ABSTRACT
This study aimed to evaluate the feasibility, safety and preliminary efficacy of high-frequency repetitive transcranial magnetic stimulation for treating chronic subjective tinnitus. Eleven adults (8 males; age: 56.70 ± 11.42 years, tinnitus duration: 12.43 ± 7.43 years) were recruited to participate in a pre-registered study. Six treatments were provided over two weeks. Feasibility was determined against pre-specified criteria addressing recruitment, adherence (protocol, 2-week follow-up, 4-week follow-up), and perceived usefulness, helpfulness and acceptability. Adverse events were recorded and preliminary efficacy was assessed using the Tinnitus Handicap Inventory. Five out of seven feasibility criteria were met and no serious adverse events occurred. One participant withdrew from the study due to stimulation being uncomfortable. Preliminary efficacy data indicated a significant effect of Time on tinnitus (p < 0.001). Compared to baseline, tinnitus reduced at mid-treatment (t(9) = −3.47, p = 0.028) and 4-weeks post treatment (t(9) = −3.62, p = 0.024). A repetitive transcranial magnetic stimulation protocol to manage chronic subjective tinnitus appears feasible, safe, and may have preliminary evidence of effectiveness. The recommendation is to proceed to trial with minor adjustments to gain further insight into the patient experience.
Introduction
Subjective tinnitus is the perception of sound in the absence of an internal or external source (Kreuzer et al., Citation2017). It can encapsulate a variety of intensities and characteristics such as high and low frequencies, be pulsatile, constant or intermittent (Møller, Langguth, DeRidder, & Kleinjung, Citation2011). The sound may be perceived unilateral, bilateral, or further inside the head (Møller et al., Citation2011), and can present as acute (apparent <6 months) or chronic (apparent ≥6 months) (Esmaili & Renton, Citation2018). Globally, tinnitus impacts more than 740 million adults, with more than 120 million people reporting their tinnitus to be a major problem (Jarach et al., Citation2022). It can negatively affect quality of life (QOL) and activities of daily living (ADL), with symptoms appearing to increase proportionally to the severity and duration of tinnitus (Moroe & Khoza-Shangase, Citation2014; Salazar et al., Citation2019; Ukaegbe, Orji, Ezeanolue, Akpeh, & Okorafor, Citation2017; Watts et al., Citation2018; Ziai, Moshtaghi, Mahboubi, & Djalilian, Citation2017). Many treatments have been explored, with one systematic review discovering 16 different therapies (Zenner et al., Citation2017). A tinnitus-specific cognitive behavioural therapy, accompanied by counselling, might be effective (Beukes, Andersson, Fagelson, & Manchaiah, Citation2022; Zenner et al., Citation2017), although others suggest evidence is limited (Fuller et al., Citation2020). Since there is no treatment that appears to work for all people with tinnitus, there is a need to explore alternative solutions.
Two characteristics of subjective tinnitus might prove valuable for exploring new treatments. First, while the cause of subjective tinnitus remains idiopathic, there are indications that hearing loss, age, trauma, ototoxicity, and several others can impact the cochlear nerve initiating maladaptive neuroplastic changes (Makar, Citation2021; Møller et al., Citation2011). Bartels, Staal, and Albers (Citation2007) and Searchfield and Zhang (Citation2021) support that there is growing evidence that neural changes likely underpin subjective tinnitus. These neural changes may increase spontaneous activity, alter temporal firing patterns and change tonotopy throughout the auditory pathway, which may invoke perceptions of ringing and buzzing without any physiological reason (Kaltenbach, Citation2000). This phenomenon of maladaptive neuroplasticity providing perceptual experiences in the absence of external stimuli is not uncommon and is thought to underpin other health issues such as central neuropathic pain (Moller, Citation2016; Møller, Citation2008). The potential role of maladaptive neuroplasticity as a therapeutic target has been explored in some capacity (Fitzgerald & Daskalakis, Citation2022). Repetitive transcranial magnetic stimulation (rTMS) has shown some early efficacy as a treatment to induce neuroplasticity and manage symptoms of tinnitus (Yang et al., Citation2021; Zenner et al., Citation2017). Several studies have applied rTMS to various cortical sites (De Ridder, Song, & Vanneste, Citation2013; Langguth et al., Citation2014; Noh et al., Citation2020; Yang et al., Citation2021; Zenner et al., Citation2017), as tinnitus appears to be a network of multiple cerebral areas including the prefrontal, parahippocampus, and anterior cingulate cortices (Noh et al., Citation2020). These studies have shown some early promise; however, the optimal location, stimulation intensity, and frequency of intervention remains inconclusive. How rTMS is administered requires further exploration.
