ABSTRACT
Growing epidemiological evidence suggests that adults with hearing loss engage in less physical activity than those without, which may increase the risk of chronic health conditions. This review aimed to systematically evaluate existing evidence assessing the effectiveness of physical activity interventions in adults with hearing loss. The review also aimed to identify the behaviour change techniques used in the included studies. A systematic search of the literature was completed and reported in line with the Preferred Reporting Items for Systematic reviews and Meta-Analysis. Two studies met the eligibility criteria, both found overall improvements for physical function, namely, aerobic fitness and muscle strength in adults with hearing loss. Limited evidence was available for the impact on psychosocial wellbeing. The evidence was judged to be of low quality and subject to bias. Further theoretically driven, high-quality intervention studies that incorporate additional behaviour techniques (e.g., providing additional environmental cues) are necessary.
Introduction
Hearing loss is a highly prevalent burden of disease; currently, 1.5 billion people are estimated to experience some degree of hearing loss worldwide (World Health Organisation [WHO], Citation2021). Communication difficulties that arise because of hearing loss can lead to social isolation, loneliness, depression, and reduced quality of life (Besser, Stropahl, Urry, & Launer, Citation2018; Shukla et al., Citation2020). Furthermore, middle- and older-aged adults with hearing loss are also at a greater risk of being diagnosed with noncommunicable (or chronic) diseases (NCDs), including diabetes, cardiovascular disease (Besser et al., Citation2018), and dementia (Livingston et al., Citation2020). NCDs are the leading cause of morbidity and mortality globally (Roth et al., Citation2020). Therefore, prevention and early identification are crucial to mitigate the negative impact of hearing loss on individuals and society.
Mounting epidemiological evidence has shown adults with hearing loss are less physically active (Curhan, Eavey, Wang, Stampfer, & Curhan, Citation2013; Engdahl, Aarhus, Lie, & Tambs, Citation2015; Gispen, Chen, Genther, & Lin, Citation2014; Kawakami et al., Citation2022; Kuo, Di, Ferrucci, & Lin, Citation2021; Wells et al., Citation2020) and exhibit greater declines in physical functioning (Chen et al., Citation2015; Huang et al., Citation2019; Martinez-Amezcua et al., Citation2021; Pupo et al., Citation2022; Yévenes-Briones et al., Citation2021). For instance, a cross-sectional study of 291 adults aged 60–69 years, found that those with hearing loss engaged in ∼30 min less physical activity per day than those with normal hearing (Kuo et al., Citation2021). Furthermore, evidence from prospective observational studies has found that physical function (e.g., gait speed, balance, sit-to-stand) declines at a faster rate in adults with hearing loss compared to those without (Chen et al., Citation2015; Martinez-Amezcua et al., Citation2021).
A possible mechanistic pathway for this association is the ‘information degradation’ model. This proposes that, because individuals with any type or severity of hearing loss are required to process degraded auditory signals, there is an increased demand on cognitive and attentional resources (Peelle, Citation2018). As a result, fewer resources may be available for other processes, such as balance and postural control. Indeed, there is evidence to suggest that the association between hearing loss and physical function is mediated by cognitive function (Huang et al., Citation2019; Pupo et al., Citation2022); the associations between hearing loss and physical function are, for instance, strongest amongst those with lower cognitive function scores (Pupo et al., Citation2022). Moreover, chronic cognitive load can increase cognitive (or mental) fatigue, which may reduce engagement in social and physical activities (Hornsby, Naylor, & Bess, Citation2016). Withdrawal from such activities could exacerbate poor psychosocial wellbeing among adults with hearing loss and may put them at greater risk of impaired physical and/or cognitive function (Dawes et al., Citation2015). Evidence from qualitative research suggests that the communication difficulties experienced during physical activity lead to fatigue in older adults with hearing loss (Kurková, Citation2016). Alternatively, the declines in physical and cognitive function with reduced hearing capacity may be reflective of a more general physiological decline and may not necessarily be causative.
The primary clinical management strategy for hearing loss is hearing aids, which have been shown to effectively improve listening abilities and quality of life (Ferguson et al., Citation2017). However, studies evaluating the provision of hearing aids on physical function/activity have demonstrated mixed findings (Chen et al., Citation2015; Gispen et al., Citation2014; Martinez-Amezcua et al., Citation2021; Wells et al., Citation2020). For instance, Martinez-Amezcua et al. (Citation2021) found hearing aid users had faster gait speed, whereas Chen et al. (Citation2015) found no differences in decline in physical function between those who did and did not use hearing aids. Potential explanations for discrepancies between studies include differences in outcome measurements, such as self-report or behavioural measures of hearing, as well as cross-sectional study designs that are subject to bias from interindividual variability or cohort effects (Gispen et al., Citation2014). As hearing aids may not improve physical activity or function, additional support for those with hearing loss may be needed. Furthermore, adults with hearing loss can be excluded from physical activity interventions, either actively (e.g., hearing loss is exclusionary criteria), or by association (e.g., exclusionary criteria based on verbal cognitive assessment, trial involves group activity). As such, high-quality studies (i.e., randomised controlled trials, RCTs) are needed to establish whether interventions for adults with hearing loss can improve physical activity participation and physical functioning.
Insufficient physical activity is a leading modifiable risk factor for NCDs and all-cause mortality (WHO, Citation2020). The WHO recommends that individuals aged 18–64 years, as well as ≥65 years should engage in at least 150 min of moderate-intensity aerobic physical activity (e.g., brisk walk, cycling); or at least 75 min of vigorous-intensity aerobic physical activity (e.g., running or sports like football and netball) each week for substantial health benefits (WHO, Citation2020). Critically, a consistent body of evidence demonstrates that NCD risk can be reduced by interventions that aim to improve physical fitness (for reviews, see Ashton et al., Citation2020; Gheysen et al., Citation2018). Physical activity interventions may be effective for improving the cardiovascular health of adults with hearing loss, as well as reducing the development of other comorbidities in mid- or later life. Improved and sustained physical activity may also attenuate the decline of hearing ability (Wattamwar et al., Citation2018). However, hearing loss is associated with limitations in mobility and physical functioning, which impacts on activities of daily living and can lead to accelerated aging (Ferguson et al., Citation2017; Kuo et al., Citation2021; WHO, Citation2021). Therefore, whether targeted physical activity/exercise interventions can also improve physical health-related outcomes in adults with hearing loss requires scrutiny (Martinez-Amezcua et al., Citation2022).
