?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background:
The National Antimicrobial Guidelines (NAG) 2014 and NAG2019 in Malaysia targeted rational and judicious use of antimicrobials. In this study, we assessed the change in antibiotic utilisation and appropriateness due to the guidelines that were implemented from 2011 to 2019.
Methods:
Interrupted time series analyses on rates of antibiotic appropriateness and utilisation were performed using prescription data from public primary care clinics in Malaysia between January 2011 and December 2019. Rates of antibiotic utilisation, reported as Defined Daily Dose (DDD) per 1000 patients per day, were stratified by antibiotic classes.
Results:
Of the 16,081,492 prescriptions recorded during the study period, 4.98% (n = 800,899) contained antibiotics. NAG2014 resulted in a significant increase in antibiotic utilisation trend by 0.029 (p < 0.0001) while NAG2019 had a substantial impact on antibiotic utilisation, decreasing DDD by 1778 and increasing appropriateness by 54.6% (p < 0.0001). Variation in the number of antibiotic molecules being prescribed also decreased after NAG2019.
Conclusion:
Our findings indicate that the introduction of NAG2019 led to a substantial improvement in antibiotic appropriateness. At the same time, antibiotic utilisation decreased. Further research is needed to ascertain and ensure the sustainability of these changes and to establish targeted improvement strategies focusing on reducing inappropriate and unnecessary prescribing.
Background
Antimicrobial resistance (AMR) has become an urgent threat to global health, with bacterial AMR accounting for an estimated 1.27 million deaths worldwide in 2019 (Bell et al., Citation2014). Antibiotic resistance not only impacts health systems significantly through increased treatment failure and prolonged hospital stays, but the need for more expensive and intensive care adds on to the healthcare costs. Antibiotic use is the primary driver of antibiotic resistance (Bell et al., Citation2014). Report on global antibiotic consumption rate showed 46% increment between 2000 and 2018, particularly in low- and middle-income countries (LMICs) which increased by 76% (Browne et al., Citation2021). The rising antibiotic consumption is alarming and thus, has been the target of interventions.
The World Health Organization (WHO) released a global strategy to contain antimicrobial resistance back in 2001 which include measures to promote the rational and judicious use of antibiotics (World Health Organization, Citation2001). Among the key strategies recommended was the antimicrobial stewardship (AMS) programmes, with the creation of antibiotic policy guidelines as a central aspect. Additionally, monitoring antibiotic prescribing is among the research priorities highlighted in the WHO global research agenda for antimicrobial resistant in human health (World Health Organisation, Citation2023).
Patient pressure, limited access to diagnostic tools, and healthcare provider misconceptions can lead to inappropriate antibiotic prescribing in Malaysian primary care. Healthcare providers may feel pressured to prescribe antibiotics to meet patient expectations or due to time constraints during consultations. Without access to rapid diagnostic tests or clear guidelines, they may resort to empirical prescribing, leading to overuse and misuse of antibiotics (Ahmad et al., Citation2021; H. P. Koh et al., Citation2021; Shamsuddin et al., Citation2016; Teng et al., Citation2004).
Antibiotic appropriateness in primary care health clinics in Malaysia have been reported to range between 37.9 and 81.4% (Lim et al., Citation2021; Shamsuddin et al., Citation2016) while physicians agreed that provision of antimicrobial guidelines helped reduce unnecessary and inappropriate prescription of antimicrobials (Akhtar et al., Citation2020; W. L. Tan et al., Citation2015). In Malaysia, the first edition of the National Antibiotic Guideline (NAG) was published in 2008 by the Ministry of Health as the first step toward the promotion of optimal antibiotic therapy. The document is continuously being reviewed and updates to the guideline was released in 2014 and 2019 in accordance to recent evidence and clinical practice (Ministry of Health, Citation2014, Citation2019). Furthermore, the Malaysian Action Plan on Antimicrobial Resistance was issued in 2017 and AMS programme was implemented in health facilities across Malaysia as part of the strategies to combat AMR (Director General of Health Malaysia, Citation2018; Ministry of Health and Ministry of Agriculture & Agro-Based Industry Malaysia, Citation2017).
By implementing evidence-based interventions tailored to the specific challenges and trends in Malaysian public primary care settings, it is possible to improve antibiotic prescription practices, mitigate antibiotic resistance, and promote rational antibiotic use for better patient outcomes. Despite the issuance and dissemination of the guidelines, little is known as to whether it has had any impact on the antibiotic utilisation in the population. Furthermore, studies have reported prevalence of inappropriate antibiotic use of varying magnitudes, which plays a substantial role in the emergence of antibiotic resistance (Sulis et al., Citation2020). Evaluations of the impact of these guidelines can help policy makers and healthcare providers make more informed decisions about antibiotic use, leading to better patient outcomes and reduced antibiotic resistance rates. As such, this study aimed to address this gap by assessing antibiotic prescriptions in public primary care clinics in Malaysia between 2011 and 2019 and evaluate the effects of implementing the antibiotic guideline on the trend in utilisation and appropriateness of antibiotic prescriptions.
Methods
This study was reported in accordance to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist (Supplemental Table S1).
