ABSTRACT
Background
Pharmacists can play an important role in the fight against tuberculosis (TB) through optimising medication use and safety, promoting adherence to anti-TB drugs, and providing patient education. Limited evidence is available on the effectiveness of pharmacist’s interventions on health outcomes in patients with pulmonary TB. This systematic review aims to assess the effectiveness of pharmaceutical care interventions in the management of pulmonary TB.
Methods
English language studies assessing the impact of pharmaceutical care interventions in TB management were searched across three electronic databases (PubMed, Embase, Cochrane), a RCT registry ClinicalTrial.gov, a peer-reviewed journal ‘The Lancet Infectious Diseases’, and the references of retrieved articles. Interventions delivered by pharmacists alone or as part of multidisciplinary teams were included in the review. Data were extracted using the modified Cochrane EPOC standardised data collection tool. The Cochrane Risk of Bias 2 and the NIH quality assessment tools were used to assess the risk of bias among included studies. Data were synthesised narratively. (PROSPERO Protocol Registration CRD42022325771).
Results
Thirteen studies, including two randomised controlled trials (RCTs) with a total of 3886 patients were included. Many of the included studies had a high risk of bias and lacked cohert reporting of treatment outcomes. The most common pharmaceutical care interventions were education and counselling regarding adverse drug reactions and resolution of drug-related problems. Five studies showed a relatively high TB completion rate yet only one study reached the targeted treatment success goal of (>90%).
Conclusion
The current evidence suggests that pharmaceutical care interventions can potentially improve treatment outcomes among patients with pulmonary TB. However, no definitive conclusion can be drawn given the low methodological quality of the included studies and lack of long-term follow-up data. Well-designed RCTs with careful attention to study methodology, standardised outcomes assessment aligned with the World Health Organization’s guidelines are warranted to guide future practice and policy.
Background
Despite advancements in healthcare, communicable diseases such as tuberculosis (TB) remain of significant public health concern globally. Active symptomatic patients diagnosed with TB can infect five to fifteen other people through close contact (Centers for Disease Control and Prevention, Citation2022; World Health Organization, Citation2022). Consequently, in 2015, the World Health Organization (WHO) led the initiative ‘End TB Strategy 2016–2035’ which was agreed upon by all member states of the WHO and the United Nations (UN) (World Health Organization, Citation2021a; World Health Organization, Citation2022). The initiative set targets for TB incidence reduction of 20% by 2020, 50% by 2025, 80% by 2030, and 90% by 2035 (World Health Organization, Citation2021a). To achieve these targets, the WHO highlighted key strategies, including: the provision of TB prevention, diagnostic, and treatment services (World Health Organization, Citation2021a). However, the WHO recently announced that as the thirteenth largest cause of death worldwide, TB caused almost 1.6 million deaths in 2021 alone, with the highest TB incidence being in South East Asia (43%) and Africa (25%) (World Health Organization, Citation2022). By 2020, there had only been an 11% decrease in TB incidence, falling short of the WHO’s aim by almost 50%. COVID-19’s disruption was cited as the primary factor for the failure to meet the target. Additionally, there is an expected global increase in the incidence rate by 2023 (World Health Organization, Citation2021a; World Health Organization, Citation2022).
The significance of pharmacist’s role in TB control initiatives is recognised on a global scale. Pharmacists are the most accessible healthcare providers and continue to be the patients’ first point of contact and highly trusted healthcare providers (Jones, Citation2011; Manolakis & Skelton, Citation2010; Tsuyuki et al., Citation2018). Pharmacist can support patients in their self-care, provide up-to-date drug information to patients and healthcare providers, as well as, refer patients to appropriate healthcare services (Manolakis & Skelton, Citation2010). In fact, pharmacists can see their patients 10 times more than other healthcare providers (Tsuyuki et al., Citation2018). Pharmacists have a unique opportunity to detect TB by identifying symptomatic patients and referring them for TB screening (Pradipta et al., Citation2023; Wong et al., Citation2023). Pharmacist involvement in TB programmes can improve continuity of care and bridge the gap between symptoms detection and monitoring of treatment (Wong et al., Citation2023). In addition, they play a crucial role in optimising therapy of patients after TB diagnosis, ensuring that appropriate patient-centred care is given, and minimising anti-TB drugs-related adverse effects (Iskandar et al., Citation2023; Miller & Goodman, Citation2020). Moreover, pharmacist play a role in directly observed therapy (DOT) strategy, which is a cornerstone in improving the outcomes of TB care (Wong et al., Citation2023). In 2011, the WHO and the International Pharmaceutical Federation (FIP) released a joint statement highlighting the essential role of pharmacists in TB control and advocating for global actions in incorporating pharmacist in TB care settings (World Health Organization, International Pharmaceutical Federation, Citation2011).