A key consideration when developing and evaluating complex health-related interventions is whether they are theory driven. Behaviour change interventions that are underpinned by theory are more likely to elicit positive outcomes (Webb, Joseph, Yardley, & Michie, Citation2010). Furthermore, a theoretical underpinning can aid in understanding why interventions are or are not effective. The application of theories of health behaviour and change within a hearing health context is advantageous, not only enabling a comprehensive understanding of the factors influencing an individual’s behaviour, but it also assists with the development and evaluation of complex hearing healthcare interventions (Coulson, Ferguson, Henshaw, & Heffernan, Citation2016). One such theory that has been employed in the context of hearing healthcare is the Capability, Opportunity, Motivation – Behaviour (COM-B) model (Maidment, Ali, & Ferguson, Citation2019; Michie, Atkins, & West, Citation2014). This model provides a framework and systematic approach to identify theoretically determined behaviour change techniques (BCTs) that can be used to create the proposed change in behaviour. On this basis, any synthesis or evaluation of complex interventions in adult aural rehabilitation should also consider incorporating an assessment of their theoretical basis.
Prior to the development and evaluation of complex healthcare interventions, existing evidence should be identified, through the completion of a systematic review (Skivingston et al., Citation2021). However, to date, no systematic review has evaluated whether interventions that aim to improve physical activity and functioning are clinically effective in adults with hearing loss. On this basis, the primary objective of this study was to synthesise the existing literature assessing the effectiveness of interventions that aim to improve physical activity and functioning in this population.
Methods
Prior to commencing the systematic review, the protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 29th October 2019, registration number CRD42019155777. Methods are reported according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) checklist (Page et al., Citation2021).
Eligibility criteria
Inclusion criteria for the review were specified in terms of participants, intervention, comparators, outcomes, and study designs (PICOS) as follows:
Participants
In accordance with Cochrane guidelines and existing systematic review of hearing loss and health (Lawrence et al., Citation2020; McKenzie et al., Citation2022; Shukla et al., Citation2020), the inclusion criteria for participants were kept broad enough to ensure a diverse range of studies were included. This also allowed for potential comparisons between interventions to be made for characteristics, such as age, as well as hearing loss type and severity. Adults (≥18 years) with a mild to profound hearing loss, defined in accordance with the WHO (Citation2021) hearing loss grades (i.e., average hearing threshold in the better hearing ear across octave frequencies 0.5–4 kHz >20 and <95 dB HL). Studies containing both children (<18 years) and adults were not included unless data were reported separately. If hearing thresholds were not specified, the study author was contacted for clarification. If hearing threshold data were not reported and could not be obtained, studies were included where the mean average hearing threshold reported fell within the range (i.e., 20–94 dB HL (WHO, Citation2021)). Studies were also included if only qualitative descriptions of hearing threshold were provided with no audiometric data. Bilateral and unilateral sensorineural, conductive, and mixed hearing losses were included.
Interventions(s)/exposure(s)
Any behaviour change intervention in which the aim was to increase the level (i.e., intensity) or amount (i.e., frequency) of physical activity. A behaviour change intervention can be defined as an intervention that aims to alter a target behaviour through at least one of the following: capability (e.g., knowledge), (ii) opportunity (e.g., environmental context), and (iii) motivation (e.g., beliefs about capabilities) (Michie et al., Citation2014). These can be targeted at the level of the individual (e.g., self-efficacy), the target population (e.g., education), or the environment (e.g., environmental restructuring).
Comparator(s)/control
The comparisons of interest were either passive (e.g., standard/usual care, wait list, no intervention) or active control (e.g., other type of physical training).
Outcomes
This review aimed to assess the effectiveness of physical activity interventions. Therefore, primary outcomes included one or more of the following: (i) level or amount of physical activity, either self-reported (e.g., daily diary) or behavioural (e.g., pedometer monitoring); (ii) functional fitness (e.g., gait speed, sit-to-stand test); and (iii) adverse effects, such as pain or injury. Secondary outcomes included any of the following: (i) cognition (e.g., executive functioning); (ii) psychosocial wellbeing (e.g., Revised-UCLA Loneliness Scale: Russell, Citation1996); (iii) hearing-specific health-related quality of life (e.g., Hearing Handicap Inventory: Ventry & Weinstein, Citation1982); (iv) general health-related quality of life (e.g., Short Form Health Survey-36: Ware & Sherbournce, Citation1992); (v) feasibility (e.g., usability, adherence); and (vi) intervention behaviour change techniques. There were no restrictions on the duration of follow-up.
Study designs
Retrospective or prospective studies, randomised controlled trials, non-randomised controlled trials, before and after studies were included. Articles reporting expert opinions, cross-sectional data, cohort data, practice guidelines, case reports, case series, conference abstracts and book chapters were excluded.
Search strategy
An initial literature review was conducted on the 7th May 2020. Searches were updated on 21st February 2023 to ensure that any newly published studies were included. The following databases were searched: APA PsycINFO (via EBSCO host), Cochrane Library, MEDLINE (via EBSCO host), PubMed, Scopus, SPORTDiscus (via EBSCO host), Web of Science, ISRCTN Registry and ClinicalTrials.gov. See Supplemental Material for full electronic search strategies for all databases.