Data source
The prescription data was obtained from the Teleprimary Care (TPC) database, Ministry of Health (MOH) Malaysia. TPC is a health clinic information system that has been implemented in public primary care clinics under the MOH since 2007. In 2019, there were 93 public primary care clinics across Malaysia that used TPC. The database includes information on clinic visits with details on patient demographic characteristics, diagnoses, treatments, procedures, and prescriptions. Drugs were coded according to the Anatomical Therapeutic Chemical (ATC) classification system and diagnoses were recorded using the International Classification of Diseases 10th Revision (ICD-10) (WHO Collaborating Centre for Drug Statistics Methodology, Citation2023). All prescriptions were anonymised before data extraction to ensure patient confidentiality.
Study design and population
This study was a retrospective analysis of antibiotic prescriptions for the period of 1 January 2011 until 31 December 2019. All patients aged 12 years and above who received a prescription for an antibiotic during the study period were included. We excluded data from clinics with incomplete data for all nine years of the study period. Based on these criteria, data from 66 clinics from seven states in Malaysia were available for analysis. Additionally, we included a control group of patients with a prescription of amlodipine from the same clinics. Amlodipine was used as a negative control series because its utilisation is unaffected by the issuance of and updates to the National Antibiotic Guidelines over the study period.
Antibiotics
All J01 coded drugs (antibiotics for systemic use) were included before aggregation into antibiotic classes. Streptomycin prescriptions were excluded as it is used specifically for treatment of tuberculosis.
Interventions: national antibiotic guidelines 2014 & national antimicrobial guidelines 2019
Between 2011 and 2019, two versions of national antibiotic guidelines were published in Malaysia to guide clinical management and antimicrobial therapy. The National Antibiotic Guidelines 2014 (NAG2014) was released in December 2014, which was an update from the first edition dated 2008. This guideline focuses on recommendations that are not only evidence-based, but also pertinent to the local situation and realities. NAG2014 was made available in both hard copies and online versions, available for download (Ministry of Health, Citation2014). Subsequently, the third edition of the guideline (National Antimicrobial Guidelines 2019, NAG2019) was made available online in March 2019, along with the Clinical Pathways 2019 (CP2019). CP2019 is a collection of flowcharts for the diagnosis and prescription of antibiotics for infective conditions most seen in primary care: acute bronchitis and pneumonia, acute otitis media, acute pharyngitis, acute rhinosinusitis, skin and soft tissue infections (impetigo, cellulitis, and abcess), urinary tract infection, and acute gastroenteritis (Ministry of Health, Citation2019). These flowcharts were then printed and displayed in consultation rooms as part of the implementation process. NAG2019 was implemented with the AMS programme for primary care along with training workshops, structural and clinical audits, and patient education/awareness campaigns. The training workshops were conducted in stages over various regions of the country in March 2019 with full implementation of the guidelines and programme in April 2019 (Director General of Health Malaysia, Citation2018).
Based on this, the study period was divided into the following segments for evaluation of the impact of guidelines on antibiotic utilisation, Segment 1: January 2011 to December 2014 – This period was before NAG2014 was introduced; Segment 2: January 2015 to March 2019 – NAG2014 was disseminated the month prior to this period; Segment 3: April 2019 to December 2019 – NAG2019 was implemented along with the AMS programme in the month prior to this period. For evaluation of the impact of guidelines on antibiotic appropriateness, the segments were the same except for the first which began later in January 2013 as mandatory input of diagnosis with every prescription order in the clinic system was not enforced until then.
Outcomes
The main outcome was antibiotic utilisation rate. The volume of antibiotic utilisation was calculated based on total number of antibiotics prescribed and its defined daily dose (DDD) as defined by the WHO (World Health Organization, Citation2023). Antibiotic utilisation rates were reported as DDD per 1000 patients per day, stratified by antibiotic classes: cephalosporins, macrolides, penicillins, sulfonamides, tetracyclines, and others. The calculation for DDD per 1000 patients was based on patient attendance at the included clinics. Diagnoses for antibiotic prescriptions were aggregated by ICD-10 codes (Supplemental Table S2).
We also assessed the appropriateness of antibiotic prescriptions for specific diagnoses. Antibiotic appropriateness was determined based on drug, dose, frequency, and duration for all the diagnoses included in CP2019 except for abscesses. Antibiotic appropriateness was not evaluated for abscesses as culture and sensitivity testing is required prior to antibiotic initiation and patients are referred to hospitals should culture and sensitivity testing be required. Prescriptions were deemed inappropriate once the drug of choice did not match guidelines for the time period. Appropriateness of antibiotic dose, frequency, and duration were only evaluated in cases of appropriate drugs. Proportions of inappropriate drug, dose, frequency, and duration were tabulated by study segments.
Statistical analysis
Descriptive analysis was performed to describe patient characteristics, antibiotic utilisation rate, and appropriate antibiotic prescriptions by diagnoses. Two monthly time series were constructed. The first series on antibiotic utilisation was constructed using the DDD per 1000 patients from January 2011 to December 2019. The second series on antibiotic appropriateness determine the trend in percentage of appropriate antibiotic prescriptions only for the period from January 2013 to December 2019.
To investigate the effects of both guidelines, we employed interrupted time series analysis (ITSA) with segmented regression analysis. ITSA is a quasi-experimental research design that allows us to evaluate the longitudinal effects of interventions or incidents. One advantage of using ITSA with observational data to assess the impact of policy changes is its ability to account for the influence of long-term trends in the outcome measures over time. When employed with an appropriate control series, ITSA is a powerful study design to evaluate population health interventions. By using the ITSA design and segmented regression, we can assess both the immediate change (i.e. change in the intercept) and the change in the trend (i.e. change in the slope) associated with the intervention or policy change while controlling for the overall pattern in the outcome rate of interest.