Several studies have reported that pharmacist-led or supported interventions could improve health outcomes (Jakeman et al., Citation2020; Jones, Citation2011; Juan et al., Citation2006; Tavitian et al., Citation2003; Wong et al., Citation2023). Jakeman et al. demonstrated high treatment completion rates and less adverse events related to TB medications when community pharmacists were included in a collaborative care model for TB patients (Jakeman et al., Citation2020). Additionally, a pharmacist-led clinic for managing latent TB among health care workers demonstrated a 90–100% completion rate (Tavitian et al., Citation2003). Nonetheless, there is currently insufficient comprehensive evidence regarding the overall impact of pharmacist-led care on pulmonary tuberculosis (TB) treatment outcomes. While individual studies have examined various aspects of pharmacist involvement in TB care, a unified perspective on the collective influence of pharmacist-led interventions is lacking. Hence the aim of this systematic review is to identify and describe the types of pharmaceutical care (PC) interventions provided to patients with TB, as well as critically evaluate the quality of the available evidence on the effectiveness of PC interventions for the management of pulmonary TB.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) protocol (Moher et al., Citation2015). The study protocol was registered in PROSPERO International prospective register of systematic reviews (CRD42022325771).
Outcome measures
The primary outcome measure of interest was the completion rate of anti-TB treatment, defined as ‘the proportion of patients who completed treatment, but who do not have negative sputum smear or culture results in the last month of treatment and on at least one previous occasion’ (World Health Organization, Citation2010). The decision to focus on completion rate is justified by its direct relevance to treatment success and its reflection of patient adherence and persistence throughout the entire course of anti-TB therapy. Secondary outcomes were those reported in the WHO guidelines: cure rate, treatment success rate, treatment failure rate, death rate, default and transfer out (World Health Organization, Citation2010). The definitions of these outcomes are available in supplementary file. Additional secondary outcomes captured in the review included time to sputum conversion, adherence to anti-TB drugs, and the number of adverse drug reactions (ADRs) or drug-related problems (DRPs) requiring pharmacist’s intervention, and patients’ satisfaction with the services provided.
Search strategy
The literature search was conducted in three databases from the date of their inception till the search date (September 2023): PubMed, EMBASE, and Cochrane. Additionally, the RCT registry available at ‘ClinicalTrial.gov’ was also searched to identify any additional RCTs, if any. The Lancet Infectious Diseases journal was hand searched as this is a well-regarded, peer-reviewed medical journal with a focus on infectious diseases. indicates the search terms used. Medical subject heading (MeSH) terms were used whenever applicable. The search was restricted to English language articles. A manual search of the references of the retrieved articles was also performed. The full details of the search strategy are available in a supplementary file. The search was conducted by three investigators (KA, MAH, MJ) and reviewed by the team. All search results were exported to Rayyan QCRI tool (a free tool that can help in title/abstract and full-text screening phases of systematic review process) (Ouzzani et al., Citation2016). Duplicates were removed from the database. Titles and abstracts were reviewed against predefined eligibility criteria, and this was followed by full-text screening for included titles/abstracts by the authors (KA, MAH, MJ) independently. Any disagreements were discussed with a mentor (AA) until reaching consensus across the team.
Table 1. Search terms used in searching the databases.
Study eligibility
Studies were included if they were: (1) published in English; (2) conducted using any of the following study desgins: randomised controlled trial (RCT), non-randomised controlled trial, cohort study, case–control study, pre–post study and quasi-experimental; (3) involving patients with confirmed diagnosis of pulmonary TB, whether active TB OR latent TB OR multi-drug resistant-TB (MDR-TB), (4) investigating pharmacist’s intervention in pulmonary TB management (including dispensing of TB medications, providing education to the participants, managing drug-related problems in TB, providing advice to healthcare providers or undertaking medication review). Qualitative studies, studies reporting on patients with extra-pulmonary TB only, and studies focusing on diagnosis, referral or tuberculin test performance as the only pharmacist’s intervention were excluded from the review.
Data extraction and synthesis
Data were extracted using the modified Cochrane EPOC standardised data collection form, and included the following information: author(s), publication year, setting, study design, number of participants, intervention details, comparator if available, pharmacist qualifications if available, and conclusion. Primary and secondary outcomes were also extracted along with methods of measurement. Data were synthesised qualitatively and presented in a tabular format as well as qualitative summaries based on outcomes of interest. For all continuous outcomes (e.g. number of ADRs reported), mean and standard deviations were extracted. For dichotomous data (e.g. treatment success, treatment completion, cure), frequencies and percentages were extracted. The primary responsibility of extracting data from the articles was undertaken by one author (KA). Following the initial extraction, another author (MAH), checked the extracted data for accuracy and consistency. Any discrepancies among the two authors were resolved through discussions.
Quality assessment
The quality of the studies was independently assessed by two of the authors (KA and MAH) and reviewed for consensus through team discussions. For the RCTs, the Cochrane Risk of Bias 2 tool (Rob-2) was used (Higgins et al., Citation2011). While for cohort studies, the NIH quality assessment tool for observational cohort and cross-sectional studies was used (National Institute of Health, Citation2013). Moreover, the NIH quality assessment tool for controlled intervention studies was used for the assessment of studies utilising quasi-experimental with control group design (National Institute of Health, Citation2013).
