ABSTRACT
In this study, information on injectable anticancer drug use and additional fee for enhanced collaboration (AEC) and additional fee for specific drug management guidance 2 (ASD2) claims from the NDB Open Data Japan (NODJ) dataset and the number of patients with cancer according to sex and age from the National Cancer Registry (NCR) dataset were integrated and evaluated to determine the current status and challenges in pharmacist interventions for patients receiving cancer treatment. The NODJ data, including receipt data billed from 2020 to 2021, were obtained from the Ministry of Health, Labour and Welfare website. The use of injectable anticancer drugs decreased relative to the number of cancer patients aged ≥ 75 years compared to those aged < 75 years. Regarding injectable anticancer drug use, the number of AEC claims was similar between men and women, but the number of ASD2 claims was lower in men than in women. The number of times community pharmacists claimed their ASD2 was approximately 5% of the number of times hospital pharmacists claimed their AEC. This study revealed that several patients did not receive sufficient guidance from community pharmacists compared to hospital pharmacists, suggesting a potential insufficiency in the collaboration between the two groups.
Background
According to the World Health Organization (WHO), cancer is the second leading cause of death globally, accounting for an estimated 10 million deaths, or 1 in 6 deaths, in 2020 (World Health Organization, Citationn.d.). In Japan, cancer has been the leading cause of death since 1981, and the estimated number of cancer deaths in Japan in 2022 was approximately 380,400, or approximately one in three people, die from cancer, and approximately one in two people will be diagnosed with cancer in their lifetime (Foundation for Promotion of Cancer Research, Citation2023). In Japan, the average length of stay for patients discharged from cancer-specialized hospitals and general clinics was 35.7 days in 2002 and 17.1 days in 2017 (Ministry of Health, Labour and Welfare, Citation2021b). The number of patients with cancer has increased from 139,400 inpatients and 119,700 outpatients in 2002 to 126,100 inpatients and 183,600 outpatients in 2017, with more patients receiving outpatient treatment (Ministry of Health, Labour and Welfare, Citation2021b). Given that side effects associated with outpatient chemotherapy are diverse and occur at different times (Ministry of Health, Labour and Welfare, Citation2009, Citation2019, Citation2021a, Citation2022a, Citation2023).
Multidisciplinary care is organised around the patient, and medical professionals collaborate and have access to a variety of shared information. In Japan, hospital pharmacists interview patients before visiting their doctors to check for side effects, suggest dosage adjustments of anticancer drugs, and provide supportive care; the efficacy of this approach has been reported (Kimura et al., Citation2017; Tanaka et al., Citation2019; Yoshimi et al., Citation2013).
There are several reports of community pharmacists monitoring adverse events and providing feedback to hospital pharmacists that have been useful (Rubira et al., Citation2021; Urakawa et al., Citation2021). A survey conducted in France reported that patients are willing to share information with community pharmacists in cancer treatment, to report side effects to the hospital, and to take effective solutions for their management (Hébert et al., Citation2018). In addition, there have been several reports on the early detection of side effects and changes in prescriptions during outpatient anticancer treatment owing to the involvement of pharmacists (Colombo et al., Citation2017; Gatwood et al., Citation2017; Herledan et al., Citation2023a, Citation2023b). To the best of our knowledge, reports about the involvement of community pharmacists with patients undergoing outpatient chemotherapy are rare.
Japan has universal health insurance; hence, all residents are required to be covered by some form of public insurance. The work of healthcare professionals is paid for by the medical and dispensing fees collected. In 2015, the Ministry of Health, Labour and Welfare (MHLW) presented the ‘Pharmacy Vision for Patients.’ It stated that it is important for community pharmacists to communicate with patients, provide patient guidance to improve adherence and avoid adverse events, and report to physicians on medication and side effects (Ministry of Health, Labour and Welfare, Citation2015). The Basic Plan for the Promotion of Cancer Control (2017) stated that the necessary measures should be taken to strengthen cooperation between hospitals and pharmacies to support outpatients undergoing drug therapy in medication management and countermeasures against adverse effects (Ministry of Health, Labour and Welfare, Citation2018). Therefore, in April 2020, an ‘additional fee for enhanced collaboration’ (AEC) was established for hospital pharmacist services as an addition to the medical treatment fee. An ‘additional fee for specific drug management guidance 2’ (ASD2) was then established for community pharmacist services aside from the pharmacy administrative fee (Ministry of Health, Labour and Welfare, Citationn.d.-e). These additional fees are paid when a pharmacist provides special patient guidance in addition to basic services for patients with cancer.
The AEC and ASD2 focus on developing facilities and equipment systems for the safe delivery of outpatient chemotherapy and collaboration between hospital and community pharmacists to manage the side effects experienced by patients. Therefore, this additional fee indicates that pharmacists contribute to safe drug treatment.
The MHLW started operating the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) by enacting the Act on Assurance of Medical Care for Elderly People in 2008. The NDB stores data on health insurance claims and specific health checkups (Ministry of Health, Labour and Welfare, Citationn.d.-d). The NDB is one of the largest health-related databases worldwide, containing digitised data on health insurance claims and specific health checkup data collected from all medical institutions, including hospitals, clinics, pharmacies, and dental clinics, covering medical information on almost all individuals (over 100 million) in Japan. The NDB has been used in multiple studies in health economics, pharmacoepidemiology, clinical epidemiology, and other fields. The MHLW released the NDB Open Data Japan (NODJ) online, which provides various summary tables from the NDB that have been freely available to the general public since 2016. The NODJ includes basic summary tables created from medical inpatient claims, medical outpatient claims, diagnosis procedure combination (DPC) claims, dispensing claims, dental inpatient claims, dental outpatient claims, and specific health checkups (Ministry of Health, Labour and Welfare, Citation2022b). The NODJ has been used in several studies to identify trends in drugs prescribed by the Japanese insurance system (Mukai et al., Citation2020; Suzuki et al., Citation2023; Tanaka et al., Citation2022; Tanito, Citation2022). Furthermore, Japan enacted the Cancer Registration Promotion Act in 2013, where the registration of diagnosed cancer cases was started in 2016 under the new National Cancer Registry (NCR) system (Ministry of Health. Labour and Welfare, Citationn.d.-a). The results of this survey are publicly available and can be obtained from the MHLW and the e-Stat website.
