ABSTRACT
Background
In the wake of the COVID-19 outbreak, many speech-language pathologists have transitioned from in-person service delivery to online environments. As such, there is an urgent need to inform clinicians on the availability of efficacious and effective telehealth interventions for childhood apraxia of speech (CAS).
Objectives
This review was informed by the following clinical question: Is providing intervention remotely through telehealth as efficacious and effective as in-person therapy for treating CAS?
Methods, eligibility criteria, and sources of evidence
Eight databases and seven search engines were searched for articles to identify intervention studies that have investigated the efficacy and/or effectiveness of treating CAS remotely. Search criteria was restricted to papers with children under 18 years of age, published in the English language between 1993 and 2020.
Results
Two studies were found to meet our inclusion criteria. A phase I study employed a multiple baseline across participants design to investigate the efficacy of the Rapid Syllable Transition treatment via telehealth. The second study assessed the feasibility of adopting a novel system for the remote administration of the Nuffield Dyspraxia Program-Third Edition. Based on the Oxford hierarchy Centre for Evidence-Based Medicine, both studies are level IV (case-series/case-control), and therefore deemed low level evidence. Results showed limited but promising outcomes when CAS therapy is conducted remotely.
Conclusion
There is limited, low-level evidence indicating positive outcomes for the remote treatment of CAS via telehealth. The scarcity of data available warrants a need for large-scale randomized control trials and controlled clinical trials.
Introduction
The American Speech–Language–Hearing Association (ASHA) defines childhood apraxia of speech (CAS) as a subtype of speech sound disorder (SSD) in children that is characterized by inconsistent speech errors, difficulties in coarticulation, and inappropriate prosody (ASHA, Citation2007). Despite the presence of an ongoing theoretical debate regarding the exact proximal and distal causes of CAS, it is generally agreed that CAS is marked by an underlying disruption of the neurophysiological processes relating to the child’s speech motor planning and/or motor programming of speech movement sequences (ASHA, Citation2007; Caruso & Strand, Citation1999; Namasivayam, Coleman, O’Dwyer, & van Lieshout, Citation2020; Terband, Maassen, & Maas, Citation2019).
Children with CAS are clinically difficult to manage as they often require specialized assessments and checklists for diagnosis (Namasivayam et al., Citation2015; Strand & McCauley, Citation2019) followed by intensive training and clinician expertise to achieve desirable therapy outcomes (Namasivayam et al., Citation2015; Strand, Citation2020). Added to such clinical challenges is the limited body of literature available on effective interventions and optimal intervention dose parameters for this population (Morgan, Murray, & Liégeois, Citation2018). CAS continues to remain a subtype of SSD that is difficult to treat, despite several efforts made by researchers to address the challenges regarding proper clinical assessment and clinician training and/or expertise (Maas, Gildersleeve-Neumann, Jakielski, & Stoeckel, Citation2014; Strand, Stoeckel, & Baas, Citation2006; Thomas, McCabe, & Ballard, Citation2014).
The current landscape for CAS consists broadly of four intervention approaches:
Sensory-motor, linguistic, combined, and multi-modal communication (Koehlinger, Citation2015; Morgan et al., Citation2018). The sensory-motor approaches utilize the principles of motor learning and may employ auditory, visual, and tactile cues in order to facilitate the child’s improvement in the accuracy, generalization, and maintenance of speech movement sequences. Some examples of the sensory-motor intervention approaches for CAS include Dynamic Temporal and Tactile Cueing (DTTC; Strand, Citation2020; Strand et al., Citation2006), Motor Speech Treatment Protocol (MSTP; Namasivayam et al., Citation2015), Rapid Syllable Transition (ReST; Murray, McCabe, & Ballard, Citation2015), Kaufman Speech to Language Protocol (K-SLP; Kaufman, Citation2014), Biofeedback Treatment (Preston, Brick, & Landi, Citation2013), and Prompts for Restructuring Oral Muscular Phonetic Targets (PROMPT; Dale & Hayden, Citation2013). The linguistic intervention approaches rely on activities that improve the use of syntactical structures and/or vocabulary, or might focus on phonology and phonological awareness, such as the child’s identification of phonemes in words, and ability to segment or blend phonemes. Two examples of the linguistic intervention for CAS include the Integrated Phonological Awareness approach (McNeill, Gillon, & Dodd, Citation2009) and Core Vocabulary Therapy (Crosbie, Holm, & Dodd, Citation2005). The combined intervention approaches integrate specific aspects of the sensory-motor approach with some principles of the linguistic approach. For instance, the Nuffield Dyspraxia Programme-Third Edition (NDP3; Williams & Stephens, Citation2004) not only targets phonological awareness skills, but also employs principles of motor learning (e.g., blocked practice and frequent feedback) to first target the child’s vowels and consonants in isolation, and then proceed to the level of syllable and syllable sequences (Maas et al., Citation2014). Other researchers have combined rhythmic approaches such as Melodic Intonation Therapy with the touch-cue method (Martikainen & Korpilahti, Citation2011), or the Stimulability Training Protocol with Core Vocabulary intervention (Iuzzini & Forrest, Citation2010). Lastly, the multi-modal communication approaches are used to facilitate communication in children with CAS who are minimally verbal. They may typically involve the use of visual picture boards, augmentative and alternative communication (AAC) devices, computers, tablets with applications, or even signs and gestures (Bornman, Alant, & Meiring, Citation2001). As each intervention approach poses unique advantages and challenges, selecting the most suitable method for treating CAS will ultimately depend on the child’s speech, language, and cognitive profile, psycho-social needs and competencies, as well as the availability of relevant resources for service delivery. A detailed review of these approaches is beyond the scope of the current manuscript, and the interested reader is directed to several comprehensive papers for further information (see Maas et al., Citation2014 or Murray, McCabe, & Ballard, Citation2014).