All database searches were completed in one day and with no time, document type or publication status limitations, but were limited to those written in English-only. The search terms were collected based on free text and controlled vocabularies (e.g., Medical Subject Headings), expert opinion, literature review, and checking the test search results. Additional information was identified manually through snowballing of the reference lists from included studies, as well as screening of related articles by shortlisted authors to identify any relevant articles that may not have been returned by the initial database searches.
Study selection
All identified references were independently screened by two investigators (MVG, DWM) to decide eligibility according to the PICOS criteria by reading the title and/or abstract. The full text was obtained for articles that appeared to meet eligibility or where there was any uncertainty (i.e., insufficient information to make a clear decision). Discrepancies were resolved through discussion between investigators. Where an agreement could not be reached, a third reviewer (EH) arbitrated.
Data collection process
A standardised data form constructed via Covidence (www.covidence.org) was used, which included study details (e.g., sponsorship source, country, setting), author’s contact details (name, institution, email, postal address), study design, population (inclusion/exclusion criteria, baseline characteristics), interventions and comparators, and outcomes. Data extraction was conducted by MVG and DWM independently but in duplicate for every included record.
Risk of bias in individual studies
The risk of bias in each included study was independently assessed by each investigator, using the Cochrane risk of bias tool version two (Sterne et al., Citation2019), which rates the studies as ‘high risk’, ‘low risk’ or ‘some concern’ in the following five domains: (i) randomisation process, (ii) deviations from the intended interventions (‘effect of assignment to interventions’ and ‘effect of adhering to interventions’), (iii) missing outcome data, (iv) measurement of the outcome, and (v) selection of the reported results. For controlled trials, the overall quality of the evidence was evaluated using the GRADE system, where evidence is up- or downgraded based on several factors including risk of bias, imprecision, and publication bias (Schünemann et al., Citation2022).
Data synthesis
Included studies were reviewed to determine suitability for meta-analysis. However, this was not possible due to high heterogeneity in the interventions evaluated and outcome measures employed. Therefore, primary and secondary outcomes were assessed at the individual study level through narrative synthesis (Popay et al., Citation2006).
Results
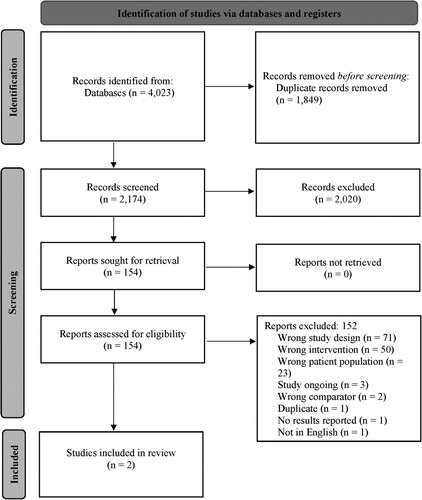
A total of 4023 records were identified for screening. Following the removal of 1849 duplicate publications, 2174 records were subjected to a three-stage screening process (). The full texts of 154 articles that passed the title and abstract screen were retrieved. Of those, 152 were judged not to meet the inclusion criteria and were excluded, resulting in only two studies being included in the review.
Figure 1. Selection of studies for the systematic review based on preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
A summary of the characteristics of the included studies can be found in . In one study, participants were randomised to a 10-week intervention consisting of either group auditory rehabilitation (GAR), exercise/walking sessions, and socialisation/health education (SHE), or GAR only (Jones et al., Citation2019). A second study non-randomly assigned participants to either a 12-week traditional dance training programme or a no-treatment (i.e., passive) control group (Tsimaras et al., Citation2010). Outcomes in both studies were assessed at baseline and immediately post-intervention only.
Table 1. Characteristics of studies included in the review.
Primary outcome
While neither study included measures of physical activity or adverse effects, the following functional fitness domains were assessed.
Aerobic capacity
Several different outcome measures were used to assess aerobic capacity across both studies. Peak minute ventilation and oxygen consumption (absolute and relative) were all found to improve in the intervention group after a 12-week traditional dance programme, but not amongst controls (Tsimaras et al., Citation2010). Tsimaras et al. (Citation2010) also found that time to exhaustion only improved in the intervention group after a 12-week traditional dance programme. Gait speed was also found to be improved to a greater extent in the intervention group compared to the control group by an average of 0.05 m/s (Jones et al., Citation2019). All other measures of aerobic capacity (Peak Heart Rate and Respiratory Exchange Rate) did not differ significantly after the intervention (Tsimaras et al., Citation2010).
Muscle strength
Tsimaras et al. (Citation2010) assessed muscle strength via peak torque of the quadriceps and hamstrings, each at 300°/s−1, 180°/s−1, and 60°/s−1. Whereas Jones et al. (Citation2019) assessed muscle strength with the 30 s sit-to-stand test and grip strength. Relative to controls, most outcomes in both studies, except for grip strength, were found to improve significantly post-intervention.
Flexibility
Assessed by Jones et al. (Citation2019) using the back scratch test and sit and reach test. Relative to the control group, only the back scratch test significantly improved post-intervention.
Balance
Assessed by Jones et al. (Citation2019) via the one-foot-balance test and the eight-foot get up and go test. No significant effect of the intervention was found for either outcome measure.
Secondary outcomes
Psychosocial wellbeing
Loneliness, social support, and depression were assessed by Jones et al. (Citation2019) using the de Jong Loneliness Scale (De Jong-Gierveld & Kamphuis, Citation1985), the Medical Outcomes Trial-Social Support Survey (Sherbourne & Stewart, Citation1991), and the Geriatric Depression Scale (Vinkers, Gussekloo, Stek, Westendorp, & Van Der Mast, Citation2004), respectively. Only the emotional-loneliness subscale of the de Jong Loneliness Scale demonstrated a significant difference, improving pre- to post-intervention in the control compared to the intervention group. All other measures did not differ statistically between groups.