Second, there are strong links between tinnitus and depression (Langguth, Landgrebe, Kleinjung, Sand, & Hajak, Citation2011; Salazar et al., Citation2019; Ziai et al., Citation2017). This might, in part, stem from the impact tinnitus has on QOL and ADL (Salazar et al., Citation2019; Ziai et al., Citation2017). However, it is interesting to observe similar neuroplastic changes that occur independently in both tinnitus and depression. Depression has been linked to a reduction of grey matter throughout the prefrontal cortices (Oakes, Loukas, Oskouian, & Tubbs, Citation2017). Additionally, similar reductions have been observed in the ventromedial and dorsomedial prefrontal cortices which were related to auditory perceptions of tinnitus (Leaver et al., Citation2012). Of note, these reductions in grey matter in individuals with tinnitus were found to be independent of depression or anxiety, suggesting they may be driven by tinnitus, rather than developing on a background of depression (Leaver et al., Citation2012). This has led to several studies attempting to target the link between depression and tinnitus, particularly the pharmacological use of anti-depressants (Zenner et al., Citation2017). However, the evidence remains inconclusive, despite the underlying physiological link between the two conditions (Baldo et al., Citation2012; Langguth & Elgoyhen, Citation2012). The use of rTMS has been shown to be highly effective in the management of depression specifically when applied to the left dorsolateral prefrontal cortex (DLPFC) (Hordacre, Comacchio, Williams, & Hillier, Citation2021; Isenberg et al., Citation2005; McNamara, Ray, Arthurs, & Boniface, Citation2001; Schutter, Citation2009), and as such has received approval from the Food and Drug Administration (FDA) and Medicare (Health, Citation2021; McClintock et al., Citation2018). The similarities in neuroanatomical structures and pathophysiology between tinnitus and depression may provide a rationale for targeting tinnitus treatments based on depression therapies (Langguth et al., Citation2011; Zöger, Svedlund, & Holgers, Citation2006). Some early work has explored rTMS therapy for the DLPFC for tinnitus. Specifically, two studies have explored either a combined stimulation of the auditory cortex and left DLPFC (Noh et al., Citation2020), or right DLPFC alone (De Ridder et al., Citation2013), and shown promising efficacy. However, it is difficult to decipher whether improvements in tinnitus were due to stimulation of the auditory cortex, DLPFC or both. We are unaware of any work employing a depression rTMS treatment targeting the left DLPFC alone to treat tinnitus. Potentially, this may be because of side effects and physical discomfort on the scale from frontal stimulation. Given the similar neurophysiology underpinning depression and tinnitus (Oakes et al., Citation2017), and the clear role of maladaptive neuroplasticity in tinnitus (Makar, Citation2021; Møller et al., Citation2011), an rTMS treatment protocol for depression applied to the left DLPFC is worth exploring. Therefore, the primary aim was to determine the feasibility of a Medicare – and FDA-approved rTMS protocol for depression in managing chronic tinnitus, our secondary aim was to explore safety and preliminary efficacy, which may support future, larger trials for what might prove to be a non-invasive and expeditious management of chronic tinnitus.
Methods
Participants
This study was pre-registered with Open Science Framework on 19th August 2022 (DOI 10.17605/OSF.IO/TM4D6) and received ethics approval from the University of South Australia Human Research Ethics Committee (ethics ID: 204857). Participants were provided with a participant information sheet prior to providing written informed consent. Whilst this was a feasibility study, a power calculation was performed based on previous work that explored the reduction of tinnitus through rTMS applied at the left auditory cortex and left DLPFC (Noh et al., Citation2020). To achieve 80% power with an allowable difference of 0.5 and population variance of 0.92 at p = 0.05 (two tailed) for a within group analysis, a total sample size of 12 participants was sought; additionally, accounting for a conservative dropout rate of 25%, we aimed to recruit 15 participants.
Recruitment of participants occurred through the University of South Australia’s (UniSA) Research Volunteers website, advertisement flyers located around UniSA’s campuses, UniSA Health and Medical Clinic as well as several private practices that specialise in Vestibular Rehabilitation. Inclusion criteria required participants to be ≥18 years of age, have a tinnitus duration ≥6 months, be medically stable and able to provide written informed consent. Participants were excluded if there were contraindications to rTMS such as history of seizures, cochlear implants, metal in the brain or skull, cardiac pacemaker (Rossi, Hallett, Rossini, & Pascual-Leone, Citation2011), previous neurosurgical treatment (i.e., craniotomy), previous neurological disease, undergone treatment for tinnitus ≤6 months prior (e.g., CBT or pharmacological intervention), or currently receiving neuroactive medications (i.e., anti-depressants).
Protocol
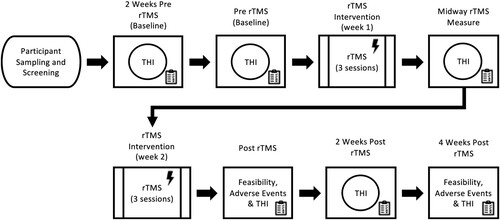
Demographics (age, sex, country of birth) and clinical characteristics (tinnitus duration, location of tinnitus, handedness, and intensity of tinnitus) were recorded. Participants received a total of six rTMS intervention sessions over two weeks.
The Tinnitus Handicap Inventory (THI) was obtained at six different time points; Baseline 1, Baseline 2, Mid-Treatment, Post-Treatment, Follow Up 1, and Follow Up 2. Specifically, Baseline 1 was performed two weeks prior to the commencement of any intervention, Baseline 2 was the day of the first treatment immediately prior to rTMS, Mid-Treatment was after three rTMS sessions, Post-Treatment was immediately following the last rTMS treatment, Follow Up 1 was 2-weeks post intervention and Follow Up 2 was 4-weeks post intervention. Feasibility data and an rTMS adverse reaction questionnaire were completed at both Post-Treatment and Follow up 2 ().
