?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Within Diagnosis Related Groups, based on service capability, efficiency, and quality safety assessment, clinical pharmacists contribute to promoting rational drug utilisation in healthcare institutions. However, a deficiency of pharmacist involvement has been observed in the total parenteral nutrition support to patients following haematopoietic cell transplantation (HCT) within DRGs.
Methods
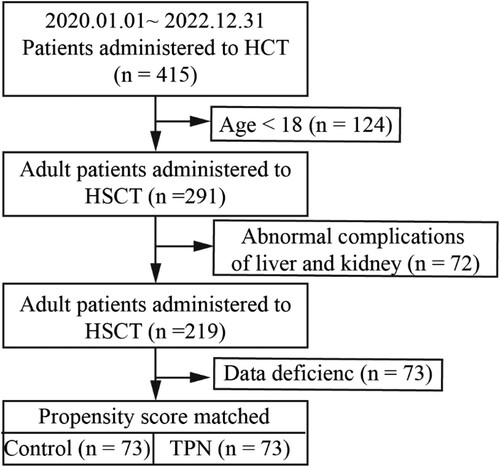
This study involved 146 patients who underwent HCT at the Department of Haematology, the Second Affiliated Hospital of Dalian Medical University, spanning from January 2020 to December 2022.
Results
Patients were allocated equally, with 73 in the control group and 73 in the pharmacist-involved group: baseline characteristics showed no statistics significance, including age, body mass index, nutrition risk screening-2002 score, liver and kidney function, etc. Albumin levels, prealbumin levels were significantly improved after a 7-day TPN support (34.92 ± 4.24 vs 36.25 ± 3.65, P = 0.044; 251.30 ± 95.72 vs 284.73 ± 83.15, P = 0.026). The body weight was increased after a 7-day support and before discharge (58.77 ± 12.47 vs 63.82 ± 11.70, P = 0.013; 57.61 ± 11.85 vs 64.92 ± 11.71, P < 0.001). The length of hospital stay, costs and the rate of re-admissions were significantly shortened (51.10 ± 1.42 vs 46.41 ± 1.86, P = 0.048; 360,162.67 ± 91,831.34 vs 324,070.16 ± 112,315.51, P = 0.035; 61.64% vs 43.84%, P = 0.046).
Conclusions
Pharmacist-joint TPN support enhances the service efficiency score of medical units, ensuring the fulfilment of orders and rational medication.
1. Introduction
Haematopoietic cell transplantation (HCT) represents a cornerstone therapeutic approach for haematologic malignancies. However, during the preparatory phase encompassing haematopoietic stem cell conditioning and stem cell infusion, patients often contend with distressing symptoms such as mucositis, severe vomiting and more. These symptoms render enteral nutrition intolerable, necessitating the adoption of parenteral nutrition support therapy (Evans et al., Citation2022). The American Society of Parenteral and Enteral Nutrition (ASPEN) strongly advocates for pharmacist involvement in the Nutrition Support Team (NST) following their completion of a standardised training programme in parenteral nutrition (PN) support, thus ensuring the delivery of tailored clinical nutrition support for each patient (Vlug et al., Citation2020). Many countries proposed to establish a team by a physician, nurse, dietitian or pharmacist to construct an interdisciplinary NST (Nieto-Gomez et al., Citation2021; Zhou et al., Citation2019). In our hospital, clinical pharmacists participate in the nutrition support especially for total parenteral nutrition (TPN) with professional theoretical knowledge in corresponding with the clinical guidelines and expert consensus. Hence, pharmacists assume a pivotal role in this endeavour, contributing significantly to optimising patient prescriptions and curbing the irrational utilisation of medications such as proton pump inhibitors (PPIs) (Wong et al., Citation2021; Yailian et al., Citation2022; Zhang et al., Citation2021), anti-infective drugs (Kooda et al., Citation2022; Pinet et al., Citation2022; Wang et al., Citation2020), anticoagulants (Bartholomew et al., Citation2023; Gurwitz et al., Citation2021; Navin et al., Citation2022).