In this study, information on injectable anticancer drug usage, AEC, and ASD2 from the NODJ dataset and the number of patients with cancer according to sex, age, and cancer site from the NCR dataset were integrated and evaluated to determine the current status and problems associated with pharmacist interventions.
Methods
Data sources
1) National Database of Health Insurance Claims and Specific Health Checkups of Japan Open Data Japan
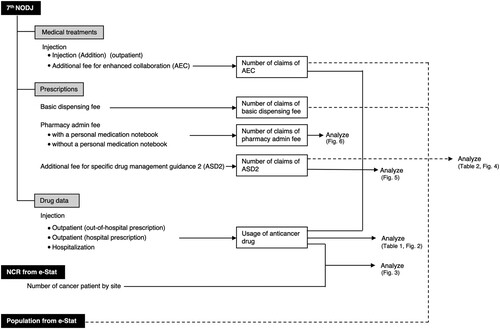
The 7th NODJ dataset, which includes receipt data billed from April 2020 to March 2021, was obtained from the MHLW website (Ministry of Health. Labour and Welfare, Citationn.d.-f). The 7th NODJ includes eight tables containing statistics on ‘medical treatments,’ ‘prescriptions,’ and ‘drug data,’ among others. In the ‘medical treatments’ table, the number of medical treatments, classified into ‘basic medical fee,’ ‘medical management,’ and other parameters, stratified by prefecture, sex, age, and month of treatment, were summarised according to the score table prepared by the MHLW.
The ‘prescriptions’ table summarises the number of claims for each dispensing act, stratified by ‘prefecture,’ ‘sex,’ and ‘age.’
In the ‘drug data’ table, the top 100 drugs regarding prescription quantity for each drug category were summarised by prefecture, sex, and age, based on information from medical inpatient/outpatient receipts, DPC receipts, and dispensing receipts. The ‘drug data’ tables were classified by dosage form: ‘oral,’ ‘topical,’ and ‘injection.’ The prescription quantities were reported as the number of days (times) multiplied by the amount used per day (per dose).
2) National Cancer Registry (NCR) system
In the NCR system, cancer sites are classified according to the WHO International Statistical Classification of Diseases and Related Health Problems (ICD) (Ministry of Health, Labour and Welfare, Citationn.d.-b). The NCR registers primary cancers; if two or more independent cancers are detected in one person, each one is counted independently.
The number of patients with cancer categorised according to cancer site in 2019 was obtained from the NCR ‘incidence by age group: by the site and sex (excluding epithelial cancers)’ (Data Table 2019_2-a), which is available on the e-Stat website. The classification of cancer sites followed the C00 – C96 coding system. The number of patients was aggregated based on 5-year age groups for each sex. The population data were obtained from the 2020 ‘Population by Year, Sex, and Age’ from the Current Population Survey available on the e-Stat website (Statistics Bureau of Japan, Citationn.d.).
Medical remuneration points
1) Additional fee for enhanced collaboration (AEC)
The AEC can be claimed when a hospital pharmacist is notified, based on the instructions provided by the physician, to provide patients receiving injectable outpatient chemotherapy with a document on the occurrence of side effects and treatment plans and provide the necessary guidance based on the patient’s condition. The contents of the document include the implementation status of the treatment regimen, dosage of anticancer agents, and the occurrence of major adverse reactions (including the severity scale [grade] of adverse reactions based on the Common Terminology Criteria for Adverse Events v5.0 Japanese translation JCOG [Japan Clinical Oncology Group] version and the results of relevant blood and biochemical tests). Hospital pharmacists instruct patients to present this document to community pharmacists. Several patients also carry a personal medication notebook. This notebook contains the patient’s medication history, including prescription medications and allergy and adverse reaction history. The personal medication notebook is also submitted when patients present a prescription at a community pharmacy. The community pharmacist may record the prescribed medication before returning the personal medication notebook to the patient. When claiming the AEC, the hospital pharmacist is supposed to use the medication notebook or other documentation to inform the community pharmacist of the patient’s treatment status. The conditions for the claim include making chemotherapy regimens available on the website and conducting training sessions for pharmacists and others working in community pharmacies at least once a year. The AEC is claimed at 150 points once a month. A point is added to each item for each medical procedure performed. The unit price per point is claimed at 0.67 USD (10 JPY).
For the AEC, the number of claims for medical practice (code: 130013570) was extracted from the data table ‘000986909.xlsx,’ which is published in ‘Number of claims by sex and age’ of item G (injection, injection [addition]) of ‘medical treatment.’ (Ministry of Health. Labour and Welfare, Citationn.d.-f, Table S1).
2) Additional fee for specific drug management guidance 2 (ASD2)
The ASD2 can be claimed when a community pharmacist confirms the patient regimen, provides the necessary pharmacological management and guidance, confirms the medication status of the patients and the presence of side effects via telephone or other means, and provides the hospital with the necessary information in writing. The conditions include that the community pharmacist must participate at least once a year in a hospital training session related to chemotherapy with antineoplastic agents to be able to guide patient in collaboration with hospital pharmacists. The ASD2 can be claimed at 100 points once a month.