Stimulation
Stimulation was delivered using a Neuro-MS/D rTMS device (Neurosoft Ltd. Ivanova, Russia) that was connected to an oil-cooled, figure-8, coil (wing diameter 70 mm). Resting motor threshold (RMT) was determined by applying adhesive disposable electromyography (EMG) electrodes over the right first dorsal interosseous (FDI) muscle and a ground strap was placed around the same wrist (surface EMG electrodes 22 × 34 mm, FIAB, Florence, Italy). Participants rested their right arm on a pillow in their lap, while sitting upright in a standard chair. The coil was systematically moved in small increments over the left primary motor cortex (M1) to determine the optimal position to evoke motor evoked potentials (MEPs) in the relaxed FDI muscle (Rossini et al., Citation2015). An automated algorithm then determined RMT, defined as the minimum stimulus intensity required to evoke a MEP in the relaxed FDI, with a peak-to-peak amplitude larger than 50 μV in at least 5 out of 10 consecutive trials (pulse frequency 0.2 Hz) (Brunoni et al., Citation2011). All subsequent TMS applications were delivered to the left DLPFC, identified using the Beam method to target the F3 location based on the 10–20 system (Beam, Borckardt, Reeves, & George, Citation2009). A high-frequency rTMS protocol with FDA approval for the management of depression was applied (McClintock et al., Citation2018). For each treatment session participants received 3000 pulses, delivered at 10 Hz (4s on and 11s off; total duration of 18.5 min) at 120% of RMT (Kito et al., Citation2019; McClintock et al., Citation2018). The coil position was constantly monitored throughout the treatment. Participants were asked verbally prior to each session about any change in their responses relating to contraindications of rTMS.
Feasibility
Feasibility of the intervention was evaluated against a pre-defined criteria (). The criteria assessed recruitment rates, treatment adherence, dropout rates and whether participants found the intervention to be useful, helpful, and acceptable. Thresholds for each criterion were defined a-priori to determine if the treatment protocol should proceed without amendment, minor amendment(s), or significant amendment(s).
Table 1. Feasibility pre-defined criteria and thresholds.
Safety
An rTMS adverse reaction questionnaire adapted from Brunoni et al. (Citation2011) and Gillick et al. (Citation2015) was administered immediately after the final rTMS session. The questionnaire sought to determine if any adverse reactions such as seizures, neck pain, sleep issues, and several others were experienced over the past two weeks, with the severity of these events and their relationship to the intervention also documented (Brunoni et al., Citation2011; Fitzgerald & Daskalakis, Citation2022). Adverse events were considered serious if they resulted in hospital admission.
Preliminary efficacy
The THI is a 25 question, self-reported measure of the impact tinnitus has on ADL and QOL (Aksoy, Firat, & Alpar, Citation2007; Zhang et al., Citation2022). The 25 questions assess the patients’ functional limitations, emotional attitude, and thoughts about their tinnitus. Participants completed the THI at six different time points, responding with yes (4-points), sometimes (2-points) or no (0-points), with the resultant 0–100 score determining the severity of tinnitus. There are four degrees of severity, THI score: 0–16 = no handicap; 18–36 = mild handicap; 38–56 = moderate handicap; 58–100 = severe handicap (Salviati et al., Citation2013). The THI questionnaire is widely recognised as a valid measurement of tinnitus severity (Salviati et al., Citation2013; Zhang et al., Citation2022). There is high internal consistency and reliability with Cronbach’s alpha coefficient (0.88) and high intraclass correlation coefficient (ICC, 0.78–0.90) (Aksoy et al., Citation2007). A 7-point change in THI was considered the minimal clinically important difference (MCID) (Zeman et al., Citation2011).
Statistical analysis
Statistical analysis was performed using SPSS software (IBM Corp., V27, Armonk, NY, USA) with the level of significance set at p < 0.05. Where appropriate, statistical assumptions were verified with a Kolmogorov–Smirnov test for normality and Levene’s test for equal variances. Feasibility, safety, participant demographics and clinical characteristics were reported descriptively. To verify test-retest reliability of THI in this sample, and confirm stability of the instrument, an intraclass correlation was performed on THI measures at 2-weeks pre-treatment and immediately pre-treatment. Preliminary treatment efficacy was analysed with a linear mixed model with dependent variables of THI, random factor of ‘Participant’ and fixed factor of ‘Time’ (six assessment time points). Covariates included in the analysis were age, sex, duration and location of tinnitus, and baseline tinnitus severity (baseline THI score). A significant effect of Time was further explored using paired t-tests to compare baseline THI scores with Mid-Treatment, Post-Treatment, Follow Up 1 (2-weeks post treatment) and Follow up 2 (4-weeks post treatment) assessments. A Bonferroni correction was applied to correct for multiple comparisons.
Results
Participants
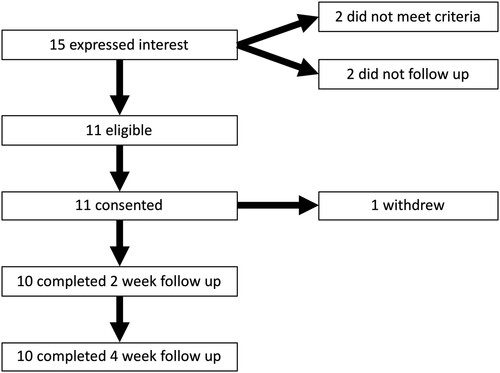
Fifteen people expressed interest in participating in the study; two participants did not meet the inclusion criteria. Of the thirteen meeting the criteria, two did not respond after the initial expression of interest. Therefore, a total of 11 participants between the ages of 26–79 years (8 males; mean age: 56.7 ± 18.4 years) were enrolled in the study, totalling 85% of eligible participants consenting to participate. Participant demographics and clinical characteristics are reported in . One participant withdrew from the study after two rTMS sessions due to stimulation intensity being too uncomfortable for the duration of each session. A total of ten of the initial eleven participants completed the treatment protocol in full (91%) and responded to two-week and four-week follow up questionnaires (91%; ).
Table 2. Participant demographics and clinical characteristics.
Participants’ country of birth included Australia (n = 6), New Zealand (n = 1), England (n = 1), Philippines (n = 1), Hong Kong (n = 1) and Canada (n = 1). Tinnitus location was predominantly experienced bilaterally (n = 8), one participant experienced it left side only, another experienced the right side only and one hearing tinnitus-related sounds coming from inside their head. Participants baseline THI scores varied considerably, ranging from very mild to severe tinnitus handicap. Therefore, baseline THI scores were included in the analysis as a covariate.