A total of 48 points were used in the first segment (24 points for antibiotic appropriateness), 51 points were used in the second segment, and 9 points were used in the third segment. Our study satisfies and surpasses the minimum number of points required for analysis as a minimum of nine points are required pre- and post-interventions in ITSA (Jandoc et al., Citation2015). An ITSA regression model was fitted for the outcome measure (antibiotic utilisation) with the following equation:
(1)
(1) Here, Yt is the DDD level of antibiotics measured monthly; Tt is the number of months since January 2011; Xt is a dummy indicator representing the impact of the guideline (pre-intervention: 0, post-intervention 1); Z is a dummy variable to denote the cohort assignment (antibiotic or control), while XtTt, ZTt, ZXt, and ZXtTt are all interaction terms among previously described variables. β0 to β3, represent the control group, and the coefficients of the upper line, β4 to β7, represent values of the antibiotic group. Specifically, β4 represents the DDD level (intercept) difference between the antibiotic and control group prior to the intervention, β5 indicates the DDD trend difference between the antibiotic and control group prior to the intervention, β6 refers to the DDD level difference between the antibiotic and control group immediately following introduction of the intervention, and β7 represents the difference in DDD trends between the antibiotic and control groups after initiation of the intervention compared with preintervention. The term ϵt, denoting the error at time t, encompasses the random fluctuations that the model is unable to account for (Turner et al., Citation2021).
Two-sided significance level of 5% was applied in all tests. Test for stationarity, a key assumption in time series analysis, was conducted using the augmented Dickey-Fuller (ADF) unit-root test. The null hypothesis of ADF test is that there is a unit root in a time series sample, which implies that the data series is not stationary (Jalil & Rao, Citation2019). Autocorrelation was also tested using the Durbin Watson D test, which depends upon two quantities; the number of observations and the number of parameters to test. R software, version 4.1.0 (http://www.R-project.org) and STATA version 15.1 were used to perform all data processing and analysis, including data cleaning (StataCorp, Citation2017).
Results
Of the 16,081,492 prescriptions recorded from 54,547,253 patients in TPC during the study period, 4.98% (n = 800,899) contained antibiotics. shows characteristics of patients with antibiotic prescriptions who were included in the analysis. Approximately half of the patients aged between 21 and 50 years. Most patients were female (53.4%) and of Malay ethnicity (59.9%). Diseases of the respiratory system, J00-J99 were the most common diagnoses at 31.1%. One in every 14 prescriptions (7.15%) included in this study did not include a diagnosis although the proportion decreased over the years. The number of patients with antibiotic prescriptions showed a steady increase over the years, from 50,687 in 2011 to 111,149 in 2019.
Table 1. Demographics of patients with antibiotic prescriptions from 2011 to 2019.
Antibiotics utilisation
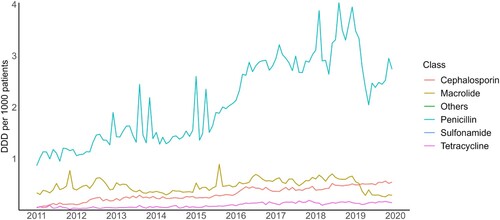
A total of 22 antibiotic molecules from six antibiotic groups were included in this study. shows monthly antibiotic utilisation rates by classes from 2011 to 2020. Penicillin was the most commonly prescribed class of antibiotics over the nine years. This was followed by macrolides, although it dropped to the third place from 2019 onwards. Use of both cephalosporins and tetracyclines showed a steady increase over the years, with use of cephalosporins surpassing macrolides in 2019. The antibiotics with the highest usage were amoxycillin, erythromycin ethyl succinate, and cloxacillin (Supplemental Figure S1). Amoxycillin was mostly used for ICD10: J00-J99 group of diagnosis (diseases of the respiratory system) and ICD10: R00-R99 (symptoms, signs and abnormal clinical and laboratory findings) while cloxacillin was most often prescribed for ICD10: L00-L99 (diseases of the skins and subcutaneous tissue). A total of 31,588 (3.94%) prescriptions for antibiotics were for diagnoses under the endocrine, nutritional and metabolic diseases group (E00-E89) while 37,167 (4.64%) prescriptions for antibiotics were for diagnoses under the neoplasms group (C00-D49) (Supplemental Table S3). Overall, our study had an antibiotic prescription rate of 1.47 per 100 patient attendances.
Figure 1. Trend in monthly antibiotic utilisation rates in defined daily dose per 1000 patients per day (DID), during 2011–2020.
The segmented linear regression model showed a baseline difference between antibiotic and amlodipine (control) utilisation of 1.679 (p < 0.0001). There was slight difference in baseline trend of antibiotic and amlodipine use by 0.013 (p = 0·0070). After NAG2014, there was a non-significant level increase in antibiotic utilisation by 0.384 (p = 0·12). This was followed by a significant increase in antibiotic utilisation trend by 0.029 (p < 0·0001). Although there was a significant decrease in antibiotic utilisation level by 1.778 (p < 0·0001) after the introduction of NAG2019 in segment 3 of the analysis, it was accompanied by an increase in antibiotic utilisation trend by 0.032 (p < 0·0001) (a, (a)).