TPN therapy entails the intravenous administration of essential nutrients, including glucose, amino acids, and intravenous fat emulsions, infused alongside electrolytes, a diverse array of micronutrients, and vitamins. ASPEN terms this approach as Total Nutrient Admixture (TNA). It is imperative to meticulously consider the concentrations of monovalent and divalent cations, non-protein energy (NPE) content, amino acids, carbohydrates/lipids, and the NPE/Nitrogen ratio when preparing TNA. Improper utilisation of TPN can precipitate metabolic dysfunction, infections, and vascular complications, thereby jeopardising patient prognosis, prolonging hospital stays, and inflating healthcare costs (Boullata et al., Citation2016). Consequently, it’s urgently needed pharmaceutical policy to standardise pharmacist-joint group in TPN application corresponding to the optimise resource allocation and healthcare service delivery.
Diagnosis Related Groups (DRGs) represent a vital facet of medical insurance payment systems, meticulously considering various patient-related factors (Mathauer & Wittenbecher, Citation2013). By categorising patients into highly homogeneous groups with similar healthcare resource consumption patterns, DRGs demands for pharmacists including service capability, service efficiency, and quality safety, collectively fostering the rational use of pharmaceuticals within healthcare institutions (Lang et al., Citation2023; Sun et al., Citation2023). HCT has increasingly become an essential treatment option for many patients undergoing life-threatening haematologic illnesses (Muskens et al., Citation2023). Nevertheless, HCT recipients often endure diarrhoea, dysphagia, which led to terrible nutrition status. Pharmacists-joint group play a pivotal role in the management of patients throughout the TPN formula medication, as well as manifesting in the reduction of TPN-related complications (Evans et al., Citation2019), curtailment of hospitalisation costs, and truncation of hospital stay durations (Tong et al., Citation2022), thereby enhancing the efficiency, quality, and safety of healthcare delivery.
Nevertheless, a notable deficiency exists in the involvement of pharmacists in the provision (Shrestha et al., Citation2022) of TPN support for patients undergoing HCT within the purview of DRGs evaluation (Shi et al., Citation2021; Wu et al., Citation2022). This study contributes to shed light on the tangible impact of pharmacist participation in TPN therapy (Katoue & Al-Taweel, Citation2016) for patients (Wang et al., Citation2019) at risk of malnutrition following HCT within the context of DRGs.
2. Methods
2.1. Pharmacist-joint parenteral nutrition programme
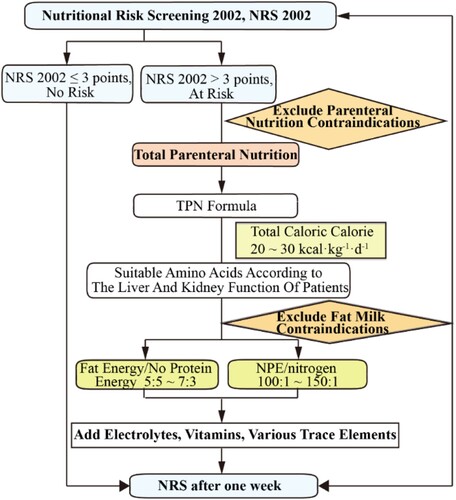
The main researchers of the project are clinical pharmacists specializing in ICU and nutrition. Both have completed a one-year clinical pharmacist training programme organised by the Pharmacy Affairs Committee of the Chinese Hospital Association and hold a clinical pharmacist position certificate. They have been involved in clinical pharmacotherapy as pharmacists for over 10 years. Pharmacists join the NST which includes other members at least a physician, nurse and dietitian. Pharmacists assumed a pivotal role in patient nutritional risk assessment, the development of personalised PN support plans, and the ongoing monitoring of key nutritional indicators throughout the course of parenteral nutritional support (Draft statement on pharmaceutical care., Citation1993). Currently, no established nutritional risk screening (NRS) tool exists specifically tailored for patients with haematological malignancies (). In accordance with robust clinical evidence and authoritative guideline recommendations (McClave et al., Citation2016), patients meeting the criteria of NRS 2002 >3 and exhibiting enteral nutrition intolerance were identified as candidates for PN therapy. Subsequently, the NST devised a standardised protocol for PN support for patients undergoing haematopoietic stem cell transplantation (). This protocol encompassed the formulation of a comprehensive total nutrient admixture support treatment plan.