Treatment experience
Mean Likert scores immediately post treatment for usefulness were 3.3 ± 1.05, for helpfulness 3.4 ± 1.26, and acceptability 4.6 ± 0.51. Two additional questions exploring whether participants would recommend rTMS and if they would use rTMS again were also asked (4.5 ± 0.52 and 4.4 ± 0.51 respectively). The proportion of participants that scored ≥4/5 for usefulness was 40%, helpfulness 50%, and acceptability 100%, recommend treatment and use again were both 100%. Four-week post treatment mean Likert scores were usefulness 3.5 ± 1.08, helpfulness 3.3 ± 1.05, acceptability 4.7 ± 0.67, participant recommendation 4.0 ± 0.66 and use treatment again 3.9 ± 0.87. Participants who scored ≥4/5 were usefulness 60%, helpfulness 60%, acceptability 90%, recommend treatment and use again were both 80% respectively.
Safety
No significant adverse events requiring hospital admission occurred. Four participants reported adverse reactions after receiving rTMS. One participant reported mild headaches and a lack of sleep; however, they were unsure if these were related to rTMS. One participant reported experiencing headaches, nausea and difficulty concentrating. The final two participants reported hearing problems and scalp pain, these participants considered their symptoms possibly related to rTMS (). All participants considered these adverse events to be mild.
Table 3. Adverse reactions reported by participants.
Preliminary efficacy
THI scores were stable between the two baseline assessments (ICC = 0.85, 95%CI 0.44–0.96, p = 0.003). Next, we investigated the change in THI across the study. There was a significant effect on Time (F(5,45.00) = 7.30, p = <0.001; ). To explore the effect of Time, we first determined that there was no change between THI baselines (t(9) = 1.087 p = 0.31). Since baseline values were consistent and similar, this response was averaged resulting in a mean baseline THI = 22.7 ± 11.44. This mean baseline was compared against the remaining 4 time points. After adjusting for multiple comparisons there was a significant decrease in THI at Mid-Treatment (t(9) = −3.47, p = 0.028) and Follow up 2 (t(9) = −3.62, p = 0.024). THI did decrease at Post-Treatment (t(9) = −2.64, p = 0.108) and Follow up 1 (t(9) = −2.86, p = 0.076), but neither reached significance after Bonferroni correction. Finally, change in THI scores at Mid-Treatment, Post-Treatment, Follow up 1 and Follow up 2 all exceeded the minimal clinical significant change score ().
Figure 3. Effect of rTMS treatment on THI scores compared from mean THI baseline. 
 : significantly different from mean BL; –: MCID reduction of 7-points from mean BL. Abbreviations: THI, Tinnitus Handicap Inventory; rTMS, Repetitive Transcranial Magnetic Stimulation; BL, Baseline; FU1, Follow Up 1 (2-weeks post treatment); FU2, Follow up 2 (4-weeks post treatment); MCID, Minimal Clinically Important Difference.
: significantly different from mean BL; –: MCID reduction of 7-points from mean BL. Abbreviations: THI, Tinnitus Handicap Inventory; rTMS, Repetitive Transcranial Magnetic Stimulation; BL, Baseline; FU1, Follow Up 1 (2-weeks post treatment); FU2, Follow up 2 (4-weeks post treatment); MCID, Minimal Clinically Important Difference.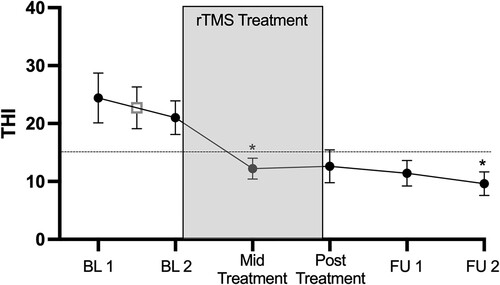
Table 4. Results for test of linear mixed model analysis.
Discussion
The purpose of this study was to explore the feasibility, safety and preliminary efficacy of an rTMS protocol, designed for depression, as a treatment for chronic subjective tinnitus in healthy adults. Recent studies have identified tinnitus as a network system involving multiple cerebral areas (Noh et al., Citation2020). Whilst studies have explored rTMS at several cortical sites (De Ridder et al., Citation2013; Langguth et al., Citation2014; Noh et al., Citation2020; Yang et al., Citation2021; Zenner et al., Citation2017), to our knowledge this is the first study that has applied rTMS solely to the left DLPFC. Modulation and suppression of tinnitus may stem from the DLPFC through an inhibitory connection with the primary auditory cortex (Noh et al., Citation2020). We found that high-frequency rTMS delivered to the left DLPFC was feasible against 5 out of 7 pre-defined feasibility criteria. rTMS delivery was safe, with no serious adverse events reported, and preliminary efficacy suggested that this protocol led to a significant reduction in tinnitus symptoms that exceeded the MCID (Zeman et al., Citation2011).
Five out of seven pre-defined feasibility criteria were met. While usefulness and helpfulness thresholds were not met, it is important to note that they were only marginally below the ≥4/5 threshold. It was difficult to determine why feasibility for usefulness and helpfulness were below this a-priori threshold, especially considering the majority of participants would recommend and use the treatment again. It is possible that perceptions of usefulness and helpfulness were guided by preconceived treatment expectations. However, this is inconclusive as determining participants knowledge of rTMS prior to the study and reasoning for perceptions of usefulness and helpfulness post intervention was not performed. We were cautious to not outline the benefits of rTMS extensively throughout the participant information sheet, allowing participants to develop their own opinions of the intervention. Future studies should continue to explore patient treatment experiences, with particular emphasis on further exploration of usefulness and helpfulness.