Figure 2. Trend in monthly (a) antibiotic utilisation rates in defined daily dose per 1000 patients per day (DID), from 2011 to 2019; (b) percentage of appropriate antibiotic prescriptions from 2013 to 2019. Time series interruption where the 2014 and 2019 National Antibiotic Guidelines (NAG) were introduced is indicated by the vertical line.
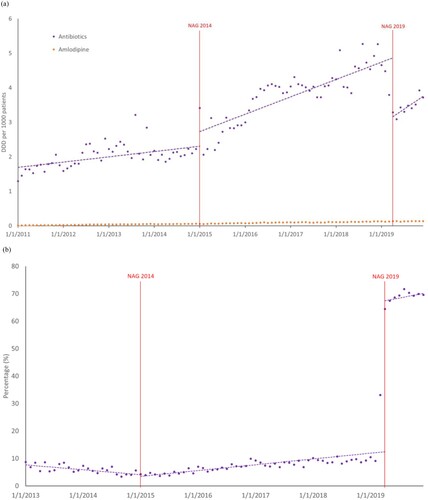
Table 2. Segmented linear regression model on (a) the change of antibiotic utilisation rates before and after the National Antibiotic Guidelines 2014 and 2019; (b) the rate of antibiotic appropriateness before and after the National Antibiotic Guidelines 2014 and 2019.
Appropriateness of antibiotics prescription
Results for time series analysis of antibiotic appropriateness are shown in (b) and (b). During the first segment of the study period (pre-NAG2014), the percentage of appropriate antibiotic prescriptions was approximately 7.77%, with a decreasing trend by 0·144 (p < 0.0001). Level change following NAG2014 was not significant but an increase by 0·317 (p < 0.0001) in antibiotic appropriateness trend was observed. The NAG2019 had a substantial impact on antibiotic appropriateness, increasing by 54.60% (p < 0.0001) after implementation with a 0·34% (p = 0.070) increase in trend. Model diagnostics did not indicate significant autocorrelation of residuals while the augmented Dickey-Fuller test showed stationarity in the data.
Overall antibiotic appropriateness was found to be 17.7%. When stratified by age groups, antibiotic utilisation and appropriateness was seen to be decrease as age of patients increased. Adolescents (12–20 year olds) were seen to be most frequently prescribed antibiotics, ranging from 7.47% to 15.20% of total annual prescriptions with antibiotic utilisation at 0.276–0.635 DDD. However, antibiotic appropriateness was also highest in this age group. The elderly (71–90 years & 81 years and above) had generally lower rates of antibiotic utilisation and appropriateness (Supplemental Figure S2).
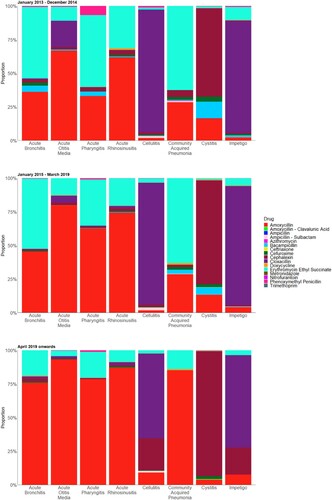
shows the rate of antibiotic appropriateness by diagnoses for each of the segmented time period. Before the implementation of NAG2019, antibiotic prescriptions for impetigo had the highest proportion of appropriateness (63.1% before NAG2014; 77.9% after NAG2014). However, after the implementation of NAG 2019, antibiotic prescriptions for acute otitis media had the highest proportion of appropriateness at 91.8%. This was followed by prescriptions for acute pharyngitis (90.2%) and acute rhinosinusitis (86.0%). Proportions of inappropriate drugs, doses, and durations were similar; only the proportion of prescriptions with inappropriate frequency was particularly lower than the aforementioned three conditions. The proportion of prescriptions with inappropriate drugs showed a decreasing trend in all diagnoses except acute rhinosinusitis, cellulitis, impetigo, and pneumonia. Prescriptions for acute bronchitis, acute otitis media, and acute pharyngitis showed significantly higher proportions of inappropriate durations compared to other diagnoses (). A total of 276 (before NAG2014), 1717 (before NAG2019) and 217 (after NAG2019) antibiotic prescriptions included acute gastroenteritis as diagnosis.
Table 3. Antibiotic appropriateness by diagnosis for each segmented time period.
The types of antibiotics prescribed for the specific diagnoses are described in for the three segments of the study period. We observed fewer variation in the number of antibiotic molecules being prescribed after NAG2019 for almost all diagnoses except for impetigo, which coincidently had the lowest rate of antibiotic appropriateness then. Upon the introduction of both guidelines, the proportion of amoxycillin use increased in acute bronchitis, acute otitis media, acute pharyngitis, acute rhinosinusitis, cellulitis and community acquired pneumonia (CAP), while the proportion of cephalexin use increased in cystitis ().
Discussion
To the best of our knowledge, this dataset is the most extensive (comprising nearly a million data entries) and covers the longest duration of individual antibiotic prescriptions ever examined to assess both antibiotic usage and its appropriateness in Malaysia. Our findings indicate that NAG2019 had a much greater impact on antibiotic utilisation and appropriateness compared to NAG2014. It is worth noting that despite an increasing trend of antibiotic utilisation following NAG2019, the rate of antibiotic appropriateness also improved, albeit non-significant trend.
