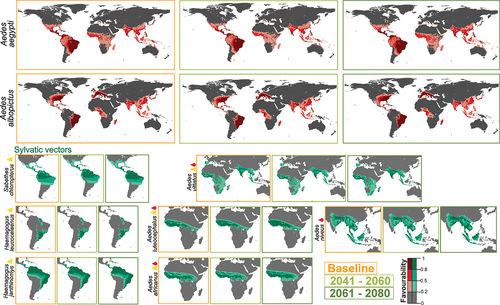
Expected changes for Ae. albopictus are much lower (). Favorable areas for this species are expected to remain unchanged (M > 0.99 in both future periods), while the global net gain in favorability could be slight, and would take place in the near future (I = 0.019 for the period 2041–2060; I = 0.020 for the period 2061–2080) (Figure S1).
Sylvatic vector models projected into the future
Changes predicted for sylvatic vectors depend on the mosquito species analyzed (). In America, favorable areas for Haemagogus janthinomys and Hg. leucocelaenus show no change with respect to the baseline (Figure S2). In contrast, high-favorability areas for Sabethes chloropterus in Central and South America seem to increase progressively north and southward (I = 0.073 for the period 2061–2080) (Figure S2). In Africa and Asia, changes could be negligible in the near future, whereas a little increase is expected in the distant future. This would be a north and southward increase in Central Africa for Ae. luteocephalus (I = 0.2) and for Ae. africanus (0.12); and a south and eastward increase in Asia for Ae. niveus (I = 0.09). Ae. vittatus, which has populations in Europe, Africa and Asia, could experience a slight favorability increase elsewhere in the more distant future (I = 0.06) ( and Figure S2).
Combined vector models projected into the future
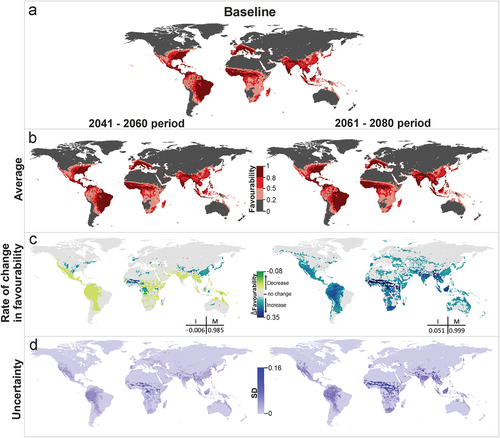
The models combining predictions for all the vectors of dengue show that areas currently favorable to the presence of these species will remain so in the near (2041–2060) and the distant (2061–2080) futures (both M > 0.98). Despite favorability decreasing globally slightly in the near future (I = −0.006), favorability could increase locally by more than 7% in the north of West Africa and at the southern limits of the Himalayas ( and S3). However, this increase would not produce a noteworthy enlargement of high-favorability areas (compare and ). In the more distant future, a 5% favorability increase is expected (I = 0.05), and so new areas could become favorable for the presence of dengue vectors in large areas of West and Central Africa and in South-East Asia, reaching Borneo ( and S3).
Figure 2. Dengue vector model projections into the future for the periods 2041–2060 and 2061–2080. (a) Vector model for the current time (2001–2017), (b) average model projections into the future for the periods 2041–2060 and 2061–2080, (c) areas where favourability increases and decreases in the future relative to the present. Difference between the future projection and the current model. I: increment rate; M: maintenance rate. Positive values of I indicate a net increase in favourability, that is, a gain in favourable areas, whereas negative values of I mean a net loss of favourable areas. M indicates the degree to which the favourable areas in the current model overlap with the favourable forecasted areas. (d) Uncertainty of the vector model in the period 2041–2060 and 2061–2080. SD: standard deviation. Zoomed maps of a regional scale can be found in the Supplemental Material.
Combined predictions for yellow fever vectors () show a similar pattern to that of dengue. Favorable areas are predicted to remain unchanged in both future periods (M > 0.97). Global favorability values could decrease slightly in the near future (I = −0.01), but might increase in the distant future (I = 0.03). This increase is expected to affect principally the Amazon basin and West and Central Africa (). The models indicate, instead, a regional favorability decrease of more than 0.01 points in coastal areas of Chile and Peru.