The basic dispensing fee, dispensing fee, and pharmacy admin fee are stated in the ‘prescriptions’ section. The number of prescriptions for the ‘basic dispensing fee’ and ASD2 were extracted from the data table ‘000987605.xlsx,’ which is published in ‘Number of claims by sex and age’ under ‘prescriptions’ (Ministry of Health. Labour and Welfare, Citationn.d.-f, Table S2). Given that the basic dispensing fee represents the points claimed upon accepting a prescription at a pharmacy, it can be considered as the number of accepted prescriptions. The total number of times the basic dispensing fee was claimed is the sum of the dispensing act codes 410004110, 410004210, 410004610, 410004810, and 410005410. ASD2 was listed as ‘pharmacy admin fee (addition and subtraction).’ For ASD2, the total number of claims was based on the dispensing act codes 440008670 and 440009370. When a patient who brought a prescription within the past three months also brings their personal medication notebook, the pharmacy claims the pharmacy admin fee using the dispensing act codes 440007810 and 440008910. However, if the patient does not bring their medication notebook, dispensing act codes 440007910 and 440009010 were used for claiming. The number of times these codes were claimed was extracted to calculate the percentage of patients who brought their personal medication books.
Injectable anticancer drug usage in outpatient (in-hospital) and inpatient settings was extracted from the data table ‘000987622.xlsx,’ which is published in ‘Number of claims by sex and age’ in the ‘drug data_ injection’ table (Ministry of Health. Labour and Welfare, Citationn.d.-f, Table S3). The therapeutic categories described in the Standard Commodity Classification Number of Japan were used (Ministry of Internal Affairs and Communications, Citationn.d.). Injectable anticancer drugs were classified according to the therapeutic category as specified by the top three digits of the therapeutic category code: alkylating agents (code: 421), antimetabolites (code: 422), antitumor antibiotics (code: 423), plant extract preparations (code: 424), and miscellaneous (code: 429). In the present study, the number of injectable anticancer drugs used was totalled for each drug category according to sex and five-year age groups.
In the data table, the number of claims < 10 or injection drugs were excluded from the analysis owing to the absence of numerical values for items with a usage < 400.
Data analysis procedures
Several analyses were performed using the data obtained from the 7th NODJ, NCR (2019), and e-Stat (). Bivariate analysis was performed to examine the relationship between the number of patients and injectable anticancer drug usage for all registered cancer sites (C00 – C96). Injectable anticancer drug usage was calculated according to inpatient and outpatient settings, sex, and 5-year age groups and graphed according to drug category. A mosaic plot was created to visualise the population and the number of claims of the basic dispensing fee, AEC, and ASD2 according to sex and age group.
A linear regression analysis was conducted to examine the relationship between the amount of injectable anticancer drugs used (outpatient) and the involvement of hospital and community pharmacists. The analysis focused on the relationship between the amount of injectable anticancer drugs used (outpatient) and the number of AEC and ASD2 claims. The ratio of the number of AEC and ASD2 claims for each 5-year age group was also calculated.
Data was analyzed using JMP Pro16 software (SAS Institute, Cary, NC, United States).
Ethics statement
The study is not subject to ethical examination as the study was an observational study without any research subjects. In addition, no consent to participate was required owing to the retrospective nature of the study.
Results
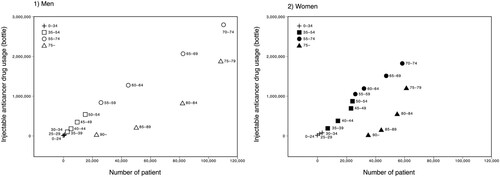
summarises the number of injectable anticancer drugs used based on the 7th NODJ dataset and the cancer incidence based on NCR. Over 95% of injectable anticancer drugs were used in hospitals for both men and women in their teenage years; however, outpatient use increased in patients starting their 20s. Among men, the percentage of outpatients exceeded 40% in the 35–39-year-old group, with the largest percentage (65.97%) in the 55–59-year-old group. Among women, outpatient usage exceeded 40% in the 25–29-year-old group, reaching a maximum of 78.44% in the 40–44-year-old group, and remained above 70% until the 75–79-year-old group.
Table 1. Injectable anticancer drugs usage and cancer incidence by age group.
presents an overview of the usage of each drug class according to 5-year age groups and sex. Regarding the drug class of injectable anticancer drugs used in the outpatient setting, ‘miscellaneous (code: 429)’ was frequently used, accounting for 42.38% and 48.34% usage in men and women, respectively. The use of antimetabolites (code: 422) was more common in men (33.26%) than in women (19.47%). Conversely, more women used alkylating agents (code: 421; 1.99% for men and 4.47% for women) and antitumor antibiotic preparations (code: 423; 2.78% for men and 6.13% for women) than men.
Figure 2. Injectable anticancer drug usage according to therapeutic category (outpatients and inpatients).
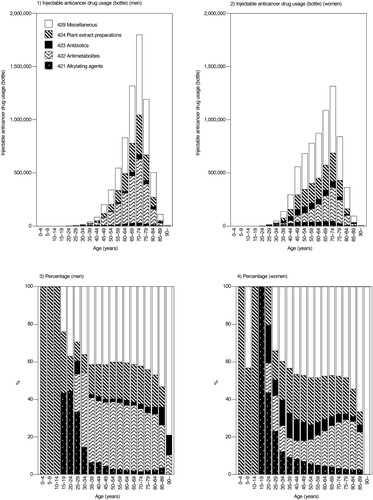
The relationship between the number of patients and injectable anticancer drugs used (total number of inpatients and outpatients) for all cancer sites (C00 – C96) is summarised in . The number of patients with cancer and the use of injectable anticancer drugs increased with age in both men and women aged < 75 years, whereas the use of injectable anticancer drugs decreased relative to the number of patients with cancer aged ≥ 75 years compared to those aged < 75 years.