Hearing-specific health-related quality of life
Jones et al. (Citation2019) reported outcome data for hearing-specific health-related quality of life using the Client-Oriented Scale for Improvement (COSI) (Dillon, James, & Ginis, Citation1997), Hearing Handicap Inventory for the Elderly (HHIE) (Ventry & Weinstein, Citation1982), and International Outcome Inventory for Hearing Aids (IOI-HA) (Cox & Alexander, Citation2002). For the COSI, post-intervention responses for each listening situation did not differ statistically between groups. For the HHIE, outcome data comparing differences between intervention and comparator groups were not reported. Instead, the HHIE was included in all analyses post hoc to account for the unanticipated differences in this outcome between groups at baseline. In addition, regardless of group, HHIE total, as well as emotional and social subscale scores, all significantly improved for participants who attended ≥80% of group auditory rehabilitation sessions compared to those that attended <80% of the sessions. For the IOI-HA, a statistically significant difference was found between groups for the hearing aid benefit subscale only.
General health-related quality of life
The 36-item Short Form Health Survey (SF-36) (Ware & Sherbournce, Citation1992) was used by Jones et al. (Citation2019), who found no statistically significant differences between groups.
Intervention feasibility
Only participants in Jones et al.’s (Citation2019) study completed a follow-up evaluation questionnaire. This assessed the acceptability of the GAR, exercise/walking sessions, and socialisation/health education sessions. Overall, participants rated all aspects of the intervention favourably. For example, 100% of participants in the intervention group agreed or strongly agreed that they were satisfied with the exercise/walking sessions, and that the socialisation/health education sessions were interesting. In addition, 94% of participants agreed or strongly agreed that the group auditory rehabilitation programme helped them to recognise and better accept their hearing loss. Responses to the group auditory rehabilitation evaluation questions did not differ statistically between groups.
Behaviour change techniques
Behaviour change techniques were mapped onto the COM-B model and associated intervention functions (see Michie et al., Citation2014). The techniques identified addressed all domains of the COM-B model (i.e., capability, opportunity, motivation) (see also, Supplemental Material). The intervention functions to support capability included enablement (self-monitoring), education (reduce negative emotions), and training (problem solving). Environmental restructuring was the only function used to aid opportunities (adding objects to the environment). For the motivation domain, enablement in the form of goal setting was the focus of interventions.
Risk of bias
The Cochrane risk of bias tool version 2 (Sterne et al., Citation2019) was used to assess risk of bias (). For the randomisation process, one study was judged as low risk (Jones et al., Citation2019) and one as high risk (Tsimaras et al., Citation2010), due to the lack of blinding of participants and non-randomisation of group allocation. The domain ‘deviation from the intended interventions’ was split into two parts. For part one ‘assignment to interventions’ was judged as ‘some concerns’ for both studies due to lack of information on deviations from the intended intervention. For part two ‘adhering to the intervention’ was judged as low for both studies. The missing outcome data and measurement of the outcome domains were also both judged as low risk for both studies, due to complete data for all participants, and the use of appropriate outcome measures that were the same for both groups. Lastly, ‘selection of the reported result’ was judged to be low for one study (Jones et al., Citation2019) and ‘some concerns’ for the other (Tsimaras et al., Citation2010), because the protocol was not pre-registered and so it was not possible to determine that it has been analysed according to a pre-specified statistical plan. Overall, one study was classified as high risk of bias (Tsimaras et al., Citation2010), and the other as ‘some concerns’ (Jones et al., Citation2019).
Table 2. Cochrane Risk of Bias version 2 assessment for each included study.
Quality assessment
The GRADE approach (Schünemann et al., Citation2022) was used to assess the quality of evidence for ‘physical function’ only since this was the only outcome measured in both studies. Several factors led to physical functioning to be downgraded to very low quality. The evidence was downgraded due to study limitations (i.e., high risk of bias) that arose from lack of clarity regarding randomisation and allocation concealment. The evidence was further downgraded due to small sample sizes (i.e., imprecision).
Discussion
The current systematic review assessed the effectiveness of physical activity interventions in adults with hearing loss. Only two studies met the eligibility criteria for inclusion in this review (Jones et al., Citation2019; Tsimaras et al., Citation2010). These studies were heterogeneous in terms of the sample recruited, type of intervention, and outcomes measured. Moreover, the studies included participants with potentially different severities of hearing, although we cannot be certain since Jones et al. (Citation2019) relied on a self-reported assessment of hearing. Moreover, Jones et al. (Citation2019) reported that 35 (53%) of their participants were hearing aid users, but Tsimaras et al. (Citation2010) did not provide any information regarding device use. Hearing technologies impact on how individuals interact with their social environment. As such, further research regarding the impact of hearing devices (e.g., hearing aids) on physical activity and function is necessary. In addition, both studies assessed physical function (e.g., aerobic capacity, muscle strength), with only one (Jones et al., Citation2019) including assessments of psychosocial wellbeing. Outcomes were measured immediately post-intervention in both studies, with no longer-term follow-up. The evidence was judged as very low to low quality, primarily due to study design limitations (i.e., participant randomisation, small sample size), indicating that there is little high-quality evidence regarding hearing loss and physical activity interventions.
Behaviour change techniques
Only one BCT identified in the intervention groups addressed hearing-specific barriers to physical activity, where additional auditory stimuli (percussion instruments) were used to aid audibility (i.e., adding objects to the environment). All other BCTs focused on general techniques, such as goal setting (e.g., a physical activity goal) and self-monitoring (e.g., encouraged to use pedometer to monitor their own activity). Qualitative evidence has shown that adults with hearing loss, irrespective of severity (i.e., mild, moderate, severe, profound), experience hearing-specific barriers to physical activity (Kurková, Citation2016). These barriers primarily stem from communication difficulties that are exacerbated by the environments that physical activity tends to occur in (e.g., sports halls, gyms, outside open space). As such, additional BCTs, such as developing communication strategies (and the self-efficacy to use them) may be necessary. These BCTs, may be particularly important for physical activity, as hearing devices cannot always be used (e.g., swimming, excessive sweating) (Kurková, Citation2016). Indeed, in Jones et al.’s (Citation2019) study, participants in both control and intervention groups received training in developing general communication strategies, highlighting the importance of ensuring adults with hearing loss are provided with targeted support to be physically active.