There have been several evidence summaries (Maas et al., Citation2014; McCabe, Murray, & Thomas, Citation2018) and systematic reviews (Morgan et al., Citation2018; Morgan & Vogel, Citation2009; Murray et al., Citation2014) on the intervention efficacy for CAS. Murray, McCabe, and Ballard’s systematic review (Citation2014) indicated that two sensory-motor interventions, Integral Stimulation/DTTC and ReST, and one linguistic intervention, the Integrated Phonological Awareness approach, had sufficient evidence for interim clinical practice and initiation of phase III efficacy trials. The most recent systemic review paper on this topic was published by Morgan and colleagues (Citation2018) in the Cochrane Library, and included studies up to April 2017. Morgan and colleagues’ Cochrane review (Citation2018) found only one well-controlled randomized controlled trial (RCT) on children with CAS (by Murray et al. (Citation2015)). The children in the RCT study were between 4–12 years of age and were randomly allocated to 12 one-hour sessions (over three weeks) of either ReST or NDP3 intervention. Results indicated that both ReST and NDP3 treatment approaches showed improvement at one-month post-treatment, but ReST demonstrated slightly greater generalization and maintenance of treatment effects at four-months post-treatment (Murray et al., Citation2015). Morgan et al. (Citation2018) concluded that other than the one RCT study ‘there is currently no available evidence for other treatments’ (p. 3). It is important to mention a caveat here in that the Cochrane reviews are limited to RCT study designs, and as such do not report on small scale single subject research design (SSRD) studies or papers with other methodological designs that might have provided evidence for CAS treatment approaches.
In recent months, the challenges in the clinical management of CAS have been exacerbated by the unexpected transition to telehealth due to the global COVID-19 pandemic (Chauhan et al., Citation2020) (). Speech Pathology Australia (SPA) defines telehealth as ‘the application of telecommunications technology to deliver clinical services at a distance by linking clinician to client, caregiver, or any person(s) responsible for delivering care to the client, for the purposes of assessment, intervention, consultation and/or supervision’ (SPA, Citation2014, p. 4; Wales, Skinner, & Hayman, Citation2017). Prior to the global pandemic, telehealth was used in the field of speech-language pathology primarily to address and improve the inequity of access to healthcare services by remote/rural populations, and to reduce expenditures (Jong, Mendez, & Jong, Citation2019; SPA, Citation2014; Wales et al., Citation2017). The sudden need to transition to the alternative telehealth service delivery model as the ‘new norm’ has left many clinicians scrambling to identify and familiarize themselves with regulatory and privacy compliant technologies, while also searching the literature for telehealth compatible and validated assessment and intervention tools to meet the evidence-based practice (EBP) requirements by the regulatory agencies (ASHA, Citation2005).
Thanks to the popularity of telerehabilitation services provided by an increasing number of clinicians, the efficacy and effectiveness of pediatric speech and language assessments and interventions delivered via telehealth have been the subject of a small number of recent review papers (Taylor, Armfield, Dodrill, & Smith, Citation2014; Wales et al., Citation2017). There is some evidence suggesting that conducting oromotor, articulation, and speech intelligibility assessments in children via telehealth are valid (Taylor et al., Citation2014). However, a slight decrease in the assessment reliability and the quality of judgements for some speech sounds and oromotor tasks has been reported when assessments are conducted online via telehealth in comparison to in-person. For example, Taylor and colleagues (Citation2014) have reported difficulties in detecting voiced versus voiceless sounds, or high frequency consonants (such as [s]), and in determining tongue lateralization and protrusion.
In terms of the efficacy of intervention delivered through telehealth services, another systematic review has indicated that speech sound therapy delivered via telehealth may be as effective as in-person intervention (Wales et al., Citation2017). This review found that the outcomes measured via standardized testing (e.g., the standard scores derived from the Goldman-Fristoe Test of Articulation-2 (GFTA-2; Goldman & Fristoe, Citation2000)) were more consistently positive in telehealth settings, whereas outcomes measured via Functional Communication Measures (FCMs; Mullen & Schooling, Citation2010) demonstrated contradictory results across studies (Gabel, Grogan-Johnson, Alvares, Bechstein, & Taylor, Citation2013; Grogan-Johnson, Alvares, Rowan, & Creaghead, Citation2010). In another study conducted by Gabel and colleagues (Citation2013), the authors found that the number of participants whose speech sound productions and intelligibility improved for at least one level on the FCMs was greater in the telehealth in comparison to the in-person condition. However, in an earlier study by Grogan-Johnson and colleagues (Citation2010) the authors found no significant difference in GFTA-2 scores (Goldman & Fristoe, Citation2000) between students in the videoconferencing versus the conventional face-to-face speech-language therapy treatment groups.
To our knowledge, there are no review papers up to this date on the efficacy and/or effectiveness of CAS intervention methods when delivered via telehealth. A review paper on this topic during this critical time may facilitate the clinicians and speech-language pathologists’ (S-LPs) evidence-based practice (EBP) decision making process in treating CAS in their young clients without interruption or delay in therapy. Given the unanticipated shift in the type of service delivery from in-person to telehealth in the past several months, and the elapsed time (∼4 years) since the last review (Morgan et al., Citation2018; Wales et al., Citation2017) there is a clear need for clinicians and caregivers to be informed about the availability of efficacious and/or effective telehealth interventions for CAS.
Research question in a clinical context
The sudden changes to children’s consistent, in-person therapy sessions have left many families in uncertainty as they weigh the ramifications of complete or partial interruptions in their children's speech therapy plans. To phrase our research question in a clinical context, consider the following case scenario: Tania (not her real name) was diagnosed with CAS when she was three and half years old after demonstrating several diagnostic features of CAS, including vowel distortions, syllable segregations, inconsistent word productions, and an overall effortful manner of speaking. She began receiving intense weekly speech therapy at a Specialty Care Center offering speech-language pathology services that was roughly an hour away from where the family lived. Tania celebrated her fourth birthday in April. She had made considerable progress over the past few months; her vocabulary had expanded significantly, and she could talk about her kindergarten adventures with three- to four-word utterances. Ever since the World Health Organization (WHO) declared the novel coronavirus a global pandemic on 11 March 2020, Tania’s regular therapy sessions have been disrupted, just when she was gaining the skills necessary to support her transition into grade one. The school setting will require Tania to rely on her expressive language skills in order to do her homework, respond to her teachers’ academic instructions, and make peer connections. The recent interruptions in Tania's therapy plan in the past several months might pose several academic and social challenges for her in the near future. To minimize Tania’s difficulties, her S-LP is consulting some colleagues and searching for research evidence on efficacious and effective intervention options for a child with CAS via telehealth, and how to tailor the therapy plan to an online format. The S-LP’s research is informed by the following clinical question: Is providing intervention remotely through telehealth as efficacious and/or effective as in-person therapy for treating CAS in children?