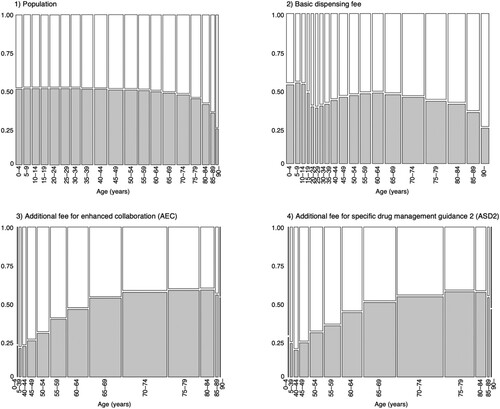
Table 2 summarises the number of claims for the AEC, ASD2, pharmacy admin fee, basic dispensing fee, and population. The total number of AEC claims was 332,480, of which 163,855 (49.28%) were for men and 168,625 (50.72%) were for women. The number of ASD2 claims was 17,938, of which 8,376 (46.69%) were for men and 9,562 (53.31%) were for women. The number of claims for the basic dispensing fee was 734,313,421, of which 321,335,654 (43.76%) were from men and 412,977,767 (56.24%) were from women. The ASD2-to-AEC ratio was 5.40% (5.11% in men and 5.67% in women).
A mosaic plot of the correlation between the population, the number of times the basic dispensing fee was claimed, the number of AEC and ASD2 claims, and age is shown in . The population remained balanced, with approximately 50% men and 50% women, until the age of 60 years. However, the frequency of claiming the basic dispensing fee was 62.01% for women in the 25–29-year-old group. Women aged ≥ 90 years had the largest difference, at 75.23%. The number of AEC claims was higher in women up to 64 years and higher in men over 65 years. The age group with the largest difference was the 35–39-year-old group, where 80.00% were women. Similar to the AEC, the number of ASD2 claims was higher among women up to 64 years and men over 65 years. The age group with the largest difference was the 40–44-year-old group, where 81.07% were women.
Figure 4. Mosaic plot of Population, Basic Dispensing Fee, AEC, and ASD2 (shaded: men; white: women).
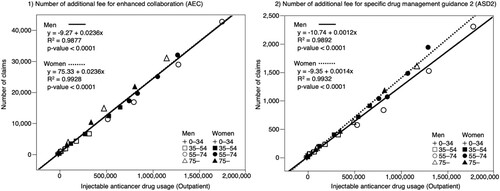
The results of the linear regression analysis of the number of injectable anticancer drugs used in outpatients and the number of AEC and ASD2 claims are summarised in . The coefficient of determination between the number of injectable drugs used and the number of AEC claims was R2 = 0.9877, p < 0.0001, regression coefficient: 0.0236 for men and R2 = 0.9928, p < 0.0001, regression coefficient: 0.0236 for women. The coefficient of determination between the number of injectable drugs used and the number of ASD2 claims was R2 = 0.9892, p < 0.0001, regression coefficient: 0.0012 for men and R2 = 0.9932, p < 0.0001, regression coefficient: 0.0014 for women.
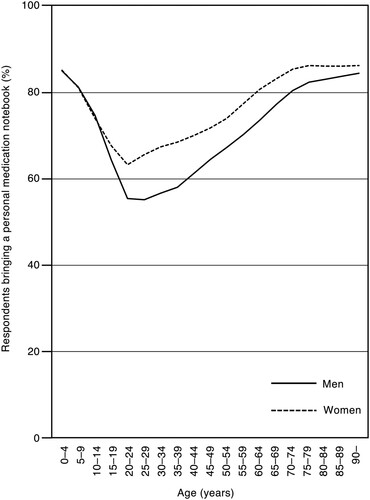
shows the percentage of individuals who brought their personal medication notebooks according to the 5-year age groups. The percentage of men aged ≥ 15 years was lower than that of women across all age groups.
Discussion
According to the Cancer Statistics 2023 published by the Foundation for Cancer Research and Progress (Foundation for Promotion of Cancer Research, Citation2023), the distribution of the causal sites of cancer mortality varies by age group. Fig. S1 illustrates the number of affected persons according to cancer site, sex, and 5-year age group from the data obtained from the NCR. In men, the number of cancer cases increased with age and was the highest among patients aged 70–74 years. Gastrointestinal cancers, such as stomach, esophagus, colon, liver, and pancreatic cancers, were more common among younger patients, whereas the prevalence of lung and prostate cancers increased with age. Among women, the number of cancer cases was the highest in the 75–79-year-old group. The distribution of cancer sites varied, with uterine cancers showing a higher percentage in the 50–54-year-olds and breast cancer being predominant in the 45–49 – and 70–74-year-olds. Uterine and breast cancers accounted for most cancer cases among women from younger age groups.
The National Comprehensive Cancer Network (NCCN) in the United States has listed Older Adult Oncology (Version: 1.2023) as a specific population in their guidelines (National Comprehensive Cancer Network, Citationn.d.-a). These guidelines state that treatment decisions for elderly patients with cancer should be based on their overall life expectancy. In this study, the use of injectable anticancer drugs decreased relative to the number of patients with cancer aged ≥ 75 years compared to those aged < 75 years. Since the cancer site varies with age, the treatment may also differ. In addition, as indicated in the NCCN guidelines, treatment options for the elderly are likely to consider prognosis. Several reports indicate that older cancer patients receive treatment less frequently than younger patients (Craigs et al., Citation2018; Haase et al., Citation2023; Okuyama & Higashi, Citation2018; Sehgal et al., Citation2014). Injectable anticancer agents are likely to be used less frequently in the elderly than in younger patients due to differences in the sites affected and from the perspective of safety.
As shows, there is no difference between men and women in the younger age groups, but the proportion of women who were dispensed drugs was higher among those in their 20s to 40s; women also accounted for a higher number of AEC and ASD2 claims. This may be because cancers specific to women, such as breast and uterine cancer, are more common in the younger age groups.
The outpatient use of injectable anticancer drugs accounted for 67% of the total use of anticancer drugs (). These findings highlight the importance of supporting patients undergoing treatment in an outpatient setting to ensure the safe administration of chemotherapy.