Table 1. NRS-2002 system (Zang et al., Citation2023).
2.2. Study subjects
The subjects of this study comprised patients admitted to the Department of Haematology at the Second Affiliated Hospital of Dalian Medical University from January 2020 to December 2022, who met the following inclusion criteria: (I) aged ≥ 18 years; (II) patients who had undergone HCT and undergone a complete nutritional assessment. Exclusion criteria included: (I) concurrent solid tumours; (II) coexisting significant organ disorders affecting the heart, lungs, liver, or kidneys; (III) concomitant digestive system diseases. Nutritional risk screening using the NRS 2002 tool was conducted by pharmacists for all patients. Patients who received pharmacist consultations in the formulation of their PN supportive treatment plans were categorised into the pharmacist-joint group, while those without pharmacist involvement in PN plan development were placed in the control group. It is worth noting that pharmacists assessed nutritional risk but did not participate in the creation and monitoring of nutritional regimens.
Parenteral nutrition was initiated when the patient’s food intake decreased by 50% within a week. For patients undergoing HCT, the daily calorie target was set at 1–1.3 times of base energy expenditure (BEE) or given adjusted body weight × 25–30 kcal with standard body weight. BEE of patients was calculated according to Harris-Benedict formula. The adjusted body weight of overweight patients with BMI > 24 kg/m2 was calculated using the following formula (Mizutani et al., Citation2023):
2.3. Propensity scoring match
Data were collected from all hospital discharge records, which include information on the patient’s demographic characteristics. To compare the outcomes of TPN and non-TPN patients, a propensity score matching procedure was performed using a 1:1 propensity score matching analysis was conducted using the nearest-neighbor method with a calliper of 0.20 with no replacement (). All baseline variables included in the matching model are listed in Supplementary Table 1. In particular, all index: age, gender, diagnosis, type of transplant, height, weight, body mass index, NRS-2002, glutamic-pyruvic transaminase, glutamic oxalacetic transaminase, creatinine, albumin, prealbumin, hemoglobin were taken into consideration. The adequacy of covariate balance in the matched sample was assessed via standardised mean differences between the two groups. Patients, for whom no match was found, were discarded from the matched analyses. Odds ratios with their 95% confidence intervals (95%CI) were computed using logistic regression models, adjusted for propensity score as covariate. 2-tailed P-values less than 0.05 were considered significant. The statistical analysis was performed using IBM SPSS Statistics v27.0 (SPSS Inc. Chicago, Illinois, USA).
2.4. Data source
Patient clinical information and pertinent parenteral nutrition details were extracted from electronic medical records, encompassing: (I) patient demographics such as age, gender, weight, transplant type, haematological assessments, length of hospital stay, hospital costs, and the incidence of unplanned readmissions; and (II) TPN prescriptions.
2.5. Cost discounting
Given the duration of this observational study exceeded one year, hospital costs were subjected to discounting, adjusted to 2022 values using a discount rate of 5% (China Guidelines for Pharmacoeconomic Evaluations, Citation2020). The formula for cost discounting was as follows: cost after discount = current cost × (1 + discount rate) (number of discount years).
2.6. Statistical analysis
Data analysis was performed using SPSS version 23.0 software. Pearson’s chi-square test was employed to assess the distribution of categorical data between groups. Continuous variables were presented as mean ± standard deviation ( ± SD) or as median (P25, P75) as appropriate. Statistical significance was defined as P < 0.05.
3. Results
3.1. Basic clinical information of patients
A total of 146 patients were enrolled in this study. Before group matching, the gender of the control group showed difference from the TPN-joint group (P < 0.001). The prealbumin of the TPN group was higher than that of the control group (250.05 ± 95.54 vs 307.87 ± 133.82, P < 0.001). The glutamic oxalacetic transaminase of the TPN group was lower than the control group (31.59 ± 23.37 vs 21.62 ± 17.21, P < 0.001). To minimise the effect of baseline nutrition status on the outcomes, propensity score matching were conducted, and 73 patients from the pharmacist-joint group were matched to 73 patients in the control group (Supplementary Table 2). The similar gender, prealbumin and glutamic oxalacetic transaminase of both groups control group vs TPN group: gender: 46.8% vs 53.2% (Male), P = 0.507; prealbumin: 292.82 ± 77.35 vs 316.39 ± 133.42, P = 0.194; glutamic oxalacetic transaminase: 26.78 ± 54.49 vs 30.54 ± 68.40, P = 0.714. The age of patients was ranged from 18 to 62 years, with an average age of (37.68 ± 12.45) years, consisted of 78 males (53.42%) and 68 females (46.58%). Other baseline characteristics, including weight, body mass index (BMI), diagnosis, type of transplant, nutrition risk screening-2002 score (NRS-2002), as well as kidney function, exhibited no significant disparities between the two groups.