Methods
The PRISMA framework
Our analyses were guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework (Page et al., Citation2021) to ensure adherence to quality review protocols. To include as many different terms associated with CAS and telehealth as possible, we extracted all the keywords related to these terms both from MEDLINE’s Scope and the Cochrane Library. As measurement outcomes are different depending on the type of therapy that clinicians opt to provide, running a systematic search on every intervention method was not feasible. Therefore, we left the therapy type unspecified in an attempt to later compare and analyze the results from a broader perspective (i.e., remote versus in-person therapy), regardless of the exact type of therapy undertaken by clinicians. Search terms related to CAS were combined with terms associated with telepractice. The specific keywords and subject headings were refined to meet each database’s search criteria and syntax. outlines a sample of the search strategy and keyword combinations used for searching the MEDLINE database.
Table 1. Outline of search strategy and keyword combinations for MEDLINE.
Inclusion and exclusion criteria
To assess study eligibility (), we used inclusion and exclusion criteria that consisted of literature reporting on the efficacy and/or effectiveness of treatment for CAS in children through telehealth.
Table 2. Study eligibility criteria.
We followed Murray et al.’s (Citation2014) definition of ‘treatment efficacy’ (adapted from McReynolds and Kearns (Citation1983)), which in this context refers to the ‘clinical cause-and-effect relationships’ (Murray et al., Citation2014, p. 487) upon the administration of the treatment (e.g., ReST therapy through telehealth) and an observed change in the client’s behaviour, such as an improvement in the child’s speech motor skills. An efficacious treatment not only consists of the child’s acquisition of the speech motor skills but extends to the adequate maintenance and generalization of the treatment effects. While ‘treatment effectiveness’ echoes the same concept of inducing a desired change in behaviour, an effectiveness research does not necessarily reflect a causal relationship, as there is often more variability and less control over the confounding factors (Rogers, Citation2009).
We limited our search to papers published between January 1993 and 20 May 2020 as our preliminary search indicated that the concept of telehealth began circulating among medical communities in 1993 after the foundation of the American Telemedicine Association (ATA; Denton, Citation1993). Additionally, we augmented our database search process by employing a grey literature search to identify documents that might have fallen outside of the indexed academic databases.
Database and grey literature search
Eight databases (MEDLINE, Scopus, CINAHL plus, PsycINFO, SpeechBITE, EMBASE, ERIC, and AMED) were searched throughout May 2020 for published journal articles to identify intervention studies that have investigated the efficacy and/or effectiveness of treating CAS remotely through telehealth. Two independent reviewers screened all titles and abstracts to exclude articles that did not meet our eligibility criteria. To identify as many relevant documents as possible, we contacted researchers and clinical colleagues for suggestions on tracing documents through standard (tracking based) and alternative (non-tracking based) search engines (Lee & Gibbon, Citation2015). Grey literature search was conducted later on in March 2021 by applying the inclusion and exclusion criteria to look for evidence in four search engines (Google, Google Scholar, Bing, and Yahoo) with active tracking systems.
Standard search engines (such as Google) track user behaviour. This makes the user susceptible to a state of ‘intellectual isolation’ due to the ‘Filter Bubble’ effect, a type of bias wherein the search engine’s tracking algorithm learns to trace the user’s clicking history and location to determine user preferences (Pariser, Citation2011). To minimize the potential confirmatory bias introduced through the Filter Bubble effect, we supplemented our search by further looking for evidence in three alternative search engines (DuckDuckGo, Mojeek, Gigablast) with no tracking feature. Where possible, we used specialized programmable search options by using Boolean algebra search operators to combine the keywords displayed in and extract multiple document formats (e.g.,.PDF,.TXT,.PPT,.PPTX, and.DOC). Inter-rater reliability for database and grey literature search between two independent blinded raters was > 80%.
Evidence quality evaluation and data reliability
The PEDro-P rating scale, a version of the Physiotherapy Evidence Database (PEDro; Blobaum, Citation2006; Maher, Sherrington, Herbert, Moseley, & Elkins, Citation2003; Sherrington, Herbert, Maher, & Moseley, Citation2000) modified by PsycBITE, was used to evaluate the methodological quality and body of evidence in group comparison studies, including RCTs. We used the Comparative Single-Case Experimental Design Rating System (CSCEDARS; Schlosser, Belfiore, Sigafoos, Briesch, & Wendt, Citation2018) for critical appraisal of SSRDs. Inter-rater reliability between two blinded raters for evidence quality was 82% and 91% for the PEDro-P and CSCEDARS scales, respectively.
Results
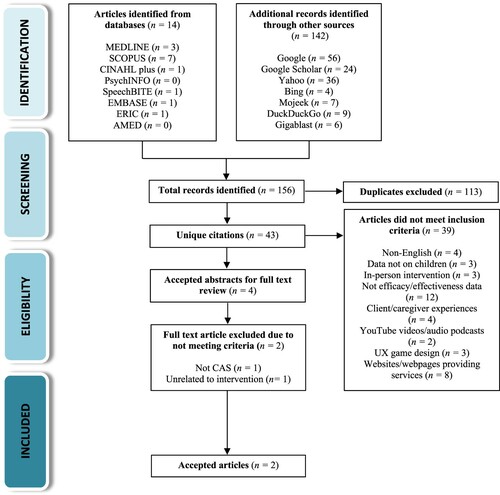
Our search results revealed that studies focusing on telepractice for treating CAS were first published in 2012 and, to date, limited research is available on the topic. The completed search yielded a total of 156 records (12 academic articles and two conference proceedings from the database search, and 142 records identified through other sources). Of the 156 identified documents, 113 were duplicates, cutting down the results to 43 in total. Thirty-nine additional papers were excluded after reviewing their abstracts as they did not meet our inclusion criteria (e.g., papers not in English, reported on adult population, etc.) or for other reasons (e.g., YouTube videos, audio podcasts, UX game designs, or websites providing therapy services). Of the remaining four papers that were accepted for full text review, an additional two were excluded; one paper by Shahin and colleagues (Citation2015) explored the effectiveness of employing a novel speech processing engine (‘TabbyTalks’), as part of a comprehensive speech assessment tool for CAS, and was unrelated to intervention per se. The other paper by Bortone and colleagues (Citation2018) investigated the efficacy of immersive wearable haptics devices for rehabilitation training of children with cerebral palsy and developmental dyspraxia, which was unrelated to speech-language pathology and CAS. The flow diagram of our study selection and review procedure is presented in . provides a summary of study screening and decisions regarding the eligibility of identified papers for inclusion in the final review.