There was a correlation between the number of AEC claims and the amount of injectable anticancer drugs used in both men and women, as well as between the number of ASD2 claims and the amount of injectable anticancer drugs used (). The slope of the regression line between the number of AEC claims and the number of injectable anticancer drugs used did not differ between men and women. However, the slope of the regression line between the number of ASD2 claims and the number of injectable anticancer drugs used differed between men and women, with men having a smaller slope. To compensate for the lack of information from the prescriptions, the AEC requires hospital pharmacists to communicate information to community pharmacists through the use of personal medication notebooks or other means. The ASD2 claim frequency may have been lower among men because they are less likely to provide a personal medication handbook (). There were also gender differences in cancer site and type of injected anticancer drugs (), which may be related to differences in the treatment regimens provided.
The number of times the community pharmacists claimed their ASD2 was approximately 5% of the number of times the hospital pharmacists claimed their AEC (). The following are possible reasons for the discrepancy between AEC and ASD2 claim counts. First, because some outpatient cancer chemotherapy regimens do not include oral anticancer agents or supportive care drugs, it is possible that some patients are treated with injectable anticancer agents but do not go to a community pharmacy. In other words, we speculate that some patients are eligible for AEC but not ASD2. Second, even if a patient receives a prescription dispensed at a community pharmacy, the community pharmacist may not have a reliable system to know that the patient is receiving anticancer drug treatment and is being instructed on AEC by the hospital pharmacist. In Japan, the disease name is not usually listed on the prescription, thereby preventing the community pharmacist from learning the name of the disease from the prescription. If no anticancer drugs are prescribed, the community pharmacist cannot know from the prescription that the patient is undergoing treatment for cancer. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Antiemesis Version 2.2023–May 24, 2023® lists aprepitant as an antiemetic agent (National Comprehensive Cancer Network, Citationn.d.-b). When prescribed, the community pharmacist knows from the information on the prescription that the patient is being treated with an injectable anticancer drug. However, for supportive care drugs, such as antidiarrheals, which are sometimes prescribed for non-cancer conditions, it is difficult for the community pharmacist to determine the treatment being received at a hospital based solely on the information on the prescription. A third factor may be that information is communicated from the hospital pharmacist to the community pharmacist via the patient. However, the decision to use a medication notebook rests with the patient. If there is sufficient cooperation between hospital pharmacists and community pharmacists, the difference in the number of AEC and ASD2 calculations could be reduced. For patients undergoing outpatient cancer chemotherapy, a system for direct communication between the hospital and community pharmacists may be required.
Table 2. The number of claims of AEC, ASD2, Pharmacy Admin Fee and Basic Dispensing Fee, and Population.
Several reports have indicated that community pharmacists lack the necessary competence to ensure the safety of patient medication used in outpatient chemotherapy (Buhl et al., Citation2023; Mensah et al., Citation2019), suggesting that community pharmacists need to acquire more knowledge in dealing with outpatient cancer chemotherapy. Efforts should be made to improve the knowledge of pharmacists regarding cancer treatment to ensure the safe administration of outpatient chemotherapy. Cancer chemotherapy has many side effects (Ministry of Health, Labour and Welfare, Citation2009, Citation2019, Citation2021a, Citation2022a, Citation2023). The percentage of injectable anticancer drugs belonging to the miscellaneous category (code: 429), which includes novel anticancer agents, such as molecular-targeted agents, to the total was 45%. Hence, it is also crucial to pay attention to side effects that differ from those of conventional cytotoxic anticancer agents owing to their mechanism of action. To prevent the side effects of anticancer drugs, the Multinational Association for Supportive Care of Cancer and the NCCN have published guidelines (Multinational Association of Supportive Care in Cancer, Citationn.d.; National Comprehensive Cancer Network, Citationn.d.-c), and the European Society for Medical Oncology has published a position paper on supportive and palliative care (Jordan et al., Citation2018). Community pharmacists need to utilise these guidelines and contribute to administering safe cancer chemotherapy for their patients. Although the terms and conditions of the AEC stipulate that training should be held at least once a year for community pharmacists, it may be necessary to increase the number of training sessions to improve knowledge and collaboration.
With a universal health insurance system, all Japanese citizens are assumed to have access to the same level of medical care. However, a discrepancy exists between the AEC and ASD2 claims, which requires immediate resolution. A strong system must be developed in which patients, hospital pharmacists, and community pharmacists can collaborate to administer safe outpatient cancer chemotherapy. Regional medical information networks are currently being established in Japan (Ministry of Health, Labour and Welfare, Citationn.d.-c). The urgent establishment of such a regional collaborative network system is desirable.
In France, to secure a path for patients to receive oral anticancer therapy, Rubira et al. reported the usefulness of a computerised tool for standardising information exchanged between ambulatory and hospital pharmacists. Conversely, insufficient communication between hospital and community pharmacies indicated that community pharmacists perceived problems in providing oral antineoplastic drugs (Cavallier et al., Citation2022). In the United States and the United Kingdom, an initiative called Collaborative Drug Therapy Management (CDTM) has been implemented with some success. In a CDTM, a physician and pharmacist enter into a contract for the treatment of a particular patient (contractual transfer of authority), and the pharmacist administers drug therapy independently according to an agreed-upon protocol. CTDM by pharmacists varies depending on the environment, such as outpatient pharmacist, and hospital pharmacist. On the contrary, in Japan, when pharmacists do not have the authority to prescribe prescriptions or order tests, they provide drug treatment using their pharmacological knowledge and skills in collaboration with physicians and other technicians based on protocols developed and agreed upon in advance by the physician and pharmacist. Therefore, more importance is placed on feedback to the hospital by community pharmacists through monitoring and tracing reports of adverse drug reactions (Urakawa et al., Citation2021). There are differences in the administrative environment surrounding pharmacists in each country, it is important that community pharmacists and hospital pharmacists support patients by treating the side effects as they occur and by sharing information related to such events.