Our study showed a lower rate of antibiotic prescriptions in primary care than that in other low- and middle-income countries such as Brazil and China with studies reporting the proportion of prescriptions containing antibiotics ranging from 17.8% to 61.0% (Lima et al., Citation2017; Zhan et al., Citation2019; Zhang et al., Citation2017). A Singaporean study reported an antibiotic prescription rate of 4.33 per 100 patient attendances which was almost three times our rate of 1.47 per 100 patient attendances (S. W. C. Koh et al., Citation2023). When compared to another neighbouring country, Indonesia, our rate of antibiotic prescriptions fared well below their reported rates in the range of 23.5–41.0% (Abdulah et al., Citation2019; Yuniar et al., Citation2017). Nevertheless, the methodological and study population differences between these studies limit a direct comparison to our results.
Despite a low proportion of prescriptions contained antibiotics, a substantial proportion were considered inappropriate antibiotic prescriptions prior to the introduction of NAG2019. This is in line with multiple local studies (both in primary care and hospitals) which have shown inappropriate use of antibiotics. A point prevalence survey conducted in local primary care clinics in 2019 immediately after introduction of NAG2019 reported an antibiotic appropriateness rate of 37.9% (Lim et al., Citation2021). Tan et al. (Citation2017) found that 40% of prescriptions from a regional secondary hospital were inappropriate. Another study evaluating antibiotic prescriptions in primary care in Yinchuan, China reported a rate of overall antibiotic appropriateness at 13.3%, similar to our 17.7% (Zhao et al., Citation2022).
A study that evaluated the impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico showed that despite the different overall usage patterns of antibiotics in these countries, the restrictions on sale of over the counter antibiotics had decreased the overall antibiotics consumption in these two countries by 1.35 and 1.17 DDD per 1000 inhabitants, respectively (Santa-Ana-Tellez et al., Citation2013). In our study, the impact of NAG2019 in reducing antibiotic consumption was greater than this over-the-counter restrictions by approximately 31% and 51%, respectively. Another study in France found that a nationwide campaign reduced unwarranted antibiotic prescriptions by 45%, especially in children, and provided an effective approach for evaluating public health strategies aimed to reduce antibiotic use (Sabuncu et al., Citation2009). This was similar to the effect of NAG2019 and AMS programmes which reduced antibiotic consumption by 35%.
Antibiotic prescriptions for two upper respiratory tract infections, acute pharyngitis and acute rhinosinusitis, were evaluated in this study. Prior to the implementation of NAG2014, antibiotic appropriateness was 2.91% for these two diagnoses. This increased slightly to 5.46% from the first to second segment, and reached 89.77% after the implementation of NAG2019. The level of antibiotic appropriateness was much higher than that of a study from Ecuador which reported a level of 9.8%. A cross-sectional study of antibiotics prescribed for acute sinusitis cases in primary care showed that only half of the cases met antibiotic prescribing criteria while Shively, et al. found that no antibiotic was indicated in 49.7% of cases in primary care clinics within a veteran affairs health care system (Shively et al., Citation2018; Truitt et al., Citation2020).
Although a large proportion of prescriptions for cellulitis prior to NAG2019 were for cloxacillin, these were deemed inappropriate as cloxacillin was only recommended for inpatient treatment (to be given intravenously) in the guidelines then. In this case, route of administration is crucial as it also affects bioavailability, hence efficacy of the drug, ultimately affecting the risk of antibiotic resistance. We also observed a reduction in the use of cefuroxime for treatment of cystitis after NAG2019. This is beneficial in reducing the risk of extended-spectrum beta-lactamases (ESBL) related infections (Shaikh et al., Citation2015). Use of bacampicillin in the treatment of CAP also decreased after NAG2019, in line with treatment guidelines (Ministry of Health, Citation2019). However, there were still antibiotics prescribed for acute gastroenteritis despite guidelines specifying antibiotics were not recommended in these cases (Ministry of Health, Citation2014, Citation2019). The lower variation in antibiotics used in most diagnoses is a positive outcome as less prescriptions contained the wrong choice of drug, which in turn, decreased risk of antibiotic resistance.
AMS programmes endeavours crafted to enhance the utilisation of antimicrobial agents, encompassing antibiotics, within medical environments. These initiatives strive to enhance patient outcomes, diminish the development of antimicrobial resistance, and decrease the negative repercussions linked to unwarranted antimicrobial usage. The effectiveness of strategies to address antimicrobial consumption and appropriateness can vary based on several factors related to their implementation. Our results indicate the difference in effectiveness of two sets of guidelines and the possible reasons behind this difference.
The difference in the impact of NAG2014 and NAG2019 is postulated to be due to the difference in deployment of the guidelines. The implementation of NAG2014 faced challenges since it was the first version produced and there were limited resources available to promote and disseminate, as well as guideline complexity and difficulty with practice integration. Subsequently, when NAG2019 was made available, the implementation process was refined and it was introduced together with structural and clinical audits as part of the AMS programme, to provide feedback to prescribers regarding their antibiotic use practices and increase accountability (Director General of Health Malaysia, Citation2018). Budgets were allocated for provision of training for healthcare providers while the AMS programme involved collaboration amongst various healthcare professionals, including infectious disease specialists, family medicine specialists, clinical pharmacists, microbiologists, and infection control personnel, providing a comprehensive approach to optimising antibiotic use and ensuring appropriate prescribing practices. This multidisciplinary approach as well as clearer guidelines and protocols proved to be a more structured approach to improve adherence to guidelines.