Table 3. Study screening and summary of decisions.
Only two studies met all our inclusion and exclusion criteria. outlines these papers’ participant characteristics, study design, methodology, procedures, and intervention outcomes.
Table 4. Summary of the two accepted papers’ participant characteristics, study design and methodology, procedures, and intervention outcomes.
A phase I study by Thomas, McCabe, Ballard, and Lincoln (Citation2016) employed a multiple baseline across participants design to investigate the efficacy of ReST treatment (Murray et al., Citation2015) via telehealth on five participants aged 5:5–11:2 years with consensus diagnosis of CAS. The children in this study were allocated to either three, four, five or six twice-weekly baseline sessions. Therapy was delivered by the real time transmission of audiovisual information through video conferencing, with the clinician and the child present at the same time on both ends of their screens. During each session, each child viewed a PowerPoint slide show with the orthography for each pseudo-word item and a picture plus orthography for the real-word item, presented with its respective sound file. A caregiver was instructed to play the sound file as the child viewed and imitated the pseudo-words. While the clinician could see and hear the child via the web camera and microphone throughout the session, the child-caregiver dyad could only hear the clinician. ReST treatment was delivered four times a week for three weeks. Each child’s performance was monitored three times during the treatment phase immediately before the fifth treatment session, immediately before the ninth treatment session, and one day post-treatment. Furthermore, the authors administered a 90-item probe list to each child to evaluate the treatment effect, generalization to related untreated items, generalization to real words with same number of syllables as treated items, and to control for maturational effects.
All five children in this study achieved significant percent accuracy scores (p< 0.05) on to-be-treated single pseudo-word items at baseline to treatment phase. Two out of four children achieved significant results (at 0.01 and 0.05) on treated pseudo-words in carrier phases (statistical analysis was not applicable to the fifth participant). Four children achieved significant results (at 0.01 and 0.05) on untreated single pseudo-word items at baseline to treatment phase. Four children achieved significant generalization (p< 0.01) to untreated real words (data was not available for the third participant). One of the children who performed significantly better in later phases compared to performance at the baseline received simultaneous therapy to improve her receptive and expressive language skills. Therefore, results should be interpreted with caution to account for the potential effect of additional therapy. Planned contrast supported maintenance of effects for four children on treatment and generalization gains four-months post-treatment. Four children demonstrated higher per cent accuracy at all follow-up points than baseline levels for (a) treated pseudowords, (b) similar but untreated words, and (c) untreated real words. One participant, who maintained some treatment and all generalization gains, lost some gain on treated pseudo-words (but not with treated pseudo-words in phrases) and had significantly lower accuracy in the follow-up phase for these items. This participant’s performance was above baseline levels at all follow-up points for untreated pseudo-words and untreated real words. The difference between the treatment and follow-up phase accuracy of these items were not statistically significant, indicating maintenance of generalization effects. Lastly, the results argued against maturational change, supporting the study’s internal validity.
Similar to the findings reported in other studies on the efficacy of in-person CAS intervention delivery (Murray et al., Citation2015; Thomas et al., Citation2014), ReST delivered through telehealth did have similar outcomes and large effect sizes regarding the acquisition and generalization of targets (both treated and untreated) in comparison to in-person therapy. These findings imply good integrity and effective intervention delivery via telehealth for ReST. However, it is important to mention that the eligibility assessment and video conferencing baseline probes were carried out in-person prior to the initiation of the intervention protocol, which could limit the generalizability of the findings to a fully remote delivery system.
This paper received a quality rating of 21/23 on the CSCEDARS scale by two independent rates who rated the study’s quality with an inter-rater reliability of > 80%. Some strengths of the study included clear description of the selection criteria and participant characteristics. The authors provided sufficient information on the critical features of the physical setting and arrangements to enable replication of the study design. The dependent variable, operationalized as the percentage of words produced with correct sounds, lexical stress and smooth connection between the syllables (accuracy of the phonemes/stress pattern and the fluency of syllable transition), was kept similar across all conditions. This variable was measured through alternating the number of baselines across the subjects to achieve randomization. Intra- and inter-rater reliability was comparable across the conditions and calculated on 20% of each baseline, probe assessment and treatment sessions. Baselines were provided for each of the compared conditions and were sufficiently consistent before introducing the intervention to enable prediction of performance.
The ReST treatment protocol was used as the study’s independent variable, following the procedures described in Murray, McCabe, and Ballard (Citation2012) with minor modifications and sufficient description to enable replicability of the design. Baseline data were compared with data gathered during the intervention phase using the same procedures as baseline for the dependent variable. The sets were randomly assigned to each condition, and a rater blinded to the treatment phase and baseline level of speech conducted the probe assessments in order to minimize bias and ensure as much equivalency across the conditions as possible. The authors demonstrated experimental control via at least three demonstrations of the experimental effect at different points in time. Lastly, the obtained data demonstrated a clear distinction between compared interventions that were consistent with the logic of the multiple baselines across participants design. One shortcoming of this study was the uncertainty around whether any of the participants had received the ReST therapy previously. This was an important point to include especially since the authors mentioned that all the participants had previously received speech therapy. Any prior familiarity with the ReST treatment protocol could have potentially added bias in the compared treatment conditions.
The second paper that met our eligibility criteria was a pilot study conducted by Parnandi, Karappa, Lan, and Shahin (Citation2015), which assessed the feasibility of adopting a novel system for the remote administration of NDP3 (Williams & Stephens, Citation2004) to six children with CAS between 4–10 years of age. The authors discussed ‘automated therapy’ as a subcategory of telehealth which enables the clinician to remotely administer the NDP3 intervention practice material to children through a tablet-based app for a determined period without requiring to commit to online meetings for real-time therapy. The novel multitier system consists of a mobile app which allows the child to practice speech exercises at home on their tablet, a therapy management interface that allows the clinician to assign therapy and monitor each child’s progress, and a built-in speech processing engine which identifies the child’s errors through its built in speech analysis algorithm. The app’s interface operates as an automated mediator which provides real-time feedback to the child while reporting the details of the child’s progress to the clinician.