Physical function
Both studies found evidence of improvement on measures of physical function in the intervention compared to control groups. In terms of aerobic capacity, most outcome measures showed improvements after the interventions, except for peak heart rate and respiratory exchange rate (Tsimaras et al., Citation2010). Aerobic fitness has most frequently been assessed in the literature with gait speed. Indeed, Jones et al. (Citation2019) included gait speed and found significant improvements in the intervention group. Population cohort studies have found that older adults with poorer hearing have significantly slower gait speeds (Chen et al., Citation2015; Huang et al., Citation2019; Martinez-Amezcua et al., Citation2021; Yévenes-Briones et al., Citation2021). Slow gait speed is concerning, as it is a risk factor for falls, disability, earlier mortality, and dementia (Peel, Alapatt, Jones, & Hubbard, Citation2019; Veronese et al., Citation2018). Gait control involves multiple domains (e.g., motor, perception, cognitive processes), and so in accordance with the ‘information degradation’ model (Peelle, Citation2018), the additional cognitive demands required may result in slower gait speeds for adults with hearing loss. However, Pupo et al. (Citation2022) found that there were no significant differences in gait speed between those with and without hearing loss. Rather, an interaction between hearing and cognitive function demonstrated that hearing loss was only significantly related to slower gait speed amongst those with poorer cognition. Nevertheless, it should be noted that neither study included in this review explicitly tested these potential mechanistic pathways for the association between hearing loss and physical activity and/or function.
Similarly, most measures of muscle strength (peak torques, sit-to-stand) showed significant improvements in the intervention groups for both included studies, except for grip strength (Jones et al., Citation2019). Muscle strength has received relatively little investigation regarding its relationship with hearing loss, but limited evidence indicates better muscular fitness is associated with a lower risk of hearing loss (Kawakami et al., Citation2021). Lack of muscle strength is also a potential marker of falls and frailty (Jehu et al., Citation2021), which are more prevalent in adults with hearing loss (Jiam, Li, & Agrawal, Citation2016). Flexibility and balance were only assessed by Jones et al. (Citation2019), with mixed results when measured via back scratch and sit-and-reach tasks, and no effect of the intervention on balance via one-foot-balance or timed get-up-and-go. The lack of improved balance following intervention may be a cause for concern given that hearing loss is strongly associated with poorer balance (Yévenes-Briones et al., Citation2021), which is a risk factor for repeated falls and frailty amongst older adults (Jehu et al., Citation2021).
Converging evidence indicates that multicomponent physical activity interventions (e.g., aerobic and resistance training) have the greatest benefit for cognitive function in healthy older adults (Sáez de Asteasu, Martínez-Velilla, Zambom-Ferraresi, Casas-Herrero, & Izquierdo, Citation2017). As such, further investigations should assess whether multicomponent physical activity interventions can also improve physical function in adults with hearing loss. The results from this study suggest that physical activity interventions for adults with hearing loss may require a more targeted approach to improve balance than for healthy adults. Improvement in balance and other physical function outcomes (e.g., gait speed, muscle strength) in adults with hearing loss could be a viable intervention pathway to prevent further declines in health, hospitalisations, and/or cognitive impairment.
Psychosocial wellbeing
The intervention by Jones et al. (Citation2019) had little effect on psychosocial wellbeing. Rather, Jones et al. (Citation2019) found the greatest improvements on the emotional-loneliness subscale were in the control (GAR only) group. This is unexpected given that the intervention also involved GAR, as well as SHE, and exercise (including group walking). Interventions to improve psychosocial wellbeing in adults with hearing loss are limited and the available evidence primarily comes from the provision of hearing aids or cochlear implants, where the evidence for better psychosocial wellbeing is mixed (Bott & Saunders, Citation2021). It might have been expected that the addition of exercise to GAR would improve psychosocial wellbeing, given that physical activity has been shown to improve psychosocial wellbeing in older adults (Taylor et al., Citation2004). However, this was not found, with Jones et al. (Citation2019) suggesting that this might be because of baseline differences in HHIE scores between groups. Indeed, greatest improvements in psychosocial wellbeing have been found following intervention (e.g., hearing aids) for those with lower HHIE baseline scores (Hickson, Worrall, & Scarinci, Citation2007). As such, it remains unclear whether baseline differences in Jones et al.’s (Citation2019) study superseded the psychosocial benefits of exercise. Even so, as only one pilot study in the current review assessed psychosocial wellbeing, further investigation is necessary.
Limitations
A key limitation of this review is the lack of intervention studies in this area, making it untenable to draw firm conclusions on the clinical effectiveness of physical activity interventions to improve health-related outcomes specifically for adults with hearing loss. Neither study in this review included a comparator group that did not have hearing loss, making it unclear whether the effectiveness of these interventions would differ for those with and without hearing loss. Furthermore, the small sample sizes of the studies included could have artificially inflated or muted the effect of the intervention on outcomes (i.e., increased likelihood of a type I or type II error, respectively). In addition, the study populations were disparate regarding age, which could impact on the findings. However, where the same outcomes were measured, results were similar, suggesting that the associations may not be age dependent.