The adverse events that participants experienced during this study were minor and are commonly associated with rTMS (Hordacre et al., Citation2021; Lehner, Schecklmann, Greenlee, Rupprecht, & Langguth, Citation2016). These minor adverse events do not necessarily pose a problem and usually subside within days, or possibly weeks, post treatment (Fitzgerald & Daskalakis, Citation2022). No serious adverse events occurred during this study which may in part have been assisted by screening for contraindications prior to any rTMS as part of the eligibility criteria. Although rare, serious adverse events such as seizures and syncope can occur. It is reported that less than 1 seizure in 60,000 rTMS sessions may occur even when following rTMS guidelines and screening for risk factors appropriately (Kim & Oh, Citation2013; Lerner, Wassermann, & Tamir, Citation2019). However, few studies have documented adverse events and their relationship to TMS. This study and previous literature exhibit similar common adverse reactions to rTMS (i.e., scalp pain, headaches, sleep problems and more) (Attal et al., Citation2021; Hordacre et al., Citation2021; Lehner et al., Citation2016; McNamara et al., Citation2001). Future studies should continue to screen for contraindications to rTMS, along with reporting any adverse events experienced by participants for the duration of the study to inform potential amendments to treatment protocol and monitor participant safety.
One participant withdrew from the study after participating in only two rTMS sessions, due to their high RMT resulting in stimulation intensity being too uncomfortable. Some studies have found operating at the high percentage of RMT i.e., 120%, to be suitable with no or minimal adverse events (Ahmadpanah et al., Citation2023; Hordacre et al., Citation2021; Lehner et al., Citation2016; Mehta et al., Citation2022). However, it is reasonable to assume lower stimulation intensities might be more comfortable for participants. Moreover, a rTMS protocol applied at 80% of RMT has still shown efficacy in stimulating the auditory cortex (De Ridder et al., Citation2013). Furthermore, Nahas et al. (Citation2001) demonstrated that a 1 Hz TMS applied at 80, 100 and 120% of RMT can initiate varied cortical activation. With regards to the participant who withdrew due to pain and discomfort from the higher stimulation intensity; it may be that reducing treatment intensity below 120% of RMT may have been better tolerated. Future studies might consider exploring whether modification of the rTMS treatment intensity below 120% of RMT could allow for greater participant adherence and safety. Even if lower stimulation intensity reduces the treatment effect (Fitzgerald, Hoy, Anderson, & Daskalakis, Citation2016), that it is better tolerated and offers at least some benefit would be promising. From a clinical perspective, this could mean patient comfort is one factor in setting treatment intensity. Alternatively, continuing to provide rTMS treatment at 120% RMT might require consideration moving forward. This would include adjusting the trial sample size for potential drop-out, and there may be a benefit in providing greater warning to participants of likely sensations experienced during stimulation.
Preliminary efficacy data suggests rTMS to the left DLPFC might be beneficial for tinnitus. There are several points to consider. First, we observed a significant effect of time that coincided with the delivery of rTMS. However, as this was a feasibility study, rather than a treatment efficacy trial; we caution about over interpreting the effect of rTMS to improve tinnitus. Without a sham rTMS control group it is difficult to be certain improvements in tinnitus were related to rTMS. In progressing to trial, our data indicates a sample of 19 participants per group might be required based on a change in THI from baseline to post-treatment (mean difference THI = 8.4 points (SD 9.3), 95% power, p < 0.05). Second, if we accept rTMS might help reduce symptoms of tinnitus, it is interesting that our data indicated the change in THI scores exceeded MCID after just three rTMS sessions and was then sustained throughout the study. One interpretation from this result may be that three rTMS sessions are as effective as six. However, we would caution against this conclusion as it may be that the sustained improvement of tinnitus observed at 4-weeks post intervention may not have occurred with only three treatment sessions. Noh et al. (Citation2020) demonstrated similar results where a clinically significant change was observed in THI scores at their 4-week follow up. They targeted cortical regions, administering 2000 pulses over the auditory cortex and 1000 pulses over the DLPFC at 110% RMT for four sessions, over four consecutive days (Noh et al., Citation2020). Two other control groups (auditory cortex only) and (sham) showed no effect on THI (Noh et al., Citation2020). Moreover, low frequency (1 Hz) TMS stimulation has shown some effect; however, treatment protocols have consisted of at least 10 sessions and have not targeted the left DLPFC independently (De Ridder et al., Citation2013; Yang et al., Citation2021). Further studies are required, in order to understand the optimal number of rTMS sessions to improve symptoms of tinnitus and maintain treatment effect. Finally, this study evaluated the stability of the THI between the two baseline assessments. We were able to demonstrate an ICC suggesting good test-retest reliability which was consistent with previous literature (Aksoy et al., Citation2007). Given good reliability, and that the THI has been widely used in previous literature (Hall et al., Citation2016), this measure appears suitable for future trials. However, additional measures might supplement the understanding of treatment response. The THI was developed to measure the impact of tinnitus on everyday function (Newman, Jacobson, & Spitzer, Citation1996). Newer measures, such as the tinnitus functional index or tinnitus primary function questionnaire, appear more sensitive to change with treatment and should be considered (Henry et al., Citation2016; Tyler et al., Citation2014). Further, given the acute change in tinnitus symptoms from interventions such as rTMS, rating scales for intensity or annoyance might also be suitable to measure immediate effect (Raj-Koziak et al., Citation2018). It would seem necessary to use several measures to provide a comprehensive assessment of treatment response.