The present study had some limitations. The original data obtained from the NODJ dataset are primarily intended for insurance billing; therefore, there may be discrepancies with the actual practice. The requirements for insurance billing are strict and complex, and even if guidance is provided to patients, it may not be billed if it does not meet the requirements for insurance billing. Additionally, not all drugs used are listed, as the list in the NODJ is limited to the top 100 drugs used in each drug category. Patients with fewer than 10 AEC and ASD2 claims are not listed in the NODJ to protect their privacy. Given that the AEC and ASD2 used in this analysis are insurance points newly established in FY 2020, they were first listed in the 7th NODJ, indicating the need for continued surveys in the future.
Conclusion
This analysis examined the number of AEC and ASD2 claims, type of injectable anticancer drug, and cancer site according to age and sex. The use of injectable anticancer drugs decreased relative to the number of patients with cancer aged ≥ 75 years compared to those aged < 75 years. The number of ASD2 claims by community pharmacists was approximately 5% of the number of AEC claims by hospital pharmacists. The percentage of ASD2 claims was lower in men than in women. Compared to the guidance provided by hospital pharmacists to patients with cancer, the guidance provided by community pharmacists was insufficient, suggesting a lack of sufficient cooperation between hospital and community pharmacists.
Abbreviations
| AEC | = | Additional fee for enhanced collaboration |
| ASD2 | = | Additional fee for specific drug management guidance 2 |
| NDB | = | National Database of Health Insurance Claims and Specific Health Checkups of Japan |
| NODJ | = | NDB Open Data Japan |
| NCR | = | National Cancer Registry |
| MHLW | = | Ministry of Health, Labor and Welfare |
| WHO | = | World Health Organization |
| DPC | = | diagnosis procedure combination |
| NCCN | = | National Comprehensive Cancer Network |
Authors' contributions
MI, MM, and MN conceived the study, participated in its design and coordination, and drafted the manuscript. KM, SH, TS, SN, and HT conceived the study, participated in its design, helped with statistical analysis, and drafted the manuscript. NI and YN performed the statistical analyses. MM, HU, YN, and KI interpreted the data. All the authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Ethical approval was not sought for this study, as this was an observational study with no research participants. All data obtained were fully anonymized prior to the authors’ access and are openly available from the MHLW website. Our research does not fall within the purview of any of the following laws and guidelines: ‘Clinical Trials Act (Act No. 16 of April 14, 2017)’, ‘Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (Law number: Act No. 145 of 1960, Last Version: Amendment of Act No. 50 of 2015)’, ‘Guideline for good clinical practice E6 (R1), https://www.pmda.go.jp/int-activities/int-harmony/ich/0076.html’, ‘Ethical guidelines for human genome and gene analysis research, https://www.mhlw.go.jp/general/seido/kousei/i-kenkyu/genome/0504sisin.html’, and ‘Ethical Guidelines for Medical and Health Research Involving Human Subjects, https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/hokabunya/kenkyujigyou/i-kenkyu/index.html#HID1_mid1’.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Material
Download Zip (3.8 MB)Disclosure statement
MI is an employee of Kifune Pharmacy. The other authors declare no conflicts of interest.
Additional information
Funding
Notes on contributors
Mari Iwata
Mari Iwata is currently working as community pharmacist at Yanaizu-Branch, Kifune Pharmacy, Gifu, Japan, and she is attending PhD program in Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Mika Maezawa
Mika Maezawa is a doctoral student in Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Koumi Miyasaka
Koumi Miyasaka is a student in Laboratory of Drug Informatics at Gifu Pharmaceutical University Gifu, Japan.
Sakiko Hirofuji
Sakiko Hirofuji is a student in Laboratory of Drug Informatics at Gifu Pharmaceutical University Gifu, Japan.
Takaaki Suzuki
Takaaki Suzuki is an employee of the Gifu Prefectural Government, Gifu, Japan, and he is attending PhD program in Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Satoshi Nakao
Satoshi Nakao is currently working as pharmacist at Kyushu University Hospital, Fukuoka, Japan, and he is attending PhD program in Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Hirofumi Tamaki
Hirofumi Tamaki is an assistant professor in the Laboratory of Community Pharmacy at Gifu Pharmaceutical University, Gifu Japan.
Nanaka Ichihara
Nanaka Ichihara is a student in Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Yuka Nokura
Yuka Nokura is a student in Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Mayuko Masuta
Mayuko Masuta is a pharmacist at Kyoto City Hospital, Kyoto, Japan, and she is a member of the Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Hiroaki Uranishi
Hiroaki Uranishi is a pharmacist at Nara Medical University Hospital, Nara, Japan, and he is a member of the Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Yuri Nishibata
Yuri Nishibata is a pharmacist at Japanese Red Cross Wakayama Medical Center, Wakayama, Japan, and she is a member of the Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
Kazuhiro Iguchi
Kazuhiro Iguchi is a professor in the Laboratory of Community Pharmacy at Gifu Pharmaceutical University, Gifu, Japan.
Mitsuhiro Nakamura
Mitsuhiro Nakamura is a professor in the Laboratory of Drug Informatics at Gifu Pharmaceutical University, Gifu, Japan.