A further potential limitation of the review was that only average hearing thresholds were reported in both studies included. It is possible that some participants were outside the specified range of mild to severe hearing loss even if the study average was between 20 and 94 dB HL. Furthermore, one study only assessed hearing via self-report (Jones et al., Citation2019). The broad inclusion criteria for hearing loss are also a potential limitation, as the communication difficulties and barriers that adults with mild hearing loss experience (aided or unaided), may be very different to those of someone with profound hearing loss. Therefore, the effectiveness of interventions and/or the strategies required to engage in physical activity will likely differ between types and degrees of hearing loss. Indeed, some evidence suggests that the relationship between hearing loss and physical activity (Kuo et al., Citation2021) and physical function (Martinez-Amezcua et al., Citation2021) may be dose-dependent; greater hearing loss associated with less physical activity and poorer physical function. In addition, the review was only able to include studies written in English; therefore, it is conceivable that relevant studies in other languages may have been missed. Nevertheless, due to limited study resources and personnel, it was not possible to translate articles published in other languages.
At present, because the studies were rated as ‘some concern’ or ‘high’ risk’ of bias, it was not possible to be confident that the effects were due to the intervention. Larger, high-quality full-scale evaluation trials (i.e., RCTs), would support greater confidence in the effect sizes and identify if they are a genuine product of the intervention. Furthermore, no study included a follow-up beyond the immediate post-intervention assessment, so it was unclear if the interventions had any long-term effects on physical function or psychosocial wellbeing.
Future directions and conclusion
In summary, there is currently no robust evidence that physical activity interventions for adults with hearing loss improve physical function or psychosocial wellbeing outcomes. Tentative conclusions suggest that interventions may be beneficial for gait speed and muscle strength in the lower extremities. In addition, the available evidence was judged as low quality and potentially subject to bias because of study design limitations. The accumulation of observational (i.e., cross-sectional, longitudinal) evidence that has found poorer physical function and physical activity in adults with hearing loss, suggests that further high-quality research in this area is needed. Specifically, RCTs investigating the impact of these interventions on long-term effects of physical function and psychosocial wellbeing are necessary to help delineate and potentially mitigate NCD risk in adults with hearing loss. Prior to this, best-practice guidance (Skivingston et al., Citation2021) states that, it is necessary to explore the barriers to physical activity in this population to identify targets of behaviour change (e.g., education, environmental restructuring), so that suitable interventions can be developed and/or evaluated. Nevertheless, hearing healthcare professionals should be aware of the range of conditions associated with hearing loss and be prepared to recommend health-promoting behaviours (e.g., physical activity) to provide a more holistic, person-centred approach (Maidment et al., Citation2023).
Supplemental Material
Download MS Word (19.4 KB)Acknowledgements
No specific funding was provided for this project. The research was undertaken as part of the first-author’s doctoral studies supported by Loughborough University. We would also like to thank Rosalyn Parker and Susannah Goggins for reviewing an earlier version of this manuscript and providing invaluable feedback.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- Ashton, R. E., Tew, G. A., Aning, J. J., Gilbert, S. E., Lewis, L., & Saxton, J. M. (2020). Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: Systematic review with meta-analysis. British Journal of Sports Medicine, 54(6), 341–348. doi:10.1136/bjsports-2017-098970
- Besser, J., Stropahl, M., Urry, E., & Launer, S. (2018). Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hearing Research, 369, 3–14. doi:10.1016/j.heares.2018.06.008
- Bott, A., & Saunders, G. (2021). A scoping review of studies investigating hearing loss, social isolation and/or loneliness in adults. International Journal of Audiology, 60(sup2), 30–46. doi:10.1080/14992027.2021.1915506
- Chen, D. S., Betz, J., Yaffe, K., Ayonayon, H. N., Kritchevsky, S., Martin, K. R., … Lin, F. (2015). Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. Journals of Geronotology: Series A, Biological Sciences and Medical Sciences, 70(5), 654–661. doi:10.1093/gerona/glu207
- Coulson, N. S., Ferguson, M. A., Henshaw, H., & Heffernan, E. (2016). Applying theories of health behaviour change to hearing health research: Time for a new approach. International Journal of Audiology, 55(sup3), 99–104. doi:10.3109/14992027.2016.1161851
- Cox, R. M., & Alexander, G. C. (2002). The international outcome inventory for hearing aids (IOI-HA): psychometric properties of the English version. International Journal of Audiology, 41(1), 30–35. doi:10.3109/14992020209101309
- Curhan, S. G., Eavey, R., Wang, M., Stampfer, M. J., & Curhan, G. C. (2013). Body mass index, waist circumference, physical activity, and risk of hearing loss in women. The American Journal of Medince, 126(12), 1142.e1–1142.e8. doi:10.1016/j.amjmed.2013.04.026
- Dawes, P., Emsley, R., Cruickshanks, K. J., Moore, D. R., Fortnum, H., Edmondson-Jones, M., … Munro, K. J. (2015). Hearing loss and cognition: The role of hearing aids, social isolation and depression. PLOS ONE, 10(3), Article e0119616. doi:10.1371/journal.pone.0119616
- De Jong-Gierveld, J., & Kamphuis, F. (1985). The development of a Rasch-type loneliness scale. Applied Psychological Medicine, 9(3), 289–299. doi:10.1177/014662168500900307
- Dillon, H., James, A., & Ginis, J. (1997). Client oriented scale of improvement (COSI) and its relationship to several other measures of benefit and satisfaction provided by hearing aids. Journal of the American Academy of Audiology, 8(1), 27–43.