Results
Study selection
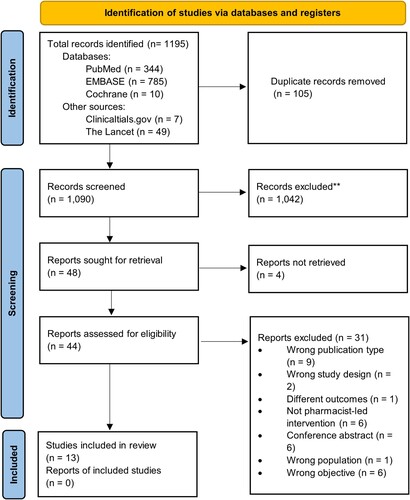
A total of 1195 studies were identified from the database search and through other sources (Clinicaltrials.gov and the Lancet). After duplicate removal, 1090 articles were screened, and 13 articles met the inclusion criteria. The search results are presented in .
Figure 1. PRISMA flow diagram. Source: Haddaway et al. (Citation2022).
Characteristics of included articles
Thirteen articles were included in this review that reported data on 3,886 patients. Four studies were conducted in USA (Carter et al., Citation2017; Hess et al., Citation2009; Jakeman et al., Citation2020; Tavitian et al., Citation2003), two studies in Brazil (Lara-Júnior et al., Citation2022; Lopes et al., Citation2017) and one study was conducted in each of the following countires: Spain (Juan et al., Citation2006), China (Tang et al., Citation2018), Turkey (Clark et al., Citation2007), Canada (Tang et al., Citation2018), Thailand (Tanvejsilp et al., Citation2017), Indonesia (Karuniawati et al., Citation2019), and India (Thomas et al., Citation2018). There were 10 cohort studies (Carter et al., Citation2017; Hess et al., Citation2009; Jakeman et al., Citation2020; Juan et al., Citation2006; Lara-Júnior et al., Citation2022; Lopes et al., Citation2017; Moadebi et al., Citation2005; Tanvejsilp et al., Citation2017; Tavitian et al., Citation2003; Thomas et al., Citation2018), two RCTs (Clark et al., Citation2007; Tang et al., Citation2018) and 1 quasi-experimental study (Karuniawati et al., Citation2019). Six studies had a comparator group (Clark et al., Citation2007; Juan et al., Citation2006; Karuniawati et al., Citation2019; Moadebi et al., Citation2005; Tang et al., Citation2018; Tanvejsilp et al., Citation2017); one study compared pharmacist care to nurse care (Moadebi et al., Citation2005), while three studies compared pharmacist care to usual practice (Clark et al., Citation2007; Hess et al., Citation2009; Tang et al., Citation2018). Tanvejsilp et al. compared pharmacist intervention to home visits and modified DOT (Tanvejsilp et al., Citation2017), while Juan et al. compared pharmacist-led DOT to self-administered therapy (Juan et al., Citation2006). On the other hand, Lara-Junior et al. compared patients who received pharmaceutical follow-up services to those who did not receive the service (Lara-Júnior et al., Citation2022). The earliest study was conducted in USA in 2003 (Tavitian et al., Citation2003), while the latest study was conducted in Brazil in 2022 (Lara-Júnior et al., Citation2022). The characteristics of the included studies are summarised in .
Table 2. Characteristics of the included studies.
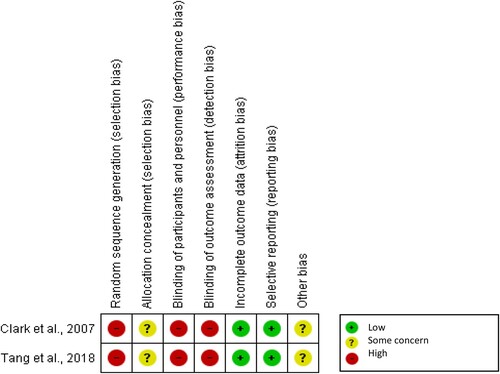
Quality assessment
The two RCTs included (Clark et al., Citation2007; Tang et al., Citation2018) were of low quality. Only one out of the 10 cohort studies was rated as high quality (Tanvejsilp et al., Citation2017). The quasi-experimental study was of fair quality (Karuniawati et al., Citation2019). Quality assessment is represented in and .
Table 3. Quality assessment for cohort and quasi-experimental studies.
Pharmacist interventions
Two studies explored pharmacist-led clinics for latent TB infection, where pharmacists screened, initiated treatment, managed medications, educated patients, ordered lab tests, and followed-up (Carter et al., Citation2017; Tavitian et al., Citation2003). Hess et al. and Lara-Junior et al. focused on pharmacist roles in patient follow-up, ADR reporting, and adherence (Hess et al., Citation2009; Lara-Júnior et al., Citation2022). Five studies addressed pharmacist-led patient education before hospital discharge, covering TB and treatment in written and verbal formats (Clark et al., Citation2007; Karuniawati et al., Citation2019; Moadebi et al., Citation2005; Tang et al., Citation2018; Thomas et al., Citation2018). Karuniawati's study focussed on patient counselling targeting anti-TB medication adherence (Karuniawati et al., Citation2019).