The children in this study were age matched in pairs and pairs were allocated randomly to one of two groups (SLP4 vs. SLP1). In the SLP4 condition, therapy was directed by a clinician four days a week for three weeks. All the 12 sessions in this condition involved the clinician providing knowledge of results (KR) feedback on each trial and giving knowledge of performance (KP) feedback to shape correct production on error trials. In the SLP1 condition, therapy was directed by a clinician once a week for three weeks and remotely by the child three or four days a week (either alone or with their parent). In this condition, the clinician followed the protocol for SLP4 for the first session during each week but provided KR feedback only on every trial for the remaining three sessions each week.
Consistent with the NDP3 program (Murray et al., Citation2012), each participant was assigned three treatment goals for practice with the tablet, based on individual speech error patterns previously identified by the clinician. Five words were selected from NDP3 stimuli to work on each goal. Each child had therapy goals addressing consonant or vowel accuracy in words, and stress production in multisyllabic words or phrases. Children in both groups underwent an in-person NDP3 session with the clinician running the CAS mobile app on a tablet. The tablet-based intervention sessions were similar to traditional sessions with the exception that paper-based therapy material was replaced by the tablet, and exercises were assigned using the clinician’s interface on the Moodle server based on the NDP3 protocol. All activities were presented to children via the tablet in both conditions, with the primary difference between the groups being the additional opportunity for the children in the SLP4 group to receive KP feedback from the clinician.
Results showed that the participants in the SLP4 condition demonstrated a substantial improvement from pre-treatment to one-week post-treatment (median 70% change from baseline) while the participants in the SLP1 condition, who received less support from the clinician, showed a smaller percent change (median 40%). The authors found that children responded better to the sound production activities compared to the stress production activities in the short-term, but stress gains continued improving to one-month post-treatment while sound accuracy showed deterioration. At four-weeks post-treatment, children’s initial gains declined to a similar degree for both groups but remained above the baseline, indicating maintenance of generalization effects.
While these initial findings seemed to support the use of tablet-based intervention for CAS with just once-a-week contact with the clinician to guide learning, Parnandi and colleagues recommended recruiting a larger sample for further analysis. The authors noted that their reported data was part of a larger and ongoing randomized clinical trial study that would enable drawing more certain conclusions on the efficacy of their proposed intervention delivery protocol. The authors were also keen to emphasize that the software was meant to ‘supplement’ (p. 10:20) traditional face-to-face (or real-time online) therapy, since intensive practice is usually required as part of the intervention plan for treating CAS. As such, the addition of this novel multitier system to the child’s therapy plan could potentially reduce the financial demands of multiple therapy sessions while also preserving S-LP resources.
This paper received a rating of 5/11 on the PEDro-P scale of methodological quality by two independent rates who rated the study’s quality with an inter-rater reliability of > 80%. In this study, the authors clearly specified their eligibility criteria. The participants were reported to be similar at baseline as they were matched in pairs by age, and the pairs were randomly allocated to the conditions. However, it was unclear whether the allocation process was concealed, and the treating clinicians were aware of the speech sound skills of each participant. Furthermore, there was no mention of blinding of the participants and the assessors who measured the study’s key outcomes. It is important to highlight here that the blinding of participants to their group conditions is by default a difficult task in the field of speech language pathology, as it is often not possible to hide (from the participants) the fact that they are receiving therapy. All of these factors were deemed as sources of bias which limited the validity of results obtained in this study.
In the results section, the measures of at least one key outcome (i.e., speech sound and stress production accuracy) were obtained from more than 85% of the subjects allocated to the groups, and all subjects with available outcome measures received the treatment or control condition as assigned. In other words, the authors analyzed the data with the ‘intention to treat’. Nonetheless, the results of between-intervention group statistical comparisons were solely reported in medians, and the study did not provide measures of variability for the participants’ speech sound and stress production accuracy.
In both studies, end user desirability and satisfaction of telehealth were explored via interviews and questionnaires. Thomas and colleagues (Citation2016) conducted partially structured telephone interviews with the clinicians and parents four-weeks post-treatment, regarding children’s motivation and all participants’ (i.e., parents, children, and clinicians) overall satisfaction with the telehealth mode of treatment. Both parents and clinicians reported high levels of satisfaction and viewed the video conferencing mode of service delivery convenient. Parents, in particular, commented being ‘very satisfied’ (Thomas et al., Citation2016, p. 668) with telehealth and added that their children were motivated to participate in these sessions.
Parnandi et al. (Citation2015) administered two questionnaires, one to the children, and the other to the caregivers and clinicians. All participants reported enjoying working with the tablet. Adults noted that children were able to maintain focus on the activities and the caregivers highlighted the app’s ‘ease of use’ (p. 10:17). Furthermore, children reported that they liked recording and listening to their speech, enjoyed the app’s built-in reward system and found the sessions to be motivating.
Based on the Oxford hierarchy Centre for Evidence-Based Medicine (OCEBM, Citation2020) both studies were level IV (case-series/case–control), and therefore deemed low level evidence.
Discussion
Our scoping review sought to find the current best evidence regarding the efficacy and/or effectiveness of treating CAS in children through telepractice. We found two studies that met our eligibility criteria. A phase I multiple baseline across participants design study by Thomas and colleagues (Citation2016) found that the ReST intervention approach had similar outcomes and large effect sizes in the acquisition and generalization of targets (both treated and untreated) when delivered through telehealth, in comparison to in-person therapy. The second study by Parnandi and colleagues (Citation2015) provided preliminary evidence that supported the use of a novel tablet-based app to supplement CAS treatment by remotely administering the NDP3 intervention material to the child’s app with just once-a-week contact with the clinician.