References
- Buhl, C., Olsen, N. L., Nørgaard, L. S., Thomsen, L. A., & Jacobsen, R. (2023). Community pharmacy staff’s knowledge, educational needs, and barriers related to counseling cancer patients and cancer survivors in Denmark. International Journal of Environmental Research and Public Health, 20(3), 2287. https://doi.org/10.3390/ijerph20032287
- Cavallier, G., Laudet, M., Vayssettes, P.-M., Balayssac, D., & Chennell, P. (2022). [Hospital– community pharmacy coordination for the dispensing of oral antineoplastic drugs: An observational study in the French county of the Aveyron]. Bulletin Du Cancer, 109(6), 692–706. https://doi.org/10.1016/j.bulcan.2022.02.007
- Colombo, L. R. P., Aguiar, P. M., Lima, T. M., & Storpirtis, S. (2017). The effects of pharmacist interventions on adult outpatients with cancer: A systematic review. Journal of Clinical Pharmacy and Therapeutics, 42(4), 414–424. https://doi.org/10.1111/jcpt.12562
- Craigs, C. L., Bennett, M. I., Hurlow, A., West, R. M., & Ziegler, L. E. (2018). Older age is associated with less cancer treatment: A longitudinal study of English cancer patients. Age and Ageing, 47(6), 833–840. https://doi.org/10.1093/ageing/afy094
- Foundation for Promotion of Cancer Research. (2023, April 26). Cancer statistics in Japan 2023. https://ganjoho.jp/public/qa_links/report/statistics/2023_en.html.
- Gatwood, J., Gatwood, K., Gabre, E., & Alexander, M. (2017). Impact of clinical pharmacists in outpatient oncology practices: A review. American Journal of Health-System Pharmacy, 74(19), 1549–1557. https://doi.org/10.2146/ajhp160475
- Haase, K. R., Sattar, S., Pilleron, S., Lambrechts, Y., Hannan, M., Navarrete, E., Kantilal, K., Newton, L., Kantilal, K., Jin, R., Wal-Huisman, H. van der, Strohschein, F. J., Pergolotti, M., Read, K. B., Kenis, C., & Puts, M. (2023). A scoping review of ageism towards older adults in cancer care. Journal of Geriatric Oncology, 14(1), 101385. https://doi.org/10.1016/j.jgo.2022.09.014
- Hébert, G., Minvielle, E., Palma, M. D., & Lemare, F. (2018). Quelles Sont Les Attentes de Coordination et d’accompagnement Des Patients Français Atteints de Cancer Vis-à-Vis de Leur Pharmacien de Ville ? Bulletin Du Cancer, 105(3), 245–255. doi: 10.1016/j.bulcan.2017.11.017
- Herledan, C., Cerfon, M.-A., Baudouin, A., Larbre, V., Lattard, C., Poletto, N., Ranchon, F., & Rioufol, C. (2023a, May 1). Impact of pharmaceutical care interventions on multidisciplinary care of older patients with cancer: A systematic review. Journal of Geriatric Oncology, 14(4), 101450. https://doi.org/10.1016/j.jgo.2023.101450
- Herledan, C., Toulemonde, A., Clairet, A.-L., Boulin, M., Falandry, C., Decker, L. D., Rioufol, C., Bayle, A., & Bertrand, N. (2023b, October 1). Enhancing collaboration between geriatricians, oncologists, and pharmacists to optimize medication therapy in older adults with cancer: A position paper from SOFOG-SFPO. Critical Reviews in Oncology/Hematology, 190, 104117. https://doi.org/10.1016/j.critrevonc.2023.104117
- Jordan, K., Aapro, M., Kaasa, S., Ripamonti, C. I., Scotté, F., Strasser, F., Young, A., Bruera, E., Herrstedt, J., Keefe, D., Laird, B., Walsh, D., Douillard, J. Y., & Cervantes, A. (2018). European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Annals of Oncology, 29(1), 36–43. https://doi.org/10.1093/annonc/mdx757
- Kimura, M., Go, M., Iwai, M., Usami, E., Teramachi, H., & Yoshimura, T. (2017). Usefulness of a pharmacist outpatient service for S-1 adjuvant chemotherapy in patients with gastric cancer. Molecular and Clinical Oncology, 7(3), 486–492. https://doi.org/10.3892/mco.2017.1337
- Mensah, K. B., Bangalee, V., & Oosthuizen, F. (2019). Assessing knowledge of community pharmacists on cancer: A pilot study in Ghana. Frontiers in Public Health, 7, 13. https://doi.org/10.3389/fpubh.2019.00013
- Ministry of Health, Labour and Welfare. (2009, May). Serious adverse reaction disease manual – peripheral neuropathy. https://www.pmda.go.jp/files/000143545.pdf.
- Ministry of Health, Labour and Welfare. (2015, October 23). Pharmacy vision for patients. https://www.mhlw.go.jp/file/04-Houdouhappyou-11121000-Iyakushokuhinkyoku-Soumuka/vision_1.pdf.
- Ministry of Health, Labour and Welfare. (2018, March). The basic plan to promote cancer control programs (3rd period). https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000196975.pdf.
- Ministry of Health, Labour and Welfare. (2021b, October 22). Central social insurance medical council 22 October, 2021. https://www.mhlw.go.jp/content/12404000/000846192.pdf.
- Ministry of Health, Labour and Welfare. (2022a, February). Manual for immune-related adverse events caused by immune checkpoint inhibitor. https://www.pmda.go.jp/files/000245271.pdf.
- Ministry of Health, Labour and Welfare. (2022b, August). Explanation of the 7th NDB Open Data Japan. https://www.mhlw.go.jp/content/12400000/001126104.pdf.
- Ministry of Health. Labour and Welfare. (n.d.-a). Cancer registration. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/gan/gan_toroku.html.
- Ministry of Health, Labour and Welfare. (n.d.-b). International statistical classification of diseases and related health problems. https://www.mhlw.go.jp/toukei/sippei/.
- Ministry of Health, Labour and Welfare. (n.d.-c). Medical information coordination network support navigator (archive). https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryou/johoka/renkei-support.html.
- Ministry of Health, Labour and Welfare. (n.d.-d). NDB Open Data Japan. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177182.html.
- Ministry of Health, Labour and Welfare. (n.d.-e). Revision of medical fee for FY 2020. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000188411_00027.html.
- Ministry of Health. Labour and Welfare. (n.d.-f). The 7th NDB Open Data Japan. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177221_00011.html.