On the other hand, Tang et al. provided post-discharge PC, including face-to-face and telephone follow-ups, ADR reporting, and treatment recommendations (Tang et al., Citation2018). Tanvejsilp et al. offered care during outpatient visits, including education, DOT, DRP identification, and adherence evaluation (Tanvejsilp et al., Citation2017). Jakeman et al. and Juan et al. investigated community pharmacies’ roles in DOT and ADR detection (Jakeman et al., Citation2020; Juan et al., Citation2006). Lopes et al. assessed PC, with pharmacists addressing DRPs and ADRs in a secondary clinic (Lopes et al., Citation2017).
Primary outcome
Treatment completion rate
Six studies reported the treatment completion rate (Carter et al., Citation2017; Hess et al., Citation2009; Jakeman et al., Citation2020; Juan et al., Citation2006; Tang et al., Citation2018; Tavitian et al., Citation2003), two of which compared pharmacist’s intervention to usual care (Juan et al., Citation2006; Tang et al., Citation2018). A study conducted in Spain among 213 patients reported that patients receiving PC with DOT had three times better completion rate compared to self-administered therapy (SAT) during a period of 6 to 9 months of follow-up (75.2% vs. 2.7%, p-value <0.0001) (Juan et al., Citation2006). The study was considered to be of fair quality and was conducted in relevance to community pharmacists follow-up. However, an RCT that compared between pharmacist’s care and usual practice (not including pharmacists) reported overall low completion rates of 19% vs. 14%, although the difference did not reach statistical significance (Tang et al., Citation2018). This RCT had a relatively high risk of bias (Tang et al., Citation2018). The other four studies reported a completion of almost 60% or more following the pharmacist’s intervention (Carter et al., Citation2017; Hess et al., Citation2009; Jakeman et al., Citation2020; Tavitian et al., Citation2003). These studies also had a relatively high risk of bias. The results of the primary outcome are presented in .
Table 4. Completion rate of pharmacist-provided interventions compared to other interventions.
Secondary outcomes
All secondary outcomes are summarised in .
Table 5. Secondary outcomes of the studies comparing pharmacist-provided interventions with usual care or other interventions.
Treatment success
Treatment success was only reported as an outcome in two studies (Tang et al., Citation2018; Tanvejsilp et al., Citation2017). In these studies, no significant difference was observed between the pharmacist’s interventions and the comparator groups (Tang et al., Citation2018; Tanvejsilp et al., Citation2017). Tanvejsilp compared the PC with modified DOT and home care (Tanvejsilp et al., Citation2017). It is of note that this study was a retrospective cohort and the only one to be considered of good quality. While Tang et al. compared PC to UC (Tang et al., Citation2018) in an RCT in China and had a relatively high risk of bias.
Treatment failure
Treatment failure was reported in two studies involving 344 participants (Juan et al., Citation2006; Tang et al., Citation2018). Juan et al. showed statistical significance between the pharmacist intervention and the comparator, whereby pharmacist’s care resulted in lower rates of treatment failure (19.8% vs. 63.3%, P < 0.0001) (Juan et al., Citation2006).
Cure
Three studies reported this outcome. Two studies showed no statistical significance between intervention and comparator group (Juan et al., Citation2006; Tang et al., Citation2018). While Laura-junior reported higher cure rate among patients who underwent pharmacy follow-up intervention (90.4%) as compared to those who did not undergo this intervention (73.5%). This difference was statistically significant, and the incidence of cure was about 2.7 times higher for patients included in the pharmacist follow-up group (HR = 2.71; 95%CI = 2.04–3.61) (Lara-Júnior et al., Citation2022).
Death
Death was assessed in 344 patients, four deaths out of 160 participants in the intervention group vs. six out of 184 comparator group arm (Juan et al., Citation2006; Tang et al., Citation2018). Juan et al. showed that patients receiving the PC with DOT had 0.69 times lower risk of death (1.9% vs. 3.5%); however, this difference was not statistically significant (Juan et al., Citation2006). This study included patients with HIV and reported any death related to TB and/or HIV cumulatively and was assessed as fair quality (Juan et al., Citation2006).
Default and transfer out
One study reported default and transfer out as two separate outcomes among 131 patients and showed no statistically significant difference between the two groups for both outcomes (Tang et al., Citation2018).
Time to sputum conversion
Only one study reported this outcome and showed no significant difference in the time needed for sputum sample to be converted into negative between the PC group and the comparator group (p = 0.708) (Tang et al., Citation2018). In both arms, approximately a mean duration of 2.8 months was required for sputum conversion.
Adherence
Adherence was reported in four studies (Clark et al., Citation2007; Karuniawati et al., Citation2019; Tang et al., Citation2018; Thomas et al., Citation2018); however, the methods of assessment varied. Urine analysis, attendance to follow-up visits and self-reported questionnaires were used to assess adherence. Despite the different assessment methods, all the studies showed a statistically significant increase in medication adherence in patients receiving pharmacist interventions compared to the comparator arm.