Furthermore, the inclusion of flowcharts within the CP2019 with NAG2019 provided clearer evidence-based guidelines for clinical decision-making to help ensure consistent and appropriate antibiotic use in addition to improved workflow integration. This knowledge is essential to policymakers in consideration of resources for the implementation of various strategies for changing antimicrobial prescribing behaviour. These approaches comprise educating prescribers on appropriate antimicrobial utilisation, establishing an antimicrobial formulary that limits the prescription of specific agents, and evaluating antimicrobial prescription patterns while providing feedback to the prescribers (Drew, Citation2009). More importantly, prescribers do not receive incentives for prescribing antibiotics as per guidelines. Therefore, the guidelines and/or interventions have to be relevant and practical to make it more feasible to be adopted in the clinical practice more naturally.
Our study provides valuable insights into the effectiveness and implementation challenges of existing antibiotic guidelines in Malaysia. Study findings will contribute as guidance policy-makers in future revisions and updates of the existing guidelines. The evaluation findings also serve as a foundation for designing and improving targeted AMS initiatives. By leveraging the evidence generated by the evaluation, stakeholders can prioritise and tailor interventions to address specific challenges identified in the local context. Moreover, the insights gained here can contribute to global efforts to combat antimicrobial resistance by knowledge sharing to facilitate the adaptation of successful strategies and interventions to diverse healthcare settings worldwide, ultimately contributing to a more coordinated and effective global response to antimicrobial resistance.
Our study was not without limitations. First, we were unable to evaluate the sustainability of NAG2019’s impact due to possible changes in antibiotic prescribing patterns caused by the COVID-19 pandemic. It would be ideal to measure the sustainability of these changes observed once the antibiotic prescribing patterns have stabilised. Second, our findings were also limited by the lack of a recorded diagnosis for a sizeable proportion of the antibiotic prescriptions. Efforts to improve the quality of documentation should therefore be encouraged. Third, the data included in this study were from 66 public clinics (approximately 6% of all public primary care clinics in Malaysia). While we are confident that the findings described the practice within public health clinics in Malaysia to certain levels, it may not represent the primary care practice in Malaysia as a whole since the practice may differ in the private primary care setting.. The study dataset, however, remains one of the largest prescription-level data for antibiotic utilisation and appropriateness with samples throughout the country in Malaysia.
Furthermore, there was no specific variable to indicate pregnancy status in the database used for this study; therefore, some cystitis diagnoses occurring in pregnancy may have been incorrectly analysed as cystitis if they used the incorrect ICD-10 code. Consequently, the appropriate choice of drug may not have been correctly identified. In this study, whether an antibiotic is required, or antibiotic necessity, was not evaluated. Given the particular diagnosis recorded in the database, the antibiotic appropriateness evaluated in this study focuses on whether the prescribed antibiotic and its use are suitable and effective for the specific case; while antibiotic necessity addresses whether antibiotics are required for a given medical condition by considering whether the underlying infection is caused by bacteria that can be effectively treated with antibiotics.
Although the public sector is performing relatively well following NAG2019, antibiotic consumption and appropriateness in the private sector remain as gaps to be addressed in future research. Additional efforts are still needed to gather further evidence on formulating suitable and efficient policy directions to decrease inappropriate antibiotic usage within the framework of healthcare system reforms in Malaysia despite the relative success of NAG2019. Strategies to promote the appropriate use of antibiotics in the community are also important to ensure ideal antibiotic consumption. The effectiveness of AMS programmes and interventions targeting antibiotic use depends on a combination of factors, including interdisciplinary collaboration, evidence-based guidelines, education, data-driven decision-making, and continuous improvement. By addressing these aspects thoughtfully and tailoring the programme to the local context, healthcare facilities can enhance their ability to optimise antimicrobial use, improve patient care, and combat the growing threat of antimicrobial resistance.
Conclusion
Our findings indicate that the introduction of a national antimicrobial guideline (namely the NAG2019) alongside the AMS programme, led to a substantial improvement in antibiotic appropriateness across public primary care clinics in Malaysia. At the same time, antibiotic utilisation decreased. Further research is needed to ascertain and ensure the sustainability of these changes, to explore factors influencing inappropriate and unnecessary prescribing, as well as to identify measures to address these issues.
List of abbreviations
DDD Defined Daily Doses
WHO World Health Organisation
MOH Ministry of Health Malaysia
AMS Antimicrobial Stewardship
ATC Anatomical Therapeutic Chemical
NAG2014 National Antibiotic Guideline 2014
NAG2019 National Antimicrobial Guideline 2019
CP2019 Clinical Pathways 2019
Authors’ contributions
Conceived and designed the study: AHL, NAR, SMO, and SS. Collected and interpreted the data: SRMA, FZMR, MI, and BKH. Performed data analysis: AHL. Writing of first draft: AHL and NAR. Critical revision and editing: SMO, SRMA, FZMR, MI, PSKT, BKH, and SS. All authors approved the final version of the manuscript.
Ethics approval
This study was registered in the National Medical Research Register (NMRR-21-33-57952) and approved by the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia.