- Ministry of Health, Labour and Welfare. (Revised 2019, September). Serious adverse reaction disease manual – hand-foot syndrome. https://www.pmda.go.jp/files/000240132.pdf.
- Ministry of Health, Labour and Welfare. (Revised 2021a, April). Serious adverse reaction disease manual – severe diarrhea. https://www.pmda.go.jp/files/000240118.pdf.
- Ministry of Health, Labour and Welfare. (Revised 2023, April). Serious adverse reaction disease manual – stomatitis caused by anticancer drug. https://www.pmda.go.jp/files/000252186.pdf.
- Ministry of Internal Affairs and Communications. (n.d.). Division 87 – Drugs and related commodities. https://www.soumu.go.jp/main_content/000294493.pdf.
- Mukai, R., Shimada, K., Suzuki, T., Nakao, S., Tanaka, M., Matsumoto, K., Yoshida, Y., Goto, F., Inoue, M., Satake, R., Nishibata, Y., Sugihara, H., & Nakamura, M. (2020). Trends associated with hemorrhoids in Japan: Data mining of medical information datasets and the national database of health insurance claims and specific health checkups of Japan (NDB) Open Data Japan. Biological and Pharmaceutical Bulletin, 43(12), 1831–1838. https://doi.org/10.1248/bpb.b20-00157
- Multinational Association of Supportive Care in Cancer. (n.d.). MASCC Guidelines. https://mascc.org/resources/mascc-guidelines/.
- National Comprehensive Cancer Network. (n.d.-a). NCCN clinical practice guidelines in oncology (NCCN Guidelines®) Older Adult Oncology Version 1. 2023. https://www.nccn.org/guidelines/guidelines-detail?category=4&id=1452.
- National Comprehensive Cancer Network. (n.d.-b). NCCN clinical practice guidelines in oncology (NCCN Guidelines®) antiemesis version 2. 2023. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1415.
- National Comprehensive Cancer Network. (n.d.-c). Supportive Care. https://www.nccn.org/guidelines/category_3.
- Okuyama, A., & Higashi, T. (2018). Patterns of cancer treatment in different age groups in Japan: An analysis of hospital-based cancer registry data, 2012–2015. Japanese Journal of Clinical Oncology, 48(5), 417–425. https://doi.org/10.1093/jjco/hyy032
- Rubira, L., Leenhardt, F., Perrier, C., & Pinguet, F. (2021). Sécurisation du parcours de soins du patient sous thérapie orale en oncologie: Expérimentation autour d’un lien pharmaceutique hôpital–ville. Annales Pharmaceutiques Françaises, 79(5), 558–565. https://doi.org/10.1016/j.pharma.2021.01.009
- Sehgal, R., Alsharedi, M., Larck, C., Edwards, P., & Gress, T. (2014). Pancreatic cancer survival in elderly patients treated with chemotherapy. Pancreas, 43(2), 306–310. https://doi.org/10.1097/MPA.0000000000000091
- Statistics Bureau of Japan. (n.d.). Vital Statistics Vital Statistics of Japan Final Data Population Yearly 2020 | File | Browse Statistics. https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00450011&tstat=000001028897&cycle=7&year=20200&month=0&tclass1=000001053058&tclass2=000001053061&tclass3=000001053072&result_back=1&tclass4val=0.
- Suzuki, T., Iwata, M., Maezawa, M., Inoue, M., Satake, R., Wakabayashi, W., Oura, K., Tanaka, H., Hirofuji, S., Miyasaka, K., Goto, F., Nakao, S., Masuta, M., Iguchi, K., & Nakamura, M. (2023). Promoting generic drug usage in Japan: Correlation between generic drug usage and monthly personal income. Journal of Pharmaceutical Policy and Practice, 16(1), 27. https://doi.org/10.1186/s40545-023-00532-5
- Tanaka, H., Onoda, T., & Ishii, T. (2022). Understanding the actual use of anti-HIV drugs in Japan from 2016 to 2019: Demonstrating epidemiological relevance of NDB Open Data Japan for understanding Japanese medical care. International Journal of Environmental Research and Public Health, 19(19), 12130. https://doi.org/10.3390/ijerph191912130
- Tanaka, K., Tachi, T., Hori, A., Osawa, T., Nagaya, K., Makino, T., Inoue, S., Yasuda, M., Mizui, T., Nakada, T., Goto, C., & Teramachi, H. (2019). Cost utility analysis of pharmacist counseling care for breast cancer chemotherapy outpatients. Die Pharmazie, 74(7), 439–442. https://doi.org/10.1691/ph.2019.9327
- Tanito, M. (2022). Nation-wide analysis of glaucoma medication prescription in fiscal year of 2019 in Japan. Journal of Personalized Medicine, 12(6), 956. https://doi.org/10.3390/jpm12060956
- Urakawa, R., Hashimoto, S., Hirohata, H., Sakai, K., Matsuura, K., Ito, Y., Tarutani, M., Kubota, K., Ueda, M., & Uejima, E. (2021). Skin disorder management in oral anticancer drugs by collaboration of hospital pharmacists and community pharmacists. Supportive Care in Cancer, 29(7), 3577–3583. https://doi.org/10.1007/s00520-020-05875-2
- World Health Organization. (n.d.). Cancer. Retrieved October 27, 2023, from https://www.who.int/news-room/fact-sheets/detail/cancer.
- Yoshimi, C., Yamada, M., Fujii, H., Nishigaki, M., Iihara, H., Kitaichi, K., Takahashi, M., Kurahashi, S., Takahashi, T., Yoshida, K., & Itoh, Y. (2013). [Evaluation of the efforts of pharmaceutical care services before medical examination at an outpatient cancer chemotherapy clinic]. Gan to Kagaku Ryoho. Cancer & Chemotherapy, 40(3), 349–354.