We identified several limitations in this study. First, although a power calculation from previous studies was performed our sample size was still considered to be small. Second, only two follow up questionnaires were administered post intervention. While therapeutic benefits appeared to persist to 4-weeks, we are unclear as to precisely how long improvements in tinnitus might last. To document longer-term treatment effects beyond 4-weeks, we recommend that future studies conduct longer follow up measures over several months. Third, we did not have access to magnetic resonance imaging data for each participant to assist in the location of the left DLFPC, instead relying on the Beam method (Beam et al., Citation2009). Although the Beam method is used extensively to locate the F3 position for rTMS, we acknowledge that this may not be as precise as individual anatomical data from neuroimaging to guide treatment. It is possible that the preliminary efficacy data may have produced an even greater treatment effect had we been able to precisely locate the left DLPFC with neuroimaging. Finally, as we did not have a sham control group in this study, the efficacy of our results should be interpreted cautiously.
Conclusion
In conclusion, rTMS applied to the left DLPFC to manage tinnitus was safe, feasible, and well tolerated with no serious adverse events. Improvement in THI scores were observed and sustained throughout the study. However, results should be interpreted cautiously as there was no sham control group. Future research may consider protocol amendments to provide further insight into patients experience with this treatment, and possibly explore adjusting stimulation intensity to maximise participant comfort during treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets generated during used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Reference
- Ahmadpanah, M., Amini, S., Mazdeh, M., Haghighi, M., Soltanian, A., Jahangard, L., Keshavarzi, A., & Brand, S. (2023). Effectiveness of repetitive transcranial magnetic stimulation (rTMS) add-on therapy to a standard treatment in individuals with multiple sclerosis and concomitant symptoms of depression—Results from a randomized clinical trial and pilot study. Journal of Clinical Medicine, 12(7), 2525.
- Aksoy, S., Firat, Y., & Alpar, R. (2007). The Tinnitus Handicap Inventory: A study of validity and reliability. The International Tinnitus Journal, 13(2), 94–98.
- Attal, N., Poindessous-Jazat, F., De Chauvigny, E., Quesada, C., Mhalla, A., Ayache, S. S., Fermanian, C., Nizard, J., Peyron, R., Lefaucheur, J. P., & Bouhassira, D. (2021). Repetitive transcranial magnetic stimulation for neuropathic pain: A randomized multicentre sham-controlled trial. Brain, 144(11), 3328–3339.
- Baldo, P., Doree, C., Molin, P., McFerran, D., Cecco, S., & Baldo, P. (2012). Antidepressants for patients with tinnitus. Cochrane Database of Systematic Reviews, 2012(9), CD003853.
- Bartels, H., Staal, M. J., & Albers, F. W. J. (2007). Tinnitus and neural plasticity of the brain. Otology & Neurotology, 28(2), 178–184.
- Beam, W., Borckardt, J. J., Reeves, S. T., & George, M. S. (2009). An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimulation, 2(1), 50–54.
- Beukes, E. W., Andersson, G., Fagelson, M., & Manchaiah, V. (2022). Internet-based audiologist-guided cognitive behavioral therapy for tinnitus: Randomized controlled trial. Journal of Medical Internet Research, 24(2), e27584–e27584.
- Brunoni, A. R., Amadera, J., Berbel, B., Volz, M. S., Rizzerio, B. G., & Fregni, F. (2011). A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology, 14(8), 1133–1145.
- De Ridder, D., Song, J. J., & Vanneste, S. (2013). Frontal cortex TMS for tinnitus. Brain Stimulation, 6(3), 355–362.
- Esmaili, A. A., & Renton, J. (2018). A review of tinnitus. Australian Journal of General Practice, 47(4), 205–208.
- Fitzgerald, P. B., & Daskalakis, Z. J. (2022). RTMS treatment for depression: A practical guide (P. B. Fitzgerald & Z. Jeff Daskalakis, Eds.; 2nd ed.). Cham, Switzerland: Springer.
- Fitzgerald, P. B., Hoy, K. E., Anderson, R. J., & Daskalakis, Z. J. (2016). A study of the pattern of response to rTMS treatment in depression. Depression Anxiety, 33(8), 746–753.
- Fuller, T., Cima, R., Langguth, B., Mazurek, B., Vlaeyen, J. W. S., & Hoare, D. J. (2020). Cognitive behavioural therapy for tinnitus. Cochrane Database of Systematic Reviews, 2020(1), CD012614.
- Gillick, B. T. P. M. P. T., Krach, L. E. M. D., Feyma, T. M. D., Rich, T. L. O. T. L. R., Moberg, K. O. T. L. R., Menk, J. M. S., Cassidy, J. D. P. T., Kimberley, T. P. P. T., & Carey, J. R. P. P. T. (2015). Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Archives of Physical Medicine and Rehabilitation, 96(4), S104–S113.
- Hall, D. A., Haider, H., Szczepek, A. J., Lau, P., Rabau, S., Jones-Diette, J., Londero, A., Edvall, N. K., Cederroth, C. R., Mielczarek, M., Fuller, T., Batuecas-Caletrio, A., Brueggemen, P., Thompson, D. M., Norena, A., Cima, R. F., Mehta, R. L., & Mazurek, B. (2016). Systematic review of outcome domains and instruments used in clinical trials of tinnitus treatments in adults. Trials, 17(1), 270.
- Health, D. o. (2021). Repetitive transcranial magnetic stimulation therapy on the MBS. Retrieved April 20, 2022.