Supplemental Material
Download MS Word (811.5 KB)Acknowledgement
The authors thank the Director General of Health Malaysia for his permission to publish this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data and materials used in this study is not publicly available but can be provided upon reasonable request from the corresponding author (Audrey Huili Lim, [email protected]).
References
- Abdulah, R., Insani, W. N., Putri, N. E., Purba, H. P., Destiani, D. P., & Barliana, M. I. (2019). Pattern of medication use in geriatric patients at primary health care facilities in Karawang, Indonesia. Drug, Healthcare and Patient Safety, 11, 1–5. https://doi.org/10.2147/dhps.s187829
- Ahmad, A., Nor, J., Abdullah, A. A., Tuan Kamauzaman, T. H., & Yazid, M. B. (2021). Patient factors in inappropriate antibiotic prescribing for upper respiratory tract infection in the emergency department. The Malaysian Journal of Medical Sciences: MJMS, 28(2), 72–83. https://doi.org/10.21315/mjms2021.28.2.7
- Akhtar, A., Khan, A. H., Zainal, H., Ahmad Hassali, M. A., Ali, I., & Ming, L. C. (2020). Physicians’ perspective on prescribing patterns and knowledge on antimicrobial use and resistance in Penang, Malaysia: A qualitative study [original research]. Frontiers in Public Health, 8, 1–8. https://doi.org/10.3389/fpubh.2020.601961
- Bell, B. G., Schellevis, F., Stobberingh, E., Goossens, H., & Pringle, M. (2014). A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infectious Diseases, 14(1), 13. https://doi.org/10.1186/1471-2334-14-13
- Browne, A. J., Chipeta, M. G., Haines-Woodhouse, G., Kumaran, E. P. A., Hamadani, B. H. K., Zaraa, S., Henry, N. J., Deshpande, A., Reiner, R. C., Day, N. P. J., Lopez, A. D., Dunachie, S., Moore, C. E., Stergachis, A., Hay, S. I., & Dolecek, C. (2021). Global antibiotic consumption and usage in humans, 2000–18: A spatial modelling study. The Lancet Planetary Health, 5(12), e893–e904. https://doi.org/10.1016/S2542-5196(21)00280-1
- Director General of Health Malaysia. (2018). Surat Pekeliling Ketua Pengarah Kesihatan Bil. 3 / Tahun 2018: Pelaksanaan Program Antimicrobial Stewardship (AMS) di Hospital dan Klinik Kesihatan. Ministry of Health Malaysia.
- Drew, R. H. (2009). Antimicrobial stewardship programs: How to start and steer a successful program. Journal of Managed Care Pharmacy: JMCP, 15(2 Suppl), S18–S23. https://doi.org/10.18553/jmcp.2009.15.s2.18
- Jalil, A., & Rao, N. H. (2019). Chapter 8 – Time series analysis (stationarity, cointegration, and causality). In B. Özcan & I. Öztürk (Eds.), Environmental kuznets curve (EKC) (pp. 85–99). Academic Press. https://doi.org/10.1016/B978-0-12-816797-7.00008-4
- Jandoc, R., Burden, A. M., Mamdani, M., Lévesque, L. E., & Cadarette, S. M. (2015). Interrupted time series analysis in drug utilization research is increasing: Systematic review and recommendations. Journal of Clinical Epidemiology, 68(8), 950–956. https://doi.org/10.1016/j.jclinepi.2014.12.018
- Koh, H. P., Ambaras Khan, R., Tay, S. L., Gill, M. K., Wong, J. Y., & Zainuddin, M. K. (2021). Appropriateness of antimicrobial prescribing in the high-burden emergency department of a tertiary hospital in Malaysia. International Journal of Clinical Pharmacy, 43(5), 1337–1344. https://doi.org/10.1007/s11096-021-01255-w
- Koh, S. W. C., Lee, V. M. E., Low, S. H., Tan, W. Z., Valderas, J. M., Loh, V. W. K., & Hsu, L. Y. (2023). Prescribing antibiotics in public primary care clinics in Singapore: A retrospective cohort study. Antibiotics (Basel), 12(4), 762. https://doi.org/10.3390/antibiotics12040762
- Lim, A. H., Wong, S. T., Thian, S. Y., Tew, W. Y., Chiew, S. Y., Lee, X., & Hor, Y. Y. (2021). Antibiotic prescribing pattern in primary care practice in federal territory Kuala Lumpur and Putrajaya, Malaysia. International Journal of Advancement in Life Sciences Research, 4(4), 15–24.
- Lima, M. G., Dutra, K. R., & Martins, U. C. M. (2017). Prescribing indicators in primary health care in Belo Horizonte, Brazil: Associated factors. International Journal of Clinical Pharmacy, 39(4), 913–918. https://doi.org/10.1007/s11096-017-0501-z
- Ministry of Health. (2014). National Antibiotic Guideline 2014. Pharmaceutical Services Programme.
- Ministry of Health. (2019). National Antimicrobial Guideline 2019. Pharmaceutical Services Programme.
- Ministry of Health and Ministry of Agriculture & Agro-Based Industry Malaysia. (2017). Malaysian Action Plan on Antimicrobial Resistance (MyAP-AMR) 2017-2021.