3.2. Comparison of TPN formulations
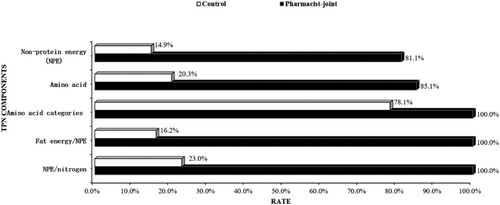
TPN formulations were compared between the two groups. Results illustrated the comparison of TPN parameters, including non-protein energy (NPE), amino acid content, energy composition, and the NPE to nitrogen ratio (NPE/N). The proportion of patients receiving NPE at the recommended range of 20–35 kcal/kg/day was significantly higher in the pharmacist-joint TPN group (81.1%) compared to the control group (14.9%) (P < 0.001). Consistent with guidelines, 85.1% of patients in the pharmacist-joint TPN group met the recommended amino acid dose of 1.0 g/kg for patients with normal renal function, while only 20.3% of patients in the control group reached this standard (P < 0.001). The energy composition ratio of fat and carbohydrates, as per the guideline recommendation of up to 50%, met by 90.5% of patients in the pharmacist-joint TPN group, contrasting with only 16.2% in the control group (P < 0.001). The proportion of TPN regimens conforming to the recommended NPE/N ratio of 100–200 kcal/g nitrogen was 93.2% in the pharmacist-joint TPN group, while it was 23.0% in the control group (P < 0.001, ).
3.3. Impact on clinical outcomes
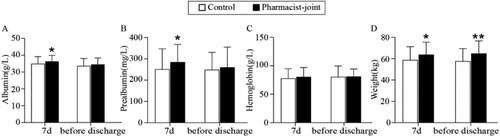
To assess the influence of pharmacist-joint TPN standardisation on clinical outcomes, the postoperative nutritional status of patients was evaluated. Following 7 days of TPN support, the pharmacist-joint group exhibited significantly higher levels of albumin (36.25 g/L) compared to the control group (34.92 g/L) (P < 0.05). Prealbumin level were also notably higher in the pharmacist-joint group (284.73 mg/L) than in the control group (251.30 mg/L) (P < 0.05). The body weight of the pharmacist-joint TPN group (63.82 kg) was significantly higher than that of the control group, which had an average weight of 58.77 kg (P < 0.05). Hemoglobin levels in the pharmacist-joint TPN group (81.12 g/L) showed an increasing trend compared to the control group (77.84 g/L), with no statistically significant difference observed. Furthermore, before discharge, the body weight of the pharmacist-joint TPN group (64.92 kg) remained significantly higher than that of the control group (57.61 kg, P < 0.05). Albumin level exhibited an increasing trend in the pharmacist-joint group (34.5 g/L) in comparison to the control group (33.63 g/L). Prealbumin level in the pharmacist-joint TPN group (260.28 mg/L) showed a higher trend than in the control group (249.06 mg/L), although no statistically significant difference was noted between the two groups. Hemoglobin levels in the TPN group (80.62 g/L) were higher than those in the control group (80.55 g/L), with no significant intergroup difference observed ().
Figure 4. Pharmacist-joint TPN improved nutritional status after 7 days of nutritional support and before discharge. The nutritional indicators of two groups including (A) albumin (g/L), (B) prealbumin (mg/L), (C) hemoglobin (g/L) and (D) weight (kg). Compared to the pharmacist-joint group, *P < 0.05 or **P < 0.01 as significant difference.