- Engdahl, B., Aarhus, L., Lie, A., & Tambs, K. (2015). Cardiovascular risk factors and hearing loss: The HUNT study. International Journal of Audiology, 54(12), 958–966. doi:10.3109/14992027.2015.1090631
- Ferguson, M. A., Kitterick, P. T., Chong, L. Y., Edmondson-Jones, M., Barker, F., & Hoare, D. J. (2017). Hearing aids for mild to moderate hearing loss in adults. Cochrane Database of Systematic Reviews, 7, CD012023. doi:10.1002/14651858.CD012023.pub2
- Gheysen, F., Poppe, L., DeSmet, A., Swinnen, S., Cardon, G., Bourdeaudhuij, I. D., … Fias, W. (2018). Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity, 15, 63. doi:10.1186/s12966-018-0697-x
- Gispen, F. E., Chen, D. S., Genther, D. J., & Lin, F. R. (2014). Association between hearing impairment and lower levels of physical activity in older adults. Journal of the American Geriatrics Society, 62(8), doi:10.1111/jgs.12938
- Hickson, L., Worrall, L., & Scarinci, N. (2007). A randomized controlled trial evaluating the active communication education program for older people with hearing impairment. Ear and Hearing, 28(2), 212–230. doi:10.1097/aud.0b013e31803126c8
- Hornsby, B. W. Y., Naylor, G., & Bess, F. H. (2016). A taxonomy of fatigue concepts and their relation to hearing loss. Ear and Hearing, 37(1supp), 136S–144S. doi:10.1097/aud.0000000000000289
- Huang, C., Sun, S., Wang, W., Li, Y., Feng, W., & Wu, Y. (2019). Cognition mediates the relationship between sensory function and gait speed in older adults: Evidence from the English longitudinal study of ageing. Journal of Alzheimer’s Disease, 70(4), 1153–1161. doi:10.3233/jad-190364
- Jehu, D. A., Davis, J. C., Falck, R. S., Bennett, K. J., Tai, D., Souza, M. F., … Liu-Ambrose, T. (2021). Risk factors for recurrent falls in older adults: A systematic review with meta-analysis. Maturitas, 114, 23–28. doi:10.1016/j.maturitas.2020.10.021
- Jiam, N. T.-L., Li, C., & Agrawal, Y. (2016). Hearing loss and falls: A systematic review and meta-analysis. The Laryngoscope, 126(11), 2587–2596. doi:10.1002/lary.25927
- Jones, C. A., Siever, J., Knuff, K., Van Bergen, C., Mick, P., Little, J., … Miller, H. (2019). Walk, talk and listen: A pilot randomised controlled trial targeting functional fitness and loneliness in older adults with hearing loss. BMJ Open, 9(4), e026169. doi:10.1136/bmjopen-2018-026169
- Kawakami, R., Sawada, S., Kato, K., Gando, Y., Momma, H., Oike, H., … Sone, H. (2021). A prospective cohort study of muscular and performance fitness and risk of hearing loss: The Niigata wellness study. The American Journal of Medicine, 134(2), 235–242. doi:10.1016/j.amjmed.2020.06.021
- Kawakami, R., Sawada, S. S., Kata, K., Gando, Y., Momma, H., Oike, H., … Sone, H. (2022). Leisure-time physical activity and incidence of objectively assessed hearing loss: The Niigata wellness study. Scandinavian Journal of Medicine & Science in Sports, 32(2), 435–445. doi:10.1111/sms.14089
- Kuo, P.-L., Di, J., Ferrucci, L., & Lin, F. R. (2021). Analysis of hearing loss and physical activity among US adults aged 60-69 years. JAMA Network Open, 4(4), e215484. doi:10.1001/jamanetworkopen.2021.5484
- Kurková, P. (2016). Physical activity among older people who are deaf and hard of hearing: Perceived barriers and facilitators. Physical Activity Review, 4, 72–80. doi:10.16926/par.2016.04.09
- Lawrence, B. J., Jayakody, D. M. P., Bennett, R. J., Eikelboom, R. H., Gasson, N., & Friedland, P. L. (2020). Hearing loss and depression in older adults: A systematic review and meta-analysis. The Gerontologist, 60(3), e137–e154. doi:10.1093/geront/gnz009
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet commission. The Lancet Commissions, 396(10248), 413–446. doi:10.1016/S0140-6736(20)30367-6
- Maidment, D. W., Ali, Y. H. K., & Ferguson, M. A. (2019). Applying the COM-B model to assess the usability of smartphone-connected listening devices in adults with hearing loss. Journal of the American Academy of Audiology, 30(5), 417–430. doi:10.3766/jaaa.18061
- Maidment, D. W., Wallhagen, M. I., Dowd, K., Mick, P., Poker, E., Spankovich, C., & Urry, E. (2023). New horizons in holistic, person-centred health promotion for hearing healthcare. Age and Ageing, 52(2), afad020. doi:10.1093/ageing/afad020
- Martinez-Amezcua, P., Dooley, E. E., Reed, N. S., Powell, D., Hornikel, B., Golub, J. S., … Palta, P. (2022). Association of hearing impairment and 24-hour total movement activity in a representative sample of US adults. JAMA Network Open, 5(3), e222983. doi:10.1001/jamanetworkopen.2022.2983
- Martinez-Amezcua, P., Powell, D., Kuo, P.-L., Reed, N. S., Sullivan, K., Palta, P., … Deal, J. A. (2021). Associations of age-related hearing impairment with physical functioning among community-dwelling older adults in the US. JAMA Network Open, 4(6), e2113742. doi:10.1001/jamanetworkopen.2021.13742
- McKenzie, J. E., Brennan, S. E., Ryan, R. E., Thomson, H. J., Johnston, R. V., & Thomas, J. (2022). Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Page, & V. A. Welch (Eds.), Cochrane handbook for systematic reviews of interventions (version 6.3). Cochrane. http://www.training.cochrane.org/handbook.
- Michie, S., Atkins, L., & West, R. (2014). The behaviour change wheel: A guide to designing interventions. London: Silverback Publishing.
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Medicine, 18(3), e1003583. doi:10.1371/journal.pmed.1003583
- Peel, N. M., Alapatt, L. J., Jones, L. V., & Hubbard, R. E. (2019). The association between gait speed and cognitive status in community-dwelling older people: A systematic review and meta-analysis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 74(6), 943–948. doi:10.1093/gerona/gly140
- Peelle, J. E. (2018). Listening effort: How the cognitive consequences of acoustic challenge are reflected in brain and behaviour. Ear and Hearing, 39(2), 204–214. doi:10.1097/AUD.0000000000000494
- Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, R., & Britten, N. (2006). Guidance on the conduct of narrative synthesis in systematic reviews. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf.