- Sabuncu, E., David, J., Bernède-Bauduin, C., Pépin, S., Leroy, M., Boëlle, P.-Y., Watier, L., & Guillemot, D. (2009). Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLOS Medicine, 6(6), e1000084. https://doi.org/10.1371/journal.pmed.1000084
- Santa-Ana-Tellez, Y., Mantel-Teeuwisse, A. K., Dreser, A., Leufkens, H. G. M., & Wirtz, V. J. (2013). Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS One, 8(10), e75550. https://doi.org/10.1371/journal.pone.0075550
- Shaikh, S., Fatima, J., Shakil, S., Rizvi, S. M., & Kamal, M. A. (2015). Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi Journal of Biological Sciences, 22(1), 90–101. https://doi.org/10.1016/j.sjbs.2014.08.002
- Shamsuddin, S., Akkawi, M. E., Zaidi, S. T., Ming, L. C., & Manan, M. M. (2016). Antimicrobial drug use in primary healthcare clinics: A retrospective evaluation. International Journal of Infectious Diseases, 52, 16–22. https://doi.org/10.1016/j.ijid.2016.09.013
- Shively, N. R., Buehrle, D. J., Clancy, C. J., & Decker, B. K. (2018). Prevalence of inappropriate antibiotic prescribing in primary care clinics within a veterans affairs health care system. Antimicrobial Agents and Chemotherapy, 62(8), e00337–e00318. https://doi.org/10.1128/AAC.00337-18
- StataCorp. (2017). Stata Statistical Software: Release 15.
- Sulis, G., Adam, P., Nafade, V., Gore, G., Daniels, B., Daftary, A., Das, J., Gandra, S., & Pai, M. (2020). Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLOS Medicine, 17(6), e1003139. https://doi.org/10.1371/journal.pmed.1003139
- Tan, G. H., Low, Q.-W., Lim, H.-C., Seah, H.-K., & Chan, H.-K. (2017). Inappropriate antibiotic utilization: Outpatient prescription review of a Regional Secondary Hospital in Kedah, Malaysia. Journal of Pharmacy Practice and Community Medicine, 3(4), 215–219. https://doi.org/10.5530/jppcm.2017.4.62
- Tan, W. L., Siti, R., Shahfini, I., & Zuraidah, A. (2015). Knowledge, attitude and practice of antibiotics prescribing among medical officers of public health care facilities in the state of Kedah, Malaysia. The Medical Journal of Malaysia, 70(5), 307–311.
- Teng, C. L., Achike, F. I., Phua, K. L., Norhayati, Y., Nurjahan, M. I., Nor, A. H., & Koh, C. N. (2004). General and URTI-specific antibiotic prescription rates in a Malaysian primary care setting. International Journal of Antimicrobial Agents, 24(5), 496–501. https://doi.org/10.1016/j.ijantimicag.2004.06.015
- Truitt, K. N., Brown, T., Lee, J. Y., & Linder, J. A. (2020). Appropriateness of antibiotic prescribing for acute sinusitis in primary care: A cross-sectional study. Clinical Infectious Diseases. https://doi.org/10.1093/cid/ciaa736
- Turner, S. L., Forbes, A. B., Karahalios, A., Taljaard, M., & McKenzie, J. E. (2021). Evaluation of statistical methods used in the analysis of interrupted time series studies: A simulation study. BMC Medical Research Methodology, 21(1), 181. https://doi.org/10.1186/s12874-021-01364-0
- WHO Collaborating Centre for Drug Statistics Methodology. (2023). ATC/DDD Index 2023. Norwegian Institute of Public Health. Retrieved 10 January.
- World Health Organisation. (2023). Global research agenda for antimicrobial resistance in human health.
- World Health Organization. (2001). WHO Global Strategy for Containment of Antimicrobial Resistance.
- World Health Organization. (2023). Defined Daily Dose (DDD). Retrieved 10 January from https://www.who.int/tools/atc-ddd-toolkit/about-ddd#:~:text=Defined%20Daily%20Dose%20(DDD)%3A,medicines%20given%20an%20ATC%20codes.
- Yuniar, C. T., Anggadiredja, K., & Islamiyah, A. N. (2017). Evaluation of rational drug Use for acute pharyngitis associated with the incidence and prevalence of the disease at two community health centers in Indonesia. Scientia Pharmaceutica, 85(2), 22. https://doi.org/10.3390/scipharm85020022
- Zhan, Q., Wang, Y. L., & Chen, X. (2019). Evaluation of antibacterial use in outpatients of township and community primary medical institutions in a district of Sichuan Province, China. Journal of Global Antimicrobial Resistance, 19, 201–206. https://doi.org/10.1016/j.jgar.2019.04.021
- Zhang, Z., Hu, Y., Zou, G., Lin, M., Zeng, J., Deng, S., Zachariah, R., Walley, J., Tucker, J. D., & Wei, X. (2017). Antibiotic prescribing for upper respiratory infections among children in rural China: A cross-sectional study of outpatient prescriptions. Global Health Action, 10(1), 1287334. https://doi.org/10.1080/16549716.2017.1287334
- Zhao, H., Wang, S., Meng, R., Liu, G., Hu, J., Zhang, H., & Zhan, S. (2022). Appropriateness of antibiotic prescriptions in Chinese primary health care and the impact of the COVID-19 pandemic: A typically descriptive and longitudinal database study in Yinchuan City [original research]. Frontiers in Pharmacology, 13, 861782. https://doi.org/10.3389/fphar.2022.861782