Patient satisfaction
Only one study reported patient satisfaction with the counselling provided by the pharmacist and compared it to the counselling provided by a nurse (Moadebi et al., Citation2005). The satisfaction was measured using the Visit-Specific Satisfaction Questionnaire (VSQ-9) (RAND Corporation). The study reported a high level of satisfaction with both services; however, there was no statistically significant difference in the overall satisfaction scores (4.2 ± 0.68 and 4.3 ± 0.73, respectively; p > 0.05) (Moadebi et al., Citation2005).
Drug-related problems and adverse drug reactions
Eight studies reported DRPs or ADRs (Carter et al., Citation2017; Clark et al., Citation2007; Hess et al., Citation2009; Jakeman et al., Citation2020; Juan et al., Citation2006; Lopes et al., Citation2017; Tang et al., Citation2018; Tavitian et al., Citation2003), However, only three of these reported the number of DRPs resolved as a result of the pharmacist interventions (Clark et al., Citation2007; Lopes et al., Citation2017; Tang et al., Citation2018). Tang et al. reported that 50 out of 57 of the pharmaceutical issues were resolved as a result of pharmacist’s interventions (Tang et al., Citation2018). Clark et al. and Lopes et al. reported 75% and 68.7% of DRPs were resolved, respectively, as a result of pharmacist’s interventions (Clark et al., Citation2007; Lopes et al., Citation2017).
Other secondary outcomes
While undertaking the data extraction, other outcomes were reported such as hospital admission and relapse that were extracted. The results are available in the Supplementary File.
Discussion
The pivotal role of pharmacists in TB control initiatives aligns with broader efforts to engage all healthcare professionals in the fight against TB. As the most accessible healthcare providers and among the most trusted, pharmacists are uniquely positioned to contribute significantly to TB management (Iskandar et al., Citation2023; Wong et al., Citation2023; World Health Organization, International Pharmaceutical Federation, Citation2011). The primary objective of this systematic review was to evaluate the impact of PC interventions on enhancing clinical outcomes in individuals diagnosed with TB. Overall, there was a scarcity of high-quality studies investigating the impact of pharmacist’s care on TB treatment outcomes. A notable concern arising from this systematic review was the overall methodological quality of the included studies. The majority of the evidence stemmed from observational cohorts, with limited data from RCTs. Moreover, the RCTs identified were rated as having a high-risk of bias, underscoring the need for more rigorous research in this domain. The lack of uniformity in outcomes reporting across studies further complicates the synthesis of evidence and underscores the necessity for high-quality research in this critical area.
The identification of various services provided by pharmacists through this review highlights the multifaceted and crucial role that pharmacists can play in the management of TB. These services extend beyond the traditional role of pharmacists as medication dispensers and underscore their potential as valuable members of the healthcare team in TB care. These findings align with a recent systematic review that explores the involvement of community pharmacists only in TB management (Wong et al., Citation2023). The most reported intervention by the pharmacist in TB management in this review was education and counselling regarding the management of DRPs and ADRs. This is consistent with the pharmacist's involvement emphasised upon in the FIP and WHO joint statement on pharmacists’ roles in TB (World Health Organization, International Pharmaceutical Federation, Citation2011), as well as Miller's review on pharmacist’s roles in low- and middle-income countries in TB control, where pharmacists are expected to be knowledgeable about TB treatments and to offer suitable medication counselling (Miller & Goodman, Citation2020; World Health Organization, International Pharmaceutical Federation, Citation2011). Nonetheless, pharmacist’s qualifications and training required for TB management were not explicitly stated, except in three studies (Clark et al., Citation2007; Jakeman et al., Citation2020; Tang et al., Citation2018). It is important for studies to report the general pharmacist’s role and qualifications to provide standardised expectations from the readers and also allow generalisability of the outcomes. Miller et al. noted that when pharmacists were given simulated cases of TB requiring referrals, 16% to 41% chose to prescribe antibiotics rather than referring the patients for additional testing, highlighting the need for training about TB management (Miller & Goodman, Citation2020).
This systematic review revealed a range of findings related to the primary outcome of treatment completion rate. Completion rate was defined differently across the six studies that reported this outcome. This included number of attended appointments, number of tablets taken, duration of treatment, and WHO definition, which focused on ‘a patient who completed treatment but who does not have a negative sputum smear or culture result in the last month of treatment and on at least one previous occasion’ (World Health Organization, Citation2010). The lack of standardised measurement contributed to variability in the reported rates. While one study conducted in Spain reported a substantial improvement in completion rates among patients receiving PC with DOT (Juan et al., Citation2006), an RCT comparing pharmacist-led interventions to usual care did not find a statistically significant difference in treatment completion rates. These contrasting results underscore the complexity of TB management and the need for further investigations to determine the optimal role of pharmacists in improving treatment completion.