3.4. Impact on DRGs assessment
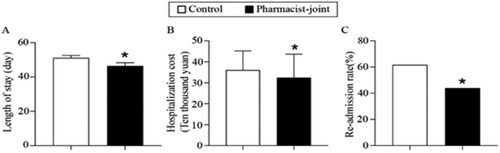
Evaluation of key healthcare metrics revealed notable differences between the two groups. The average length of hospital stay in the pharmacist-joint TPN group (46.41 days) was significantly shorter than that in the control group (51.10 days) (P < 0.05). Hospital costs for the pharmacist-joint group (¥ 324,070.16) demonstrated a marked reduction compared to the control group (¥ 360,162.67) (P < 0.05, ).
4. Discussion
This study demonstrates the substantial benefits of pharmacists’ involvement in TPN support for patients undergoing HCT, aligning with the evolving direction of healthcare institutions following the implementation of DRGs. After a 7-day period of PN support, patients in the pharmacist-joint group exhibited significant improvements in albumin levels, prealbumin levels, and body weight. Furthermore, before discharge, patients in the pharmacist-joint group experienced a noticeable increase in body weight. Importantly, the length of hospital stay was significantly shorter, and hospital costs were notably reduced in the pharmacist-joint group. Additionally, pharmacist involvement in TPN support during HCT was found to significantly reduce the rate of re-admissions.
DRGs, initially originating in the United States, represent a prevalent method for classifying diseases, grouping cases with similar clinical treatment processes or resource consumption. Since the implementation of DRGs has demonstrated positive outcomes in reducing medical costs and shortening average hospital stays, many countries have sought to adopt its mechanisms and adapt them to their specific contexts. In China, the internal mechanism of DRGs, often referred to as ‘balance retained’ and ‘postpaid to prepaid,’ has gained prominence. Chinese public hospitals rely on generating revenue through service provision rather than relying on public funds or government budgets, making them particularly responsive to the financial incentives introduced by DRGs (Zou et al., Citation2020). The implementation of DRGs has incentivised hospitals to enhance the quality and efficiency of medical services while concurrently reducing medical costs. The misuse of TPN, considered a high-alert medication, can lead to complications such as parenteral nutrition-related liver injury, fat overload syndrome, and glucose metabolism disorders, all of which contribute to prolonged hospitalisations, increased costs, and elevated re-admission rates – factors directly impacting the ‘balance retained.’ Rational utilisation of TPN support therapy not only significantly enhances patient nutritional levels but also mitigates TPN-related adverse reactions, thus favourably contributing to the quality and efficiency of medical services. This aligns with the needs of high-quality healthcare development and financial incentives in hospitals operating under the backdrop of DRGs (Trissel, Citation2012).
The implementation of DRGs places considerable emphasis on three key medical indicators: length of hospital stay, hospital costs, and re-admission rates, all of which directly affect the ‘balance retained.’ Our study found that, compared with the control group, patients receiving TPN support from pharmacists experienced significantly shorter hospital stays, indicating a marked improvement in hospital service efficiency. Furthermore, the hospital costs for HCT patients were significantly reduced when pharmacists were involved in TPN support, aligning with the imperative of cost control in hospitals post-DRGs implementation. Re-admission rates, closely linked to increased hospital costs, were also examined as an outcome measure of inpatient healthcare utilisation. We observed that pharmacist-joint TPN support for HCT patients could effectively reduce the re-admission rate. In summary, pharmacist-joint TPN support enhances service efficiency and reduces hospital costs, congruent with the evolving objectives of hospitals post-DRGs implementation.
Nutritional status plays a pivotal role in the quality of life and overall well-being of cancer patients. Malnutrition and weight loss are common contributors to mortality in this patient population (LeCompte & Brawley, Citation2023). Nutrition support therapy is especially relevant for HCT patients experiencing moderate to severe graft-versus-host disease (GVHD), coupled with poor oral intake and significant malabsorption (August & Huhmann, Citation2009). TPN, a well-established method of nutritional support, has been shown to enhance the nutritional status, overall survival, time to recurrence, and disease-free survival of HCT patients compared to 5% glucose solution hydration containing electrolytes, minerals, trace elements, and vitamins. Our results showed that PN support significantly improved albumin levels, prealbumin levels, and body weight. TPN support comprehensively improves the nutritional status of