- Henry, J. A., Griest, S., Thielman, E., McMillan, G., Kaelin, C., & Carlson, K. F. (2016). Tinnitus functional index: Development, validation, outcomes research, and clinical application. Hearing Research, 334, 58–64.
- Hordacre, B., Comacchio, K., Williams, L., & Hillier, S. (2021). Repetitive transcranial magnetic stimulation for post-stroke depression: A randomised trial with neurophysiological insight. Journal of Neurology, 268(4), 1474–1484.
- Isenberg, K., Downs, D., Pierce, K., Svarakic, D., Garcia, K., Jarvis, M., North, C., & Kormos, T. C. (2005). Low frequency rTMS stimulation of the right frontal cortex is as effective as high frequency rTMS stimulation of the left frontal cortex for antidepressant-free, treatment-resistant depressed patients. Annals of Clinical Psychiatry, 17(3), 153–159.
- Jarach, C. M., Lugo, A., Scala, M., van den Brandt, P. A., Cederroth, C. R., Odone, A., Garavello, W., Schlee, W., Langguth, B., & Gallus, S. (2022). Global prevalence and incidence of tinnitus: A systematic review and meta-analysis. JAMA Neurology, 79(9), 888–900.
- Kaltenbach, J. A. (2000). Neurophysiologic mechanisms of tinnitus. Journal of the American Academy of Audiology, 11(3), 125–137.
- Kim, Y. K., & Oh, S. Y. (2013). Chapter 1 - Transcranial magnetic stimulation: A brief conspectus and area of therapeutic application. In L. Alba-Ferrara (Ed.), Transcranial magnetic stimulation: Methods, clinical uses and effects on the brain (pp. 1–26). Nova Science Publishers.
- Kito, S., Miyazi, M., Nakatani, H., Matsuda, Y., Yamazaki, R., Okamoto, T., & Igarashi, Y. (2019). Effectiveness of high-frequency left prefrontal repetitive transcranial magnetic stimulation in patients with treatment-resistant depression: A randomized clinical trial of 37.5-minute vs 18.75-minute protocol. Neuropsychopharmacology Reports, 39(3), 203–208.
- Kreuzer, P. M., Poeppl, T. B., Rupprecht, R., Vielsmeier, V., Lehner, A., Langguth, B., & Schecklmann, M. (2017). Individualized repetitive transcranial magnetic stimulation treatment in chronic tinnitus? Frontiers in Neurology, 8, 126.
- Langguth, B., & Elgoyhen, A. B. (2012). Current pharmacological treatments for tinnitus. Expert Opinion on Pharmacotherapy, 13(17), 2495–2509.
- Langguth, B., Landgrebe, M., Frank, E., Schecklmann, M., Sand, P. G., Vielsmeier, V., Hajak, G., & Kleinjung, T. (2014). Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. The World Journal of Biological Psychiatry, 15(4), 276–285.
- Langguth, B., Landgrebe, M., Kleinjung, T., Sand, G. P., & Hajak, G. (2011). Tinnitus and depression. The World Journal of Biological Psychiatry, 12(7), 489–500.
- Leaver, A. M., Seydell-Greenwald, A., Turesky, T. K., Morgan, S., Kim, H. J., & Rauschecker, J. P. (2012). Cortico-limbic morphology separates tinnitus from tinnitus distress. Frontiers in Systems Neuroscience, 6, 21–21.
- Lehner, A., Schecklmann, M., Greenlee, M. W., Rupprecht, R., & Langguth, B. (2016). Triple-site rTMS for the treatment of chronic tinnitus: A randomized controlled trial. Scientific Reports, 6, 22302.
- Lerner, A. J., Wassermann, E. M., & Tamir, D. I. (2019). Seizures from transcranial magnetic stimulation 2012–2016: Results of a survey of active laboratories and clinics. Clinical Neurophysiology, 130(8), 1409–1416.
- Makar, S. K. (2021). Etiology and pathophysiology of tinnitus-A systematic review. The International Tinnitus Journal, 25(4), 87–97.
- McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., Cook, I. A., O'Reardon, J., Husain, M. M., Wall, C., Krystal, A. D., Sampson, S. M., Morales, O., Nelson, B. G., Latoussakis, V., George, M. S., Lisanby, S. H. (2018). Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. Journal of Clinical Psychiatry, 79(1).
- McNamara, B., Ray, J. L., Arthurs, O. J., & Boniface, S. (2001). Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychological Medicine, 31(7), 1141–1146.
- Mehta, S. S., Downar, J. J., Mulsant, B. H. B. H., Voineskos, D. D., Daskalakis, Z. J. Z. J., Weissman, C. R. C. R., Vila-Rodriguez, F. F., & Blumberger, D. M. D. M. (2022). The effect of high frequency versus theta-burst repetitive transcranial magnetic stimulation on suicidality in patients with treatment resistant depression: The effect of 10 Hz rTMS vs. iTBS on suicidality. Acta Psychiatrica Scandinavica, 145(5), 529–538.
- Møller, A. R. (2008). Neural plasticity: For good and bad. Progress of Theoretical Physics Supplement, 173, 48–65.
- Moller, A. R. (2016). Sensorineural tinnitus: Its pathology and probable therapies. International Journal of Otolaryngology, 2016, 1–13.
- Møller, A. R., Langguth, B., DeRidder, D., & Kleinjung, T. (2011). Textbook of tinnitus (A. R. Møller, B. Langguth, D. DeRidder, & T. Kleinjung, Eds., 1st 2011 ed.). New York, NY: Springer.
- Moroe, N. F., & Khoza-Shangase, K. (2014). The impact of tinnitus on daily activities in adult tinnitus sufferers: A pilot study. The South African Journal of Communication Disorders, 61(1).