The outcomes reported in the studies did not consistently align with the recommendations of the WHO for TB treatment outcomes. In fact, only Tang et al. study applied the same outcomes as per the WHO guidelines, while all other included studies used different outcomes and different outcome measures (Tang et al., Citation2018). These inconsistencies limit the ability to compare the results in a meaningful way and to draw robust conclusions. This discrepancy further complicates the comparison of results and limits our ability to draw meaningful conclusions. Standardisation of outcomes reporting in future studies is crucial for advancing our understanding of the pharmacist's role in TB management.
The WHO recommends the DOT since it has been shown to be successful in improving medication adherence and TB treatment outcomes (Nowak, Citation1995; World Health Organization, Citation2021b; Wright et al., Citation2004; Zhang et al., Citation2016; Zvavamwe & Ehlers, Citation2009). DOT ensures that patients take their medications as prescribed, reducing the risk of treatment failure, relapse, and the development of drug-resistant TB strains. However, only two studies used this strategy besides the pharmacists’ services provided (Jakeman et al., Citation2020; Juan et al., Citation2006). The provision of DOT by pharmacists is a cornerstone in TB control efforts (Pradipta et al., Citation2023). This service is particularly essential for patients with latent TB infection and those who may face challenges in adhering to their treatment regimens independently.
This review included different types of study design and the search was performed in a comprehensive manner to ensure the highest possible number of articles matching the review’s inclusion criteria. PubMed and EMBASE are noted to cover almost 80% of the literature (Falagas et al., Citation2008). Despite these, there were some limitations that should be acknowledged in this systematic review. First, the search strategy did not include grey literature, which could have been of relevance. Second, the search was restricted to studies published in English only, which might have resulted in missing relevant articles. Resources to translate non-English articles, however, were not available for our review. Third, referral and TB screening were not investigated in this review since there was an existing registered protocol in PROSPERO (CRD42021230818) covering this intervention in community pharmacies. Finally, the studies included in this review did not specify the source of funding or the provision of incentives for pharmacy-led services. This omission posed challenges in assessing the practicality and sustainability of the interventions provided by pharmacists. In fact one systematic review highlighted that incentives are of great importance to ensure continuity of service provision (Pradipta et al., Citation2023).
Conclusion
In conclusion, this systematic review offers valinsights into the potential benefits of pharmacist-led interventions and pharmaceutical care in improving outcomes for patients with pulmonary tuberculosis. However, the quality of evidence remains limited. The majority of included studies had a high risk of bias and inconsistent reporting of outcomes, complicating synthesis and interpretation of the data. Although the findings imply a favourable influence of pharmacist involvement on treatment adherence and completion rates, the heterogeneity observed among the included studies and the predominance of observational designs hinder the broad applicability of these results. Well-designed randomised controlled trial using standardised outcomes assessments aligned with WHO guidelines will be key to investigating which specific pharmacist interventions offer the greatest patient impact.
Supplemental Material
Download MS Word (18.9 KB)Acknowledgements
KA and MAH contributed to the conception and design of the study, acquisition of data, and analysis. MJ conducted an updated search. All authors contributed to the interpretation of data, drafting the article, revising it critically for important intellectual content, and final approval of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed during this study are included in the published article [and its supplementary information files]
Additional information
Notes on contributors
Kheloud Awad
Kheloud Awad completed her BSc in Pharmacy and PharmD degrees from Qatar University. She is currently working as a research assistant at the College of Pharmacy, Qatar University. She has a research interest in evidence synthesis.
Myriam Jaam
Myriam Jaam completed her MSc in Clinical Pharmacy and Practice from Qatar University. She is working as a teaching assistant in the Department of Clinical Pharmacy and Practice, College of Pharmacy, Qatar University. She has co-authored over 15 peer-reviewed publications. Her research interests are in the fields of medication adherence and safety.
Ahmed Awaisu
Professor Ahmed Awaisu is a Professor of Clinical Pharmacy and Practice in the Department of Clinical Pharmacy and Practice, College of Pharmacy, Qatar University. He has co-authored over 200 papers in international peer-reviewed journals. His main areas of research include medication safety, medicines optimization, and pharmacy education.
Derek Stewart
Professor Derek Stewart is a Professor of Clinical Pharmacy and Practice in the Department of Clinical Pharmacy and Practice, College of Pharmacy, Qatar University. He has co-authored over 200 papers in international peer-reviewed journals. His main areas of research include evidence synthesis, medication safety, and pharmaceutical health services research.
Hassaan Anwer Rathore
Dr. Hassaan Anwer Rathore is an Associate Professor in the Department of Pharmaceutical Sciences, College of Pharmacy, Qatar University. He has co-authored over 70 peer-reviewed papers. His main areas of research include evidence synthesis and understanding the pathophysiological basis of disease.
Muhammad Abdul Hadi
Dr. Muhammad Abdul Hadi is an Associate Professor of Clinical Pharmacy and Practice in the Department of Clinical Pharmacy and Practice, College of Pharmacy, Qatar University. He has co-authored over 80 papers in international peer-reviewed journals. He is a research methodologist and likes to work across clinical disciplines. He has a special interest in the development and evaluation of pharmaceutical health services.