Implications for practice
The ongoing social distancing restrictions which may well continue into the upcoming months (and possibly years) require clinicians to find creative ways to tailor their therapy plans while being mindful of the existing evidence on what does and does not work if the therapy is to be administered remotely. As discussed at the beginning of this paper, positive outcomes have been reported in relation to the efficacy of both ReST and NDP3 treatments when they are administered in person (Murray et al., Citation2015). Regarding the remote administration of these intervention approaches, however, the small number of existing studies on this topic and the level of evidence provided in the two studies outlined above limit the clinical utility of the findings. In addition, the in-person components of these studies pose additional barriers to the generalizability of treatment results to a fully remote/online intervention protocol. The availability of high-quality evidence (Murray et al., Citation2015) and preliminary data (Parnandi et al., Citation2015; Thomas et al., Citation2016) taken together could provide some therapy planning choices for the S-LPs currently treating CAS. For example, incorporating ReST into the child’s remote therapy plan is one option to explore, provided the child meets the eligibility criteria for this treatment method (i.e., the suggested age range to qualify for ReST is between 4–12 years (Murray et al., Citation2015) and the child is expected to have at least four consistent vowels and consonants (McCabe, Thomas, Murray, Crocco, & Madill, Citation2017)). For more information, refer to the complete readiness checklist available on the ReST website at https://rest.sydney.edu.au/.
For the time being, it is not possible to comment on the efficacy of NDP3 treatment method if it is delivered in real-time via telehealth, but the reported positive outcomes on its remote administration through the tablet-based therapy tool (Parnandi et al., Citation2015) appears to be a promising starting point. While the level of evidence is deemed low, the tablet-based app could be an alternative option that practicing clinicians might opt to experiment with, given that complementing traditional therapy sessions with the app could potentially reduce the number of in-person sessions that are required.
On a broader note, clinicians might want to review the details of different CAS treatment methods to examine which intrinsic aspects of each approach are telehealth compatible or might pose barriers, while keeping in mind that there is currently no research evidence for the efficacy and effectiveness of any service delivery modifications that clinicians choose to implement. For example, there are preliminary data by Dale and Hayden (Citation2013) on the delivery of full PROMPT intervention with tactile-kinaesthetic-proprioceptive (TKP) cues versus treatment that included all PROMPT components with the exception of TKP cues in a group of four children with CAS (3:6–4:8 years of age). Even though all children made significant gains on both standardized tests and on the production of untreated words during intervention, the addition of TKP cues provided larger effect sizes (i.e., greater gains). These results suggest that clinicians could anticipate a potential reduction in the effectiveness of sensory-motor approaches based on the number of physical (especially TKP) cues necessary. Methods such as MSTP (Namasivayam et al., Citation2015), PROMPT (Dale & Hayden, Citation2013) or DTTC (Strand, Citation2020; Strand et al., Citation2006) rely heavily on multisensory tactile and gestural cues to support the child’s speech motor movements, and so may not be ‘telehealth friendly’ treatment options. Moreover, since some of these approaches administer temporal and audiovisual cueing (i.e., "listen to me, watch me, do what I do") any audio lag or out of sync video/audio could render the therapy session frustrating and potentially less effective (Bortone, Leonardis, Mastronicola, Crecchi, & Bonfiglio, Citation2018). Further studies are warranted to test the efficacy of sensory-motor approaches when delivered via telehealth, in the absence of TKP cueing. Future research should also investigate how to modify and adapt the remotely delivered multisensory intervention methods so that they become equally as effective as in-person therapy.
Sensory-motor approaches that do not depend heavily on TKP cues like ReST (Murray et al., Citation2015), or the more linguistically inclined methods such as the Integrated Phonological Awareness approach (McNeill et al., Citation2009), and the Stimulability Training Protocol with Core Vocabulary intervention (Iuzzini & Forrest, Citation2010) may be more suited to be delivered via telehealth. The Integrated Phonological Awareness approach, in particular, is based on the assumption that children with CAS are more likely to experience concurrent literacy difficulties such as decoding or spelling errors (McNeill et al., Citation2009). This intervention method cues the child by using visual prompts (i.e., colour blocks) or by drawing attention to the phonological structure of words in order to guide speech production. Similarly, the Stimulability Training Protocol with Core Vocabulary intervention targets the child’s phonological planning difficulties through a series of drill activities. Both intervention methods may be more easily adaptable to telehealth formats.
For clinicians seeking further training on any of the CAS intervention approaches mentioned throughout this paper, it should be pointed out that certain methods, such as the Biofeedback Treatment (Preston et al., Citation2013) and MSTP (Namasivayam et al., Citation2015), either require the child to be physically present in the clinic to use the equipment (i.e., the ultrasound probe) or the S-LP typically needs to attend in-person workshops to acquire the necessary skills. Interested readers are referred to Appendix A for a brief list of online CAS intervention resources that have been made available for clinicians. For S-LPs aiming to supplement their remote CAS therapy plans, the availability of such online resources for training could aid them in acquiring the clinical skills at a time when attending in person workshops is risky or not feasible.
Lastly, although some caregivers and clinicians may be apprehensive on transitioning to online environments for service delivery, research indicates high user satisfaction and an overall positive experience with telehealth (Parnandi et al., Citation2015; Thomas et al., Citation2016). Some aspects of telehealth that are reportedly appealing to children are the interactivity, built-in reward system, the possibility of accessing practice material from the comfort of home, and the fact that in tablet-based therapy, children could take charge of their therapy routine by recording and hearing their speech.
Limitations
Our results were limited in that we did not look for papers that were published in any language other than English. While every effort was made to limit bias during grey literature search, we are aware of the potential risk of confirmatory bias introduced as a result of the ‘Filter Bubble effect’.
Conclusion
The present review examined the existing studies to elucidate the efficacy and/or effectiveness of different intervention methods for CAS when delivered via telehealth. Given the ongoing climate of uncertainty among the speech-language pathology communities, we felt the need to provide clinicians with timely information in order to guide them in making informed, evidence-based decisions as they transition to online services. Our review indicated two level IV (case-series/case–control) studies that showed limited but promising outcomes when CAS therapy was conducted remotely, either through the use of a tablet-based app with the help of children’s caregivers (NDP3), or through real-time online therapy (ReST). The scarcity of data available on this topic warrants an immediate need for large-scale randomized control trials and controlled clinical trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- American Speech-Language-Hearing Association. (2005). Evidence-based practice in communication disorders. Position statement. Retrieved May 2, 2020 from https://www.asha.org/.