- Nahas, Z., Lomarev, M., Roberts, D. R., Shastri, A., Lorberbaum, J. P., Teneback, C., McConnell, K., Vincent, D. J., Li, X., George, M. S., & Bohning, D. E. (2001). Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biological Psychiatry, 50(9), 712–720.
- Newman, C. W., Jacobson, G. P., & Spitzer, J. B. (1996). Development of the Tinnitus Handicap Inventory. Archives of Otolaryngology–Head & Neck Surgery, 122(2), 143–148.
- Noh, T.-S., Kyong, J.-S., Park, M. K., Lee, J. H., Oh, S. H., & Suh, M.-W. (2020). Dual-site rTMS is more effective than single-site rTMS in tinnitus patients: A blinded randomized controlled trial. Brain Topography, 33(6), 767–775.
- Oakes, P., Loukas, M., Oskouian, R. J., & Tubbs, R. S. (2017). The neuroanatomy of depression: A review. Clinical Anatomy, 30(1), 44–49.
- Raj-Koziak, D., Gos, E., Swierniak, W., Rajchel, J. J., Karpiesz, L., Niedzialek, I., Wlodarczyk, E., Skarzynski, H., & Skarzynski, P. H. (2018). Visual analogue scales as a tool for initial assessment of tinnitus severity: Psychometric evaluation in a clinical population. Audiology and Neurootology, 23(4), 229–237.
- Rossi, S., Hallett, M., Rossini, P. M., & Pascual-Leone, A. (2011). Screening questionnaire before TMS: An update. Clinical Neurophysiology, 122(8), 1686.
- Rossini, P. M., Burke, D., Chen, R., Cohen, L. G., Daskalakis, Z., Di Iorio, R., Di Lazzaro, V., Ferreri, F., Fitzgerald, P. B., George, M. S., Hallett, M., Lefaucheur, J. P., Langguth, B., Matsumoto, H., Miniussi, C., Nitsche, M. A., Pascual-Leone, A., Paulus, W., Rossi, S., ... Ziemann, U. (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology, 126(6), 1071–1107.
- Salazar, J. W., Meisel, K., Smith, E. R., Quiggle, A., McCoy, D. B., & Amans, M. R. (2019). Depression in patients with tinnitus: A systematic review. Otolaryngology—Head and Neck Surgery, 161(1), 28–35.
- Salviati, M. M. D., Macrì, F. M. D., Terlizzi, S. M. D., Melcore, C. M. D., Provenzano, A. M. D., Capparelli, E., Altissimi, G. M. D., & Cianfrone, G. M. D. (2013). The Tinnitus Handicap Inventory as a screening test for psychiatric comorbidity in patients with tinnitus. Psychosomatics, 54(3), 248–256.
- Schutter, D. J. L. G. (2009). Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: A meta-analysis. Psychological Medicine, 39(1), 65–75.
- Searchfield, G. D., & Zhang, J. (2021). The behavioral neuroscience of tinnitus. (G. D. Searchfield & J. Zhang, Eds.). Cham, Switzerland: Springer.
- Tyler, R., Ji, H., Perreau, A., Witt, S., Noble, W., & Coelho, C. (2014). Development and validation of the tinnitus primary function questionnaire. American Journal of Audiology, 23(3), 260–272.
- Ukaegbe, O. C., Orji, F. T., Ezeanolue, B. C., Akpeh, J. O., & Okorafor, I. A. (2017). Tinnitus and its effect on the quality of life of sufferers: A Nigerian cohort study. Otolaryngology—Head and Neck Surgery, 157(4), 690–695.
- Watts, E. J., Fackrell, K., Smith, S., Sheldrake, J., Haider, H., & Hoare, D. J. (2018). Why Is tinnitus a problem? A qualitative analysis of problems reported by tinnitus patients. Trends in Amplification, 22, 2331216518812250.
- Yang, H., Cheng, G., Liang, Z., Deng, W., Huang, X., Gao, M., & Zheng, Y. (2021). Efficacy of repetitive transcranial magnetic stimulation (rTMS) for tinnitus: A retrospective study. Ear, Nose, & Throat Journal, 102(10), 1455613211016896.
- Zeman, F., Koller, M., Figueiredo, R., Aazevedo, A., Rates, M., Coelho, C., Kleinjung, T., de Ridder D., Langguth, B., & Landgrebe, M. (2011). Tinnitus Handicap Inventory for evaluating treatment effects: Which changes are clinically relevant? Otolaryngology—Head and Neck Surgery, 145(2), 282–287.
- Zenner, H.-P., Delb, W., Kröner-Herwig, B., Jäger, B., Peroz, I., Hesse, G., Mazurek, B., Goebel, G., Gerloff, C., Trollmann, R., Biesinger, E., Seidler, H., & Langguth, B. (2017). A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. European Archives of Oto-Rhino-Laryngology, 274(5), 2079–2091.
- Zhang, J., Huo, Y., Lui, G., Li, M., Tyler, R. S., & Ping, H. (2022). Reliability and validity of the Tinnitus Handicap Inventory: A clinical study of questionnaires. The Journal of international Advanced Otology, 18(6), 522–529.
- Ziai, K., Moshtaghi, O., Mahboubi, H., & Djalilian, H. R. (2017). Tinnitus patients suffering from anxiety and depression: A review. The International Tinnitus Journal, 21(1), 68–73.
- Zöger, S., Svedlund, J., Holgers, K.-M., 2006. The effects of sertraline on severe tinnitus suffering – A randomized, double-blind, placebo-controlled study. Journal of Clinical Psychopharmacology 26(1), 32–39.