- Pupo, D. A., Small, B. J., Deal, J. A., Armstrong, N. M., Simonsick, E. M., Resnick, S. M., … Tian, Q. (2022). Hearing and mobility in aging – the moderating role of neuropsychological function. The Journals of Gerontology: Series A, glac047. doi:10.1093/gerona/glac047
- Roth, G. A., Mensah, G. A., Johnson, C. A., Addolorato, G., Ammirati, E., Baddour, L. M., … Fuster, V. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. Journal of the American College of Cardiology, 76(25), 2982–3021. doi:10.1016/j.jacc.2020.11.010
- Russell, D. W. (1996). UCLA loneliness scale (version 3): reliability, validity, and factor structure. Journal of Personality Assessment, 66(1), 20–40. doi:10.1207/s15327752jpa6601_2
- Sáez de Asteasu, M. L., Martínez-Velilla, N., Zambom-Ferraresi, F., Casas-Herrero, Á, & Izquierdo, M. (2017). Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Research Reviews, 37, 117–134. doi:10.1016/j.arr.2017.05.007
- Schünemann, H. J., Higgins, J. P. T., Vist, G. E., Glasziou, P., Akl, E. A., Skoetz, N., & Guyatt, G. H. (2022). Chapter 14: Completing ‘summary of findings’ tables and grading the certainty of the evidence. In J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Page, & V. A. Welch (Eds.), Cochrane handbook for systematic reviews of interventions (version 6.3). Cochrane. http://www.training.cochrane.org/handbook
- Sherbourne, C. D., & Stewart, A. L. (1991). The MOS social support survey. Social Science and Medicine, 32(6), 705–714. doi:10.1016/0277-9536(91)90150-b
- Shukla, A., Harper, M., Pedersen, E., Goman, A., Suen, J. J., Price, C., … Reed, N. S. (2020). Hearing loss, loneliness, and social isolation: A systematic review. Otolaryngology – Head and Neck Surgery, 162(5), 622–633. doi:10.1177/0194599820910377
- Skivingston, K., Matthews, L., Simpson, S. A., Craig, P., Baird, J., Blazeby, J. M., … Moore, L. (2021). A new framework for developing and evaluating complex interventions: Update of medical research council guidance. BMJ, 30(374), 2061. doi:10.1136/bmj.n2061
- Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., … Higgins, J. P. T. (2019). Rob 2: A revised tool to assess risk of bias in randomized trials. BMJ, 366, 4898. doi:10.1136/bmj.l4898
- Taylor, A. H., Cable, N. T., Faulkner, G., Hillsdon, M., Narici, M., & Van der Bij, A. K. (2004). Physical activity and older adults: A review of health benefits and the effectiveness of interventions. Journal of Sports Sciences, 22(8), 703–725. doi:10.1080/02640410410001712421
- Tsimaras, V. K., Kyriazis, D. A., Christoulas, K. I., Fotiadou, E. G., Kokaridas, D. G., & Angelopoulou, N. A. (2010). The effect of a traditional dance training program on the physical fitness of adults with hearing loss. Journal of Strength and Conditioning Research, 24(4), 1052–1058. doi:10.1519/jsc.0b013e3181ca501c
- Ventry, I. M., & Weinstein, B. E. (1982). The hearing handicap inventory for the elderly: A new tool. Ear and Hearing, 3(3), 128–134. doi:10.1097/00003446-198205000-00006
- Veronese, N., Stubbs, B., Volpato, S., Zuliani, G., Maggi, S., Cesari, M., … Cereda, E. (2018). Association between gait speed with mortality, cardiovascular disease and cancer: A systematic review and meta-analysis of prospective cohort studies. Journal of the American Medical Directors Association, 19(11), 981–988. doi:10.1016/j.jamda.2018.06.007
- Vinkers, D. J., Gussekloo, J., Stek, M. L., Westendorp, R. G. J., & Van Der Mast, R. C. (2004). The 15-item geriatrics depression scale (GDS-15) detects changes in depressive symptoms after a major negative life event. The Leiden 85-plus study. International Journal of Geriatrics Psychiatry, 19(1), 80–84. doi:10.1002/gps.1043
- Ware, J. E., & Sherbournce, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual Framework and Item Selection. Medical Care, 30(6), 473–483.
- Wattamwar, K., Qian, J. Z., Otter, J., Leskowitz, M. J., Caruana, F. F., Siedlecki, B., … Lalwani, A. K. (2018). Association of cardiovascular comorbidities with hearing loss in the older old. JAMA Otolaryngology: Head & Neck Surgery, 144(7), 623–629. doi:10.1001/jamaoto.2018.0643
- Webb, T. L., Joseph, J., Yardley, L., & Michie, S. (2010). Using the internet to promote health behavior change: A systematic review and meta-analysis of the impact of the theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. Journal of Medical Internet Research, 12(1), e4. doi:10.2196/jmir.1376
- Wells, T. S., Nickels, L. D., Rush, S. R., Musich, S. A., Wu, L., Bhattarai, G. R., & Yeh, C. S. (2020). Characteristics and health outcomes associated with hearing loss and hearing aid use among older adults. Journal of Aging and Health, 32(7-8), 724–734. doi:10.1177/0898264319848866
- World Health Organisation. (2020). WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organisation.
- World Health Organisation. (2021, March 3). Word report on hearing. https://www.who.int/publications/i/item/world-report-on-hearing.
- Yévenes-Briones, H., Caballero, F. F., Struijk, E. A., Rey-Martinez, J., Montes-Jovellar, L., Graciani, A., … Lopez-Garcia, E. (2021). Association between hearing loss and impaired physical function, frailty, and disability in older adults: A cross-sectional study. JAMA Otolaryngology Head & Neck Surgery, 147(11), 951–958. doi:10.1001/jamaoto.2021.2399