References
- Carter, K. L., Gabrellas, A. D., Shah, S., & Garland, J. M. (2017). Improved latent tuberculosis therapy completion rates in refugee patients through use of a clinical pharmacist. The International Journal of Tuberculosis and Lung Disease, 21(4), 432–437. https://doi.org/10.5588/ijtld.16.0575
- Centers for Disease Control and Prevention. (2022). How TB spreads. CDC. https://www.cdc.gov/tb/topic/basics/howtbspreads.htm
- Clark, P. M., Karagoz, T., Apikoglu-Rabus, S., & Izzettin, F. V. (2007). Effect of pharmacist-led patient education on adherence to tuberculosis treatment. American Journal of Health-System Pharmacy, 64(5), 497–505. https://doi.org/10.2146/ajhp050543
- Falagas, M. E., Pitsouni, E. I., Malietzis, G. A., & Pappas, G. (2008). Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. The FASEB Journal, 22(2), 338–342. https://doi.org/10.1096/fj.07-9492LSF
- Haddaway, N. R., Page, M. J., Pritchard, C. C., & McGuinness, L. A. (2022). PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Systematic Reviews, 18(2), e1230. https://doi.org/10.1002/cl2.1230
- Hess, K., Goad, J., Wu, J., & Johnson, K. (2009). Isoniazid completion rates for latent tuberculosis infection among college students managed by a community pharmacist. Journal of American College Health, 57(5), 553–555. https://doi.org/10.3200/jach.57.5.553-556
- Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., Savović, J., Schulz, K. F., Weeks, L., & Sterne, J. A. (2011). The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343(2), Article d5928. https://doi.org/10.1136/bmj.d5928
- Iskandar, D., Suryanegara, F. D. A., van Boven, J. F. M., & Postma, M. J. (2023). Clinical pharmacy services for tuberculosis management: A systematic review. Frontiers in Pharmacology, 14, Article 1186905. https://doi.org/10.3389/fphar.2023.1186905
- Jakeman, B., Logothetis, S. J., Roberts, M. H., Bachyrycz, A., Fortune, D., Borrego, M. E., Ferreira, J., & Burgos, M. (2020). Addressing latent tuberculosis infection treatment through a collaborative care model with community pharmacies and a health department. Preventing Chronic Disease, 17, E14. https://doi.org/10.5888/pcd17.190263
- Jones, J. M. (2011). Record 64% rate honesty, ethics of members of congress low: Ratings of nurses, pharmacists, and medical doctors most positive. Gallup. https://news.gallup.com/poll/151460/record-rate-honesty-ethics-members-congress-low.aspx
- Juan, G., Lloret, T., Perez, C., Lopez, P., Navarro, R., Ramón, M., Cortijo, J., & Morcillo, E. J. (2006). Directly observed treatment for tuberculosis in pharmacies compared with self-administered therapy in Spain. The International Journal of Tuberculosis and Lung Disease, 10(2), 215–221.
- Karuniawati, H., Putra, O. N., & Wikantyasning, E. R. (2019). Impact of pharmacist counseling and leaflet on the adherence of pulmonary tuberculosis patients in lungs hospital in Indonesia. Indian Journal of Tuberculosis, 66(3), 364–369. https://doi.org/10.1016/j.ijtb.2019.02.015
- Lara-Júnior, C. R., Ahouagi, A. E. O., Pinto, I. V. L., Braga, D. G., Andrade, T. R., Ramalho-de-Oliveira, D., & Nascimento, M. M. G. D. (2022). Implementation and effectiveness of a pharmacotherapeutic follow-up service for people with tuberculosis in primary healthcare. International Journal of Environmental Research and Public Health, 19(21), 14552. https://doi.org/10.3390/ijerph192114552
- Lopes, A., Miranda, S., Ceccato, M., Silveira, M., Resende, N., & Carvalho, W. (2017). Evaluation of the impact of pharmaceutical care for tuberculosis patients in a Secondary Referral Outpatient Clinic, Minas Gerais, Brazil. Anais da Academia Brasileira de Ciências, 89(4), 2911–2919. https://doi.org/10.1590/0001-3765201720170301
- Manolakis, P. G., & Skelton, J. B. (2010). Pharmacists’ contributions to primary care in the United States collaborating to address unmet patient care needs: The emerging role for pharmacists to address the shortage of primary care providers. American Journal of Pharmaceutical Education, 74(10), S7. https://doi.org/10.5688/aj7410s7
- Miller, R., & Goodman, C. (2020). Quality of tuberculosis care by pharmacies in low- and middle-income countries: Gaps and opportunities. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases, 18, Article 100135. https://doi.org/10.1016/j.jctube.2019.100135
- Moadebi, S., Stark, G., Elwood, R. K., White, R., & Marra, F. (2005). Patient satisfaction with antituberculosis medication counselling: A comparison of services provided by pharmacists and nurses. The Canadian Journal of Hospital Pharmacy, 58(3), 136–141.
- Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P., & Stewart, L. A. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4(1), 1. https://doi.org/10.1186/2046-4053-4-1
- National Institute of Health. (2013). Study quality assessment tools. NIH. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Nowak, R. (1995). WHO calls for action against TB. Science, 267(5205), 1763. https://doi.org/10.1126/science.7892595
- Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan – a web and mobile app for systematic reviews. Systematic Reviews, 5(1), 210–220. https://doi.org/10.1186/s13643-016-0384-4
- Pradipta, I. S., Yanuar, E. O., Nurhijriah, C. Y., Maharani, N. P., Subra, L., Destiani, D. P., & Diantini, A. (2023). Practical models of pharmaceutical care for improving tuberculosis patient detection and treatment outcomes: A systematic scoping review. Tropical Medicine and Infectious Disease, 8(5), 287. https://doi.org/10.3390/tropicalmed8050287
- RAND Corporation. Visit-specific satisfaction instrument (VSQ-9). RAND. https://www.rand.org/health-care/surveys_tools/vsq9.html
- Tang, Z. Q., Jiang, R. H., & Xu, H. B. (2018). Effectiveness of pharmaceutical care on treatment outcomes for patients with first-time pulmonary tuberculosis in China. Journal of Clinical Pharmacy and Therapeutics, 43(6), 888–894. https://doi.org/10.1111/jcpt.12746
- Tanvejsilp, P., Pullenayegum, E., Loeb, M., Dushoff, J., & Xie, F. (2017). Role of pharmaceutical care for self-administered pulmonary tuberculosis treatment in Thailand. Journal of Clinical Pharmacy and Therapeutics, 42(3), 337–344. https://doi.org/10.1111/jcpt.12519
- Tavitian, S. M., Spalek, V. H., & Bailey, R. P. (2003). A pharmacist-managed clinic for treatment of latent tuberculosis infection in health care workers. American Journal of Health-System Pharmacy, 60(18), 1856–1861. https://doi.org/10.1093/ajhp/60.18.1856
- Thomas, A., Joy, J., Kurian, A., & Sivakumar, V. (2018). Socio-epidemiological evaluation of tuberculosis and impact of pharmaceutical care on medication adherence among tuberculosis patients. Asian Journal of Pharmaceutical and Clinical Research, 11(2), 265–268. https://doi.org/10.22159/ajpcr.2018.v11i2.20503
- Tsuyuki, R. T., Beahm, N. P., Okada, H., & Al Hamarneh, Y. N. (2018). Pharmacists as accessible primary health care providers: Review of the evidence. Canadian Pharmacists Journal / Revue des Pharmaciens du Canada, 151(1), 4–5. https://doi.org/10.1177/1715163517745517
- Wong, Y. J., Ng, K. Y., & Lee, S. W. H. (2023). Community pharmacists-led interventions in tuberculosis care: A systematic review. Research in Social and Administrative Pharmacy, 19(1), 5–15. https://doi.org/10.1016/j.sapharm.2022.09.001
- World Health Organization. (2010). Treatment of tuberculosis: Guidelines (4th ed.). WHO. https://apps.who.int/iris/handle/10665/44165
- World Health Organization. (2021a). Global tuberculosis report 2021. WHO. https://www.who.int/publications/digital/global-tuberculosis-report-2021/tb-disease-burden/incidence
- World Health Organization. (2021b). New WHO recommendations issued to improve access to rapid molecular tests for the detection of TB and drug-resistant TB. WHO. https://www.who.int/news/item/07-07-2021-new-who-recommendations-issued-to-improve-access-to-rapid-molecular-tests-for-the-detection-of-tb-and-drug-resistant-tb
- World Health Organization. (2022). Tuberculosis, key facts. WHO. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- World Health Organization, International Pharmaceutical Federation. (2011). The role of pharmacists in tuberculosis care and control India. WHO, FIP. https://www.fip.org/files/fip/WHO/Signing%20ceremony_WHOFIPJointStatement.pdf
- Wright, J., Walley, J., Philip, A., Pushpananthan, S., Dlamini, E., Newell, J., & Dlamini, S. (2004). Direct observation of treatment for tuberculosis: A randomized controlled trial of community health workers versus family members. Tropical Medicine & International Health, 9(5), 559–565. https://doi.org/10.1111/j.1365-3156.2004.01230.x
- Zhang, H., Ehiri, J., Yang, H., Tang, S., & Li, Y. (2016). Impact of community-based DOT on tuberculosis treatment outcomes: A systematic review and meta-analysis. PLoS One, 11(2), e0147744. https://doi.org/10.1371/journal.pone.0147744
- Zvavamwe, Z., & Ehlers, V. J. (2009). Experiences of a community-based tuberculosis treatment programme in Namibia: A comparative cohort study. International Journal of Nursing Studies, 46(3), 302–309. https://doi.org/10.1016/j.ijnurstu.2008.09.013