- American Speech-Language-Hearing Association. (2007). Childhood Apraxia of Speech [technical report]. Ad Hoc Committee on Apraxia of Speech in Children. Retrieved March 23, 2020, from http://www.asha.org/policy/TR2007-00278/.
- Blobaum, P. (2006). Physiotherapy Evidence Database (PEDro). Journal of the Medical Library Association, 94(4), 477–478.
- Bornman, E., Alant, E., & Meiring, J. (2001). The use of a digital voice output device to facilitate language development in a child with developmental apraxia of speech: A case study. Disability and Rehabilitation, 23(14), 623–634. doi:10.1080/09638280110036517
- Bortone, I., Leonardis, D., Mastronicola, N., Crecchi, A., & Bonfiglio, L. (2018). Wearable haptics and immersive virtual reality rehabilitation training in children with neuromotor impairments. IEEE Transactions on Neural Systems & Rehabilitation Engineering, 26(7), 1469–1478. doi:10.1109/TNSRE.2018.2846814
- Caruso, A. J., & Strand, E. A. (1999). Clinical management of Motor Speech disorders in children. New York, NY: Thieme.
- Chauhan, V., Galwankar, S., Arquilla, B., Garg, M., Somma, S. D., El-Menyar, A., … Stawicki, S. P. (2020). Novel coronavirus (COVID-19): leveraging telemedicine to optimize care while minimizing exposures and viral transmission. Journal of Emergencies, Trauma, and Shock, 13(1), 20–24. doi:10.4103/JETS.JETS_32_20
- Crosbie, S., Holm, A., & Dodd, B. (2005). Intervention for children with severe speech disorder: A comparison of two approaches. International Journal of Language & Communication Disorders, 40(4), 467–491. doi:10.1080/13682820500126049
- Dale, P. S., & Hayden, D. A. (2013). Treating speech subsystems in childhood apraxia of speech with tactual input: The PROMPT approach. American Journal of Speech-Language Pathology, 22(4), 644–661. doi:10.1044/1058-0360(2013/12-0055)
- Denton, I. (1993). Telemedicine: A new paradigm. Healthcare Informatics: The Business Magazine for Information and Communication Systems, 10(11), 44–50.
- Gabel, R., Grogan-Johnson, S., Alvares, R., Bechstein, L., & Taylor, J. (2013). A field study of telepractice for School intervention using the ASHA NOMS K-12 database. Communication Disorders Quarterly, 35(1), 44–53. doi:10.1177/1525740113503035
- Gillon, G. T., & McNeill, B. C. (2007). Integrated Phonological Awareness: An intervention program for preschool children with Speech-language impairment. University of Canterbury. 2007. Retrieved May 24, 2020, from www.canterbury.ac.nz/education/research/phonological-awareness-resources.
- Goldman, R., & Fristoe, M. (2000). Goldman–Fristoe Test of Articulation-2. Guidance Service.
- Grogan-Johnson, S., Alvares, R., Rowan, L., & Creaghead, N. (2010). A pilot study comparing the effectiveness of speech language therapy provided by telemedicine with conventional on-site therapy. Journal of Telemedicine and Telecare, 16(3), 134–139. doi:10.1258/jtt.2009.090608
- Helm-Estabrooks, N., Nicholas, M., & Morgan, A. (1989). Melodic intonation therapy. Austin, TX: Pro-Ed., Inc.
- Iuzzini, J., & Forrest, K. (2010). Evaluation of a combined treatment approach for childhood apraxia of speech. Clinical Linguistics & Phonetics, 24(4-5), 335–345. doi:10.3109/02699200903581083
- Jong, M., Mendez, I., & Jong, R. (2019). Enhancing access to care in northern rural communities via telehealth. International Journal of Circumpolar Health, 78(2), 1–3. https://doi.org/10.1080/22423982.2018.1554174
- Kaufman, N. (2014). The Kaufman Speech to Language Protocol [Powerpoint].
- Koehlinger, K. M. (2015). Improving speech intelligibility in children with childhood apraxia of speech: Employing evidence-based practice. EBP Briefs, 9(5), 1–10. Pearson.
- Lee, A. S., & Gibbon, F. E. (2015). Non-speech oral motor treatment for children with developmental speech sound disorders. The Cochrane Database of Systematic Reviews, 2015(3), 3. https://doi.org/10.1002/14651858.CD009383.pub2
- Maas, E., Gildersleeve-Neumann, C., Jakielski, K. J., & Stoeckel, R. (2014). Motor-based intervention protocols in treatment of childhood apraxia of speech (CAS). Current Developmental Disorders Reports, 1(3), 3. https://doi.org/10.1007/s40474-014-0016-4
- Maher, C. G., Sherrington, C., Herbert, R. D., Moseley, A. M., & Elkins, M. (2003). Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy, 83(8), 713–721.
- Martikainen, A.-L., & Korpilahti, P. (2011). Intervention for childhood apraxia of speech: A single-case study. Child Language Teaching and Therapy, 27(1), 9–20. doi:10.1177/0265659010369985
- McCabe, P., Murray, E., & Thomas, D. (2018). 2018 Evidence Brief: Childhood Apraxia of Speech. Apraxia-kids.org. Retrieved May 2, 2020, from https://www.apraxia-kids.org/wp content/uploads/2019/01/McCabe-Evidence-Brief.pdf.
- McCabe, P., Thomas, D., Murray, E., Crocco, L., & Madill, C. (2017). Rapid Syllable Transition Treatment – ReST. The University of Sydney. Retrieved May 24, 2020, from https://rest.sydney.edu.au/.
- McNeill, B. C., Gillon, G. T., & Dodd, B. (2009). Effectiveness of an integrated phonological awareness approach for children with childhood apraxia of speech (CAS). Child Language Teaching and Therapy, 25(3), 341–366. doi:10.1177/0265659009339823
- McReynolds, L. V., & Kearns, K. P. (1983). Single-subject experimental designs in communicative disorders. Baltimore: University Park Press.
- Morgan, A. T., Murray, E., & Liégeois, F. J. (2018). Interventions for childhood apraxia of speech. Cochrane Database of Systematic Reviews, 5, 1–64. https://doi.org/10.1002/14651858.CD006278.pub3
- Morgan, A. T., & Vogel, A. P. (2009). A Cochrane review of treatment for childhood apraxia of speech. European Journal of Physical and Rehabilitation Medicine, 45(1), 103–110.