Figure 3. Yellow fever vector model future projections for the periods 2041–2060 and 2061–2080. (a) Vector model for the current time (2001–2017), (b) average model projections into the future for the periods 2041–2060 and 2061–2080, (c) areas where favourability increases and decreases in the future relative to the present. Difference between the future projection and the current model. I: increment rate; M: maintenance rate. Positive values of I indicate a net increase in favourability, that is, a gain in favourable areas, whereas negative values of I mean a net loss of favourable areas. M indicates the degree to which the favourable areas in the current model overlap with the favourable forecasted areas. (d) Uncertainty of the vector model in the period 2041–2060 and 2061–2080. SD: standard deviation. Zoomed maps of a regional scale can be found in the Supplemental Material.
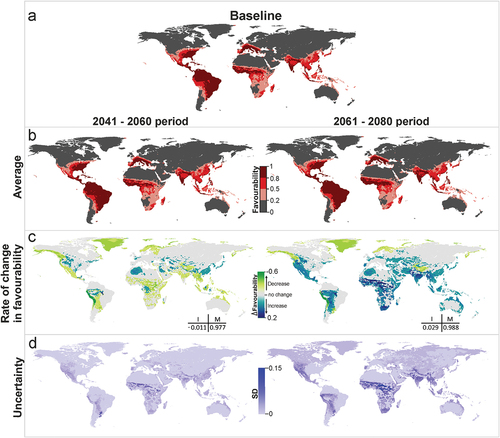
According to the uncertainty analysis, variations between scenario-model combinations in the near future mostly occur in the areas where average favorability is predicted to increase. In the more distant future, there is more consistency between projections, and high variations only occur in some areas of West and Central Africa (). Some uncertainty is also observed at the southern limits of the Himalayas in predicted values regarding vectors transmitting the yellow fever virus.
Discussion
This study is the first to analyze the effect of climate change on the distribution of all mosquito species transmitting dengue and yellow fever. Our projections for Ae. albopictus and Ae. aegypti predict that climate change could allow these species to expand into some previously unoccupied temperate areas (). In addition, our projections have also detected species of sylvatic mosquitoes that may experience distribution changes, and could thus contribute to increasing disease transmission risk. This has implications for surveillance in the future.
Despite the insights gained from a macro-scale analysis (spatial resolution: 7,774 km2), there are limitations to its ability to capture micro-scale environmental conditions that may influence vector distribution. It is important to recognize that the results here cannot comprehensively address local-level complexities but rather provide a biogeographic overview. This could partially explain gaps between our results and those of other authors that are mentioned below. It is also necessary to interpret with caution the relevance of these models regarding human health, as vectors alone do not allow us to predict areas prone to disease transmission to humans. For this, the effects of climate change on vectors should be combined with that on other factors influencing anthroponotic and zoonotic transmission (see Aliaga-Samanez et al. [Citation14,Citation19]).
The favorability models on which our projections have been based are diverse regarding the climatic predictor variables involved. While some species could be mostly affected by precipitation (e.g. Ae. albopictus, Ae africanus), others could be mostly influenced by temperature (e.g. Ae. luteocephalus) or by both temperature and precipitation (e.g. Ae. aegypti) (Table S2). Besides, there is substantial heterogeneity between species in the fine-scale spatial patterns derived from climate change. For example, currently, Ae. aegypti has stable populations in all South American countries, while Ae. albopictus has not yet been detected in Peru, Ecuador, Chile, Bolivia and Uruguay, yet is already present in neighboring countries. Aliaga-Samanez et al. [Citation14] describe an increase in favorable territories for both Aedes species, from the Brazilian coast to the Amazon basin during the last 20 years. According to our model projections, the Amazon basin could remain favorable for both species in the two future periods analyzed. In particular, the Amazon could become even more favorable for Ae. aegypti, reaching intermediate-high values in Colombia, Peru and Brazil (). These results suggest that temperature changes might have already favored the recent spread of Aedes species in the area, despite other studies predicting no new relevant variations in distribution under climate change in South America [Citation33]. In North America, in contrast to Kraemer et al. (2019) [Citation34], who predict an expansion of Ae. aegypti between 2020 and 2050 in the United States, our projections are more conservative. We only predict a relevant increase in favorability for this species in the distant future, after 2060 (Fig. S1). Instead, both our projections and Kraemer and collaborators suggest an increase in favorability for Ae. albopictus starting in the near future (Fig. S1).
In Asia, our models forecast an increase of favorability for Ae. aegypti in territories to the east and south of China (), which is consistent with Liu et al.’s [Citation35] results. De Guilhem de Lataillade et al. [Citation36] observed that Ae. aegypti populations in Singapore, Taiwan, Thailand and New Caledonia are competent vectors for yellow fever transmission in that region of Asia and the Pacific, and our models also predict a slight increase in those countries, especially in Thailand (). Bonnin et al. [Citation37] found that Ae. aegypti and Ae. albopictus densities will increase in Southeast Asia due to future temperature increases. They also predict Ae. aegypti densities will increase from 25% (with climate mitigation measures) to 46% without, with smaller increases of 13% and 21% for Ae. albopictus. On Reunion Island, our projections predict a slight increase in favorability for Ae. albopictus in both future time periods, agreeing with Lamy et al. [Citation38] who found that Ae. albopictus abundance will increase in 2070–2100. Central Africa could also see a spread of favorable areas for Ae. aegypti (). Gaythorpe et al. [Citation39] report that Central Africa Republic is one of the countries most likely to see an increase in yellow fever transmission. Unfortunately, Africa lacks uniform surveillance policies [Citation40]. The results of this work could therefore be used to highlight territories where surveillance efforts should be focused. In Europe, in the distant future, our projections predict that Ae. aegypti could find favorable areas in Spain, the Netherlands and Portugal and, more intensively, in Italy, Turkey and Greece (Fig. S1). This agrees with Kraemer et al.’s [Citation34] suggestion that, around 2080, this species could become established in Italy and Turkey. Ae. albopictus is already established in many European countries and will surely continue to find favorable areas in the future. Favorability is predicted to increase after 2040 in central Europe, in countries such as France, Germany, Belgium and United Kingdom. Kraemer et al. [Citation34] also forecasted Ae. albopictus to spread widely across Europe, reaching large parts of France and Germany.
Focusing on the response of sylvatic mosquitoes to climate changes (), Ae. niveus and Ae. vittatus should be subject to survey as dengue vectors in Asia, where areas favorable to their presence could spread in the hinterlands of India, in the south-east of China, and in the South-Asian countries, especially in Borneo. In west and central sub-Saharan Africa, according to our results, attention should be paid to the three sylvatic vectors of dengue and yellow fever, Ae. vittatus, Ae. luteocephalus and Ae. africanus, especially in Cameroon, Central Africa Republic and north of Democratic Republic of Congo. However, the current entomological capacity in Africa is primarily focused on malaria vectors, and most countries lack routine surveillance programs, trained personnel and control activities focusing on Aedes and the viruses they transmit [Citation41]. As outbreaks of Aedes-borne arboviruses continue to increase in Africa, it would be critical to establish a solid public health entomology infrastructure for Aedes mosquitoes to contain and prevent further outbreaks [Citation42].
Both in Africa and in Asia, the most relevant changes are forecasted for the distant future (2061–2080), but in South America, the increase in favorability for the yellow fever vector Sabetes chloropterus, forecasted for the jungle areas of Bolivia, Peru and Brazil, could start in the near future. The climatic conditions could become, however, less favorable for this species in western Peru and Bolivia. On the other hand, our models suggest no change in favorable zones for Hg. janthinomys. Sadeghieh et al. [Citation43] suggest that many areas in Brazil will become unsuitable for Haemagogus spp. to survive and suboptimal for yellow fever virus replication under climate change. We therefore suggest that surveillance and prevention measures planned by the Pan American Health Organization (PAHO) for urban mosquitoes also include specifications and survey planning for Sa. chloropterus. In Brazil, sylvatic yellow fever outbreaks that have occurred since 2017 highlight the need to strengthen surveillance for zoonotic yellow fever in non-human primates [Citation19,Citation44].
Such variation has implications for how climate change may influence establishment success in areas currently absent from urban mosquito species. For example, taking into account that some of the most important ports in Europe are located in Barcelona, Rotterdam, Valencia, and Antwerp [Citation45], these could be important points for establishment of Ae. aegypti in case of accidental entry. There is already evidence of accidental arrivals of this mosquito species in northern Europe. For instance, in 2010 national surveillance in the Netherlands detected the presence of Ae. aegypti in a shipment of tires from Miami [Citation46,Citation47], and in 2016, Ae. aegypti was again detected in the Netherlands at the Schiphol International airport [Citation48]. Da Re et al. [Citation49] assessed the probability that Ae. aegypti, which currently occurs just on the east coast of the Black Sea, Turkey, Bulgaria and Russia [Citation50], spread through continental Europe. They selected five European ports taking into account environmental conditions and the economic importance of the harbors, and suggested that the city most likely to be at risk of establishment was Barcelona, followed by Algeciras. Our results concur. In Europe our model suggests that Barcelona, Algeciras, Venice, Sardinia, Antwerp, Naples and the west coast of Portugal are favorable areas (0.2<F < 0,5) for the presence of Ae. aegypti (). In addition, we detect areas with intermediate-high favorability values (0.5<F < 0.8) in Valencia, Rotterdam, Istanbul and Naples (). For Ae. albopictus, favorable conditions are predicted to occur in the 7 countries where it is not yet established, which will continue to be favorable through to 2080 ().
For Ae. aegypti, re-introduction in Europe is possible with many pathways and entry points. The question is whether, once the introduction has happened, environmental conditions are suitable for the species to establish successfully. Although Da Re et al. [Citation49] detected that the areas of Genoa, Venice and Rotterdam are not suitable for establishment of Ae. aegypti populations now, they suggested that climate change could create conditions for a future invasion because temperatures could reach values above the threshold limiting population establishment [Citation51]. Both Campbell et al. [Citation8] and Ryan et al. [Citation11] concur that the potential distribution of Ae. aegypti under future conditions could increase in most of Europe, even reaching the north of the continent as a consequence of climate change. However, our model projections for 2060 and 2080 continue to predict the same favorable areas that are currently suitable. According to our models, the risk of Ae. aegypti new introduction in Europe would remain concentrated principally in urban areas. Most of the cities outlined by these models are equipped with large harbors and/or airports, which could eventually favor the arrival of this species more than climate changes.
Similarly, the introduction of Ae. aegypti in the Canary Islands, northwest of Africa, has so far not lead to establishment. In early 2022, Ae. aegypti larvae were detected in La Palma, one of the Canary Islands, but extensive entomological surveillance work has not detected any life-stage since [Citation52]. Also in 2022, this species was detected on another of the Canary Islands, in Tenerife, but reinforced entomological surveillance has not detected further occurrence there [Citation53]. The high level of regular communication with nearby endemic regions such as Madeira Island (Portugal) and, to a lesser extent, with the archipelago of the Republic of Cape Verde, means that there is a real risk of introduction of the vector to the islands [Citation53].
In South America, Ae. aegypti has been introduced and established in new regions of Peru in recent years, and also at new altitudes. This happened in Piura (North of the country) and in Huanuco (Centre-East), at 1,959 and 2,227 meters above sea level, respectively [Citation54]. In 2022, the number of dengue cases in Peru exceeded that of the previous year [Citation55]. Our models predict an increase in favorability for Ae. aegypti in different regions of Peru for both future periods (), which could increase the number of dengue cases in the following years. In this scenario, the Pan American Health Organization (PAHO) has established, since 2019, a manual for indoor residual spraying in urban areas for the control of Ae. aegypti in American regions [Citation44].
The situation for Ae. albopictus is different. The establishment of Ae. albopictus in western South America, in countries such as Peru, could further favor the risk of dengue and yellow fever transmission, in addition to favoring the link between the urban and sylvatic cycle, since Ae. albopictus acts as a ‘bridge vector’. Our models do not predict a change in the favorable zones with respect to the baseline model, predicting that it will continue to be favorable in both future periods in southeastern Peru (). Peru has reinforced surveillance measures on the borders with Brazil and Colombia, has developed a technical standard for entomological surveillance and control of Ae. aegypti and surveillance of the entry of Ae. albopictus into the national territory [Citation56]. In Ecuador, this species was reported for the first time in 2017 in Guayaquil, and is currently expanding its distribution in the country [Citation57,Citation58]. Guayaquil is a coastal city and has the main port in the country, responsible for more than 80% of exports, and vulnerable to invasive species [Citation58]. In Australia, our models predict that Ae. albopictus will find favorable areas in eastern Australia in the future (), agreeing with Laporta et al. [Citation59]. However, the Australian government has had a well-structured control plan in place since 2005 which has resulted in the successful suppression of Ae. albopictus populations at major transport hubs in the Torres Strait [Citation60]. In Europe, this species has established stable populations since 1979, probably introduced via the Durres Port (Albania) through the entry of goods from China [Citation61]. Ae. albopictus has now been introduced in 33 European countries and is established in 26. This species might have caused dengue outbreaks already in France, Italy and Croatia and autochthonous cases in Spain [Citation62,Citation63]. In 2022, an increase in imported cases of dengue from Cuba was detected in Europe due to limited fumigation in Cuba [Citation64], and, at the beginning of 2023, autochthonous cases were detected for the first time in Ibiza (Balearic Islands).
According to the WHO, funding is needed in south-eastern Asia for strengthening surveillance, reporting and vector management [Citation65], as a response to the increased burden of dengue experienced since 2011 [Citation66], and although no autochthonous yellow fever cases have been reported in the Asia-Pacific regions, surveillance for imported cases is recommended. The combination of repeated introductions of imported cases, an immunologically naïve local population in an environment suitable for transmission, and climate change that will continue to favor its presence in the future could increase the risk of yellow fever occurrence in Asia.
Conclusions
Climate change could potentially modify and increase the risk of zoonotic diseases through its effects on vector distributions. Our study suggests that most of the area favorable to the presence of these species could remain the same, but increases in favorability could occur particularly in Thailand, Peru, Colombia, Brazil, China, India, Central African Republic, Spain, Italy, Netherlands, and Portugal. Ae. aegypti, Ae. albopictus, Ae. vittatus, Ae. luteocephalus, Ae. africanus, Ae. niveus and Sa. chloropterus, according to our analysis, will find new favorable areas or favorability will increase in currently occupied areas. Our results suggest the need to reinforce entomological surveillances in areas in which populations of vectors that are usually neglected could establish as climate changes.
Author contributions
AA-S, RD, RR and OJ designed and implemented the study. AA-S and OJ managed the curation of the database and analyzed the data. AA-S and performed and applied the modeling procedures. AA-S, RD and OJ interpreted the data, wrote, reviewed, and edited the manuscript. SM interpreted and reviewed about the health interpretation of the main results. MK, RR and OJ revised the different versions of the manuscript. RR and OJ managed the funding acquisition and project administration. All authors read and approved the final version of the manuscript.
Availability of data and materials
All the data relevant generated or analyzed during this study are included in this published article and its Supplemental Material.
Supplemental material.docx
Download MS Word (2.4 MB)Acknowledgements
This study was supported by the Project PID2021-124063OB-I00, of the Spanish Ministry of Science and Innovation and European Regional Development Fund (ERDF). AA-S was supported by a postdoctoral contract of the Plan Propio de Investigación, Transferencia y Divulgación Científica of the University of Malaga. RD is supported by the incorporation Doctor program of the Plan Propio de Investigación, Transferencia y Divulgación Científica of the University of Malaga, UMA-2022/REGSED-64576.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20477724.2024.2369377
Additional information
Funding
References
- Kuno G, Halstead SB. A Re-examination of the history of etiologic confusion between dengue and chikungunya. PLOS Negl Trop Dis. 2015;9(11):1–11. doi: 10.1371/journal.pntd.0004101
- Colón-González FJ, Sewe MO, Tompkins AM, et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet Heal. 2021;5:e404–e414. doi: 10.1016/S2542-5196(21)00132-7
- WHO. A global brief on vector-borne diseases. Geneva, Switzerland: World Health Organization Press; 2014. Available from: https://apps.who.int/iris/handle/10665/111008int
- Rupasinghe R, Chomel BB, Martínez-López B. Climate change and zoonoses: a review of the current status, knowledge gaps, and future trends. Acta Trop. 2022;226:106225. doi: 10.1016/J.ACTATROPICA.2021.106225
- Hanley KA, Monath TP, Weaver SC, et al. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292–311. doi: 10.1016/J.MEEGID.2013.03.008
- Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci USA. 2006;103(16):6242–6247. doi: 10.1073/pnas.0508391103
- Haines A. Global warming and health. BMJ. 1991;302(6778):669–670. doi: 10.1136/bmj.302.6778.669
- Campbell-Lendrum D, Manga L, Bagayoko M, et al. Climate change and vector-borne diseases: what are the implications for public health research and policy? Philos Trans R Soc B Biol Sci. 2015;370(1665):20130552–20130558. doi: 10.1098/rstb.2013.0552
- Messina JP, Brady OJ, Pigott DM, et al. The many projected futures of dengue. Nat Rev Microbiol. 2015;13(4):230–239. doi: 10.1038/nrmicro3430
- Iwamura T, Guzman-Holst A, Murray KA. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat Commun. 2020;11:1–10. Available from: https://www.nature.com/articles/s41467-020-16010-4
- Ryan SJ, Carlson CJ, Mordecai EA, et al. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLOS Negl Trop Dis. 2018;13(3):e0007213. doi: 10.1371/JOURNAL.PNTD.0007213
- Campbell LP, Luther C, Moo-Llanes D, et al. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc B Biol Sci. 2015;370(1665):20140135–20140139. doi: 10.1098/rstb.2014.0135
- Watts N, Amann M, Arnell N, et al. The 2020 report of the lancet countdown on health and climate change: responding to converging crises. Lancet. 2021;397(10269):129–170. doi: 10.1016/S0140-6736(20)32290-X
- Aliaga-Samanez A, Cobos-Mayo M, Real R, et al. Worldwide dynamic biogeography of zoonotic and anthroponotic dengue. PLOS Negl Trop Dis. 2021;15(6):e0009496. doi: 10.1371/JOURNAL.PNTD.0009496
- Khan SU, Ogden NH, Fazil AA, et al. Current and Projected Distributions of Aedes aegypti and Ae. albopictus in Canada and the U.S. Environ Health Perspect. 2020;128. doi: 10.1289/EHP5899
- Marrama Rakotoarivony L, Schaffner F. ECDC guidelines for the surveillance of invasive mosquitoes in Europe. Eurosurveillance. 2012. Available from: https://www.eurosurveillance.org/content/10.2807/ese.17.36.20265-en
- Filho WL, Ternova L, Parasnis SA, et al. Climate change and zoonoses: a review of concepts, definitions, and bibliometrics. Int J Environ Res Public Heal. 2022;19(2):893. Available from: https://www.mdpi.com/1660-4601/19/2/893/htm
- de Souza WM, Weaver SC. Effects of climate change and human activities on vector-borne diseases. Nat Rev Microbiol. 2024;1(16). doi: 10.1038/s41579-024-01026-0
- Aliaga-Samanez A, Real R, Segura M, et al. Yellow fever surveillance suggests zoonotic and anthroponotic emergent potential. Commun Biol. 2022;5(1):530. doi: 10.1038/s42003-022-03492-9
- Meyer Steiger DB, Ritchie SA, Laurance SGW. Mosquito communities and disease risk influenced by land use change and seasonality in the Australian tropics. Parasites Vectors. 2016;9(1):1–13. doi: 10.1186/s13071-016-1675-2
- Carvalho BM, Rangel EF, Vale MM. Evaluation of the impacts of climate change on disease vectors through ecological niche modelling. Bull Entomol Res. 2017;107(4):419–430. Available from: https://www.cambridge.org/core/journals/bulletin-of-entomological-research/article/evaluation-of-the-impacts-of-climate-change-on-disease-vectors-through-ecological-niche-modelling/6AED53FF15736045E4ECEEF077390175
- Acevedo P, Real R. Favourability: Concept, distinctive characteristics and potential usefulness. Naturwissenschaften. 2012;99:515–522. doi: 10.1007/s00114-012-0926-0
- Romero D, Olivero J, Márquez AL, et al. Uncertainty in distribution forecasts caused by taxonomic ambiguity under climate change scenarios: a case study with two newt species in mainland Spain. J Biogeogr. 2014;41(1):111–121. doi: 10.1111/jbi.12189
- Stocker TF, Qin D, Plattner GK, et al. Climate change 2013 the physical science basis: working Group I contribution to the fifth assessment report of the intergovernmental panel on climate change. Vol. 9781107057. Cambridge University Press; 2013. p. 1–1535. doi: 10.1017/CBO9781107415324
- McSweeney CF, Jones RG, Lee RW, et al. Selecting CMIP5 GCMs for downscaling over multiple regions. Clim Dyn. 2015;44(11–12):3237–3260. doi: 10.1007/s00382-014-2418-8
- Sanderson BM, Knutti R, Caldwell P. A representative democracy to reduce interdependency in a multimodel ensemble. J Clim. 2015;28(13):5171–5194. doi: 10.1175/JCLI-D-14-00362.1
- Nikolaus Karger D, Schmatz DR, Dettling G, et al. High-resolution monthly precipitation and temperature time series from 2006 to 2100. Sci Data. 2020;7(1):248. doi: 10.1038/s41597-020-00587-y
- Karger DN, Wilson AM, Mahony C, et al. Global daily 1 km land surface precipitation based on cloud cover-informed downscaling. Sci Data. 2021;8(1):307. doi: 10.1038/s41597-021-01084-6
- Karger DN, Conrad O, Böhner J, et al. Climatologies at high resolution for the earth’s land surface areas. Sci Data. 2017;4(1):1–20. doi: 10.1038/sdata.2017.122
- Knutti R, Masson D, Gettelman A. Climate model genealogy: generation CMIP5 and how we got there. Geophys Res Lett. 2013;40(6):1194–1199. doi: 10.1002/grl.50256
- Estrada A, Real R, Vargas JM. Using crisp and fuzzy modelling to identify favourability hotspots useful to perform gap analysis. Biodivers Conserv. 2008;17(4):857–871. doi: 10.1007/s10531-008-9328-1
- Kuncheva LI. Using measures of similarity and inclusion for multiple classifier fusion by decision templates. Fuzzy Sets Syst. 2001;122(3):401–407. doi: 10.1016/S0165-0114(99)00161-X
- Kamal M, Kenawy MA, Rady MH, et al. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLOS ONE. 2018;13(12):e0210122. doi: 10.1371/journal.pone.0210122
- Kraemer MUG, Reiner RC, Brady OJ, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y
- Liu B, Gao X, Ma J, et al. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci Total Environ. 2019;664:203–214.doi: 10.1016/j.scitotenv.2019.01.301
- de Guilhem de Lataillade L, Vazeille M, Obadia T, et al. Risk of yellow fever virus transmission in the Asia-Pacific region. Nat Commun. 2020;11:1–10. Available from: https://www.nature.com/articles/s41467-020-19625-9
- Bonnin L, Tran A, Herbreteau V, et al. Predicting the effects of climate change on dengue vector densities in Southeast Asia through process-based modeling. Environ Health Perspect. 2022;130(12). doi: 10.1289/EHP11068
- Lamy K, Tran A, Portafaix T, et al. Impact of regional climate change on the mosquito vector Aedes albopictus in a tropical island environment: La Réunion. Sci Total Environ. 2023;875:162484. doi: 10.1016/J.SCITOTENV.2023.162484
- Gaythorpe KAM, Hamlet A, Cibrelus L, et al. The effect of climate change on yellow fever disease burden in Africa. Elife. 2020;9:1–27. doi: 10.7554/ELIFE.55619
- Weetman D, Kamgang B, Badolo A, et al. Aedes mosquitoes and aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health. 2018;15(2):220. doi: 10.3390/ijerph15020220
- Buchwald AG, Hayden MH, Dadzie SK, et al. Aedes-borne disease outbreaks in West Africa: a call for enhanced surveillance. Acta Trop. 2020;209:105468. doi: 10.1016/J.ACTATROPICA.2020.105468
- Dadzie SK, Akorli J, Coulibaly MB, et al. Building the capacity of West African countries in aedes surveillance: inaugural meeting of the West African aedes surveillance network (WAASuN). Parasites Vectors. 2022;15(1):381. doi: 10.1186/s13071-022-05507-0
- Sadeghieh T, Sargeant JM, Greer AL, et al. Yellow fever virus outbreak in Brazil under current and future climate. Infect Dis Model. 2021;6:664–677. doi: 10.1016/J.IDM.2021.04.002
- PAHO. Plan de acción sobre entomología y control de vectores 2018–2023 (56 Consejo Directivo). Washington (USA); 2019. Available from: https://www.paho.org/es/documentos/cd5611-plan-accion-sobre-entomologia-control-vectores-2018-2023
- Eurostat. Top 20 EU ports handling containers, 2010, 2019 and 2020 (million TEUs). 2021 [cited 2022 Jun 28]. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Top_20_EU_ports_handling_containers,_2010,_2019_and_2020_(million_TEUs)_V2.png
- Scholte EJ, den Hartog W, Dik M, et al. Introduction and control of three invasive mosquito species in the Netherlands, July–October 2010. Eurosurveillance [Internet]. 2010;15(45):1–4. doi: 10.2807/ese.15.45.19710-en
- Brown JE, Scholte EJ, Dik M, et al. Aedes aegypti mosquitoes imported into the Netherlands, 2010. Emerg Infect Dis. 2011;17(12):2335. doi: 10.3201/eid1712.110992
- Ibañez-Justicia A, Gloria-Soria A, Den Hartog W, et al. The first detected airline introductions of yellow fever mosquitoes (Aedes aegypti) to Europe, at Schiphol International airport, the Netherlands. Parasites Vectors. 2017;10(1):1–9. doi: 10.1186/s13071-017-2555-0
- Da Re D, Montecino-Latorre D, Vanwambeke SO, et al. Will the yellow fever mosquito colonise Europe? Assessing the re-introduction of Aedes aegypti using a process-based population dynamical model. Ecol Inf. 2021;61:101180. doi: 10.1016/J.ECOINF.2020.101180
- ECDC. Aedes Aegypti - Current Known Distribution: march 2022. [cited 2022 May 7]. Available from: https://www.ecdc.europa.eu/en/publications-data/aedes-aegypti-current-known-distribution-march-2022
- Reinhold JM, Lazzari CR, Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: a review. Insects. 2018;9(4):158. doi: 10.3390/insects9040158
- Centro de Coordinación de Alertas y Emergencias Sanitarias del Ministerio de Sanidad de España. Evaluación rápida de riesgo: Identificación del mosquito Aedes aegypti en la isla de La Palma. 2022. Available from: https://www.sanidad.gob.es/areas/alertasEmergenciasSanitarias/alertasActuales/vectores/docs/20220504_Ae_aegypti_ERR.pdf
- Centro de Coordinación de Alertas Y Emergencias Sanitarias del Ministerio de Sanidad de España. Evaluación rápida de riesgo: Identificación del mosquito Aedes aegypti en Santa Cruz de Tenerife. 2023. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/20171226_Aedes-aegypti_en_Fuerteventura_ERR.pdf
- More M, Castañeda C, Suyón M. Nuevo registro altitudinal de Aedes aegypti en la región de Piura, Perú. Rev Peru Med Exp Salud Publica. 2018;35(3):536–537. doi: 10.17843/rpmesp.2018.353.3791
- Ministerio de Salud de Perú. Minsa: Más de 58 mil casos de dengue se han notificado en regiones del país en 2022. 2022. Available from: https://www.gob.pe/institucion/minsa/noticias/653797-minsa-mas-de-58-mil-casos-de-dengue-se-han-notificado-en-regiones-del-pais-en-2022
- DIGESA. Fronteras seguras para prevenir el ingreso del mosquito “Aedes albopictus”, transmisor de chikungunya, en el Perú. 2023. Available from: http://www.digesa.minsa.gob.pe/noticias/marzo2023/nota18.asp
- Carrazco-Montalvo A, Ponce P, Villota SD, et al. Establishment, genetic diversity, and habitat suitability of aedes albopictus populations from Ecuador. Insects. 2022;13(3):305. doi: 10.3390/insects13030305
- Ponce P, Di M, Argoti A, et al. First report of Aedes (stegomyia) albopictus (skuse) (Diptera: Culicidae), the asian tiger mosquito, in ecuador. J Med Entomol. 2018;55(1):248. doi: 10.1093/jme/tjx165
- Laporta GZ, Potter AM, Oliveira JFA, et al. Global distribution of Aedes aegypti and Aedes albopictus in a climate change scenario of Regional Rivalry. Insects. 2023;14(1):49. doi: 10.3390/insects14010049
- Muzari MO, Devine G, Davis J, et al. Holding back the tiger: successful control program protects Australia from aedes albopictus expansion. PLOS Negl Trop Dis. 2017;11(2):e0005286. doi: 10.1371/JOURNAL.PNTD.0005286
- Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc [Internet]. 1998;14(3):340–343. Available from: https://pubmed.ncbi.nlm.nih.gov/9813831/
- Gossner CM, Ducheyne E, Schaffner F. Increased risk for autochthonous vector-borne infections transmitted by aedes albopictus in continental europe. Eurosurveillance. 2018;23(24):2–7. doi: 10.2807/1560-7917.ES.2018.23.24.1800268
- Centro de Coordinación de Alertas y Emergencias Sanitarias. Ministerio de Sanidad Consumo y Bienestar. Evaluación rápida de riesgo. Dengue autóctono en España 2a actualización. 2019. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/analisisituacion/doc/ERR_Dengue_autoctono_mayo2019.pdf
- EL Pais. España: Los hospitales españoles detectan un fuerte incremento de casos de dengue en turistas procedentes de Cuba. 2022. Available from: https://elpais.com/sociedad/2022-09-01/los-hospitales-espanoles-detectan-un-fuerte-incremento-de-casos-de-dengue-en-turistas-procedentes-de-cuba.html
- Nagpal B, Knox T, Risintha P, et al. Strengthening of vector control in south-East Asia: outcomes from a WHO regional workshop. J Vector Borne Dis. 2018;55(4):247. doi: 10.4103/0972-9062.256559
- Bangert M, Latheef AT, Dev Pant S, et al. Economic analysis of dengue prevention and case management in the Maldives. PLOS Negl Trop Dis. 2018;12(9):e0006796. doi: 10.1371/JOURNAL.PNTD.0006796