- Mullen, R., & Schooling, T. (2010). The national outcomes measurement system for pediatric speech-Language pathology. Language, Speech, and Hearing Services in Schools, 41(1), 44–60. doi:10.1044/0161-1461%282009/08-0051%29
- Murray, E., McCabe, P., & Ballard, K. J. (2012). A comparison of two treatments for childhood apraxia of speech: Methods and treatment protocol for a parallel group randomised control trial. BMC Pediatrics, 12, 112. doi:10.1186/14712431-12-112
- Murray, E., McCabe, P., & Ballard, K. J. (2014). A systematic review of treatment outcomes for children with childhood apraxia of speech. American Journal of Speech-Language Pathology, 23(3), 486–504. doi:10.1044/2014_AJSLP-13-0035
- Murray, E., McCabe, P., & Ballard, K. J. (2015). A randomized controlled trial for children with childhood apraxia of speech comparing Rapid Syllable Transition treatment and the Nuffield Dyspraxia programme–Third edition. Journal of Speech, Language, and Hearing Research, 58(3), 669–686. DOI: 10.1044/2015_JSLHR-S-13-0179
- Namasivayam, A. K., Coleman, D., O’Dwyer, A., & van Lieshout, P. (2020). Speech sound disorders in children: An articulatory phonology perspective. Frontiers in Psychology, 10(2998), 1–20. doi:10.3389/fpsyg.2019.02998
- Namasivayam, A. K., Pukonen, M., Goshulak, D., Hard, J., Rudzicz, F., Rietveld, T., … van Lieshout, P. (2015). Treatment intensity and childhood apraxia of speech. International Journal of Language & Communication Disorders, 50(4), 529–546. doi:10.1111/1460-6984.12154
- OCEBM Levels of Evidence Working Group. (2020). The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. Retrieved April 17, 2020, from https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372(71), 1–9. https://doi.org/10.1136/bmj.n71
- Pariser, E. (2011). The filter bubble: What the internet is hiding from you. New York: Penguin Press.
- Parnandi, A., Karappa, V., Lan, T., & Shahin, M. A. (2015). Development of a remote therapy tool for childhood apraxia of speech. ACM Transactions on Accessible Computing (TACCESS), 7(3), 1–23. DOI: 10.1145/2776895
- Preston, J. L., Brick, N., & Landi, N. (2013). Ultrasound biofeedback treatment for persisting childhood apraxia of speech. American Journal of Speech-Language Pathology, 22(4), 627–643. doi:10.1044/1058-0360(2013/12-0139)
- Rogers, M. A. (2009). Phases of intervention research. Access Academics and Research Newsletter. Retrieved May 20, 2020 from https://academy.pubs.asha.org/.
- Schlosser, R. W., Belfiore, P. J., Sigafoos, J., Briesch, A. M., & Wendt, O. (2018). Appraisal of comparative single-case experimental designs for instructional interventions with non-reversible target behaviors: Introducing the CSCEDARS (“cedars”). Research in Developmental Disabilities, 79, 33–52. doi:10.1016/j.ridd.2018.04.028
- Shahin, M., Ahmed, B., Parnandi, A., Karappa, V., McKechnie, J., Ballard, K. J., & Gutierrez-Osuna, R. (2015). Tabby talks: An automated tool for the assessment of childhood apraxia of speech. Speech Communication, 70, 49–64. doi:10.1016/j.specom.2015.04.002
- Sherrington, C., Herbert, R. D., Maher, C. G., & Moseley, A. M. (2000). PEDro. A database of randomized trials and systematic reviews in physiotherapy. Manual Therapy, 5(4), 223–226. doi:10.1054/math.2000.0372
- Speech Pathology Australia. (2014). Telepractice in Speech Pathology. Statement. 2014. Retrieved May 5, 2020 from https://www.telemedecine-360.com.
- Strand, E. A. (2020). Dynamic Temporal and Tactile Cueing: a treatment strategy for childhood apraxia of speech. American Journal of Speech-Language Pathology, 29(1), 30–48. doi:10.1044/2019_AJSLP-19-0005
- Strand, E. A., & McCauley, R. J. (2019). Dynamic Evaluation of Motor Speech skill (DEMSS) manual. Baltimore: Paul H. Brookes Publishing Company.
- Strand, E. A., Stoeckel, R., & Baas, B. (2006). Treatment of severe childhood apraxia of speech: A treatment efficacy study. Journal of Medical Speech-Language Pathology, 14(4), 297–307.
- Taylor, O. D., Armfield, N. R., Dodrill, P., & Smith, A. C. (2014). A review of the efficacy and effectiveness of using telehealth for paediatric speech and language assessment. Journal of Telemedicine and Telecare, 20(7), 405–412. doi:10.1177/1357633X14552388
- Terband, H., Maassen, B., & Maas, E. (2019). A psycholinguistic framework for diagnosis and treatment planning of developmental speech disorders. Folia Phoniatrica et Logopaedica, 71(5-6), 216–227. doi:10.1159/000499426
- Thomas, D. C., McCabe, P., & Ballard, K. J. (2014). Rapid Syllable Transitions (ReST) treatment for childhood apraxia of speech: The effect of lower dose-frequency. Journal of Communication Disorders, 51, 29–42. doi:10.1016/j.jcomdis.2014.06.004
- Thomas, D. C., McCabe, P., Ballard, K. J., & Lincoln, M. (2016). Telehealth delivery of Rapid Syllable Transitions (ReST) treatment for childhood apraxia of speech. International Journal of Language & Communication Disorders, 51, 654–671. doi:10.1111/1460-6984.12238
- Wales, D., Skinner, L., & Hayman, M. (2017). The efficacy of telehealth-delivered speech and Language intervention for primary school-Age children: A systematic review. International Journal of Telerehabilitation, 9(1), 55–70. doi:10.5195/ijt.2017.6219
- Williams, P., & Stephens, H., (Eds) (2004). The Therapy Manual of The Nuffield Centre Dyspraxia Programme (3rd Edition). London: The Nuffield Centre Dyspraxia Programme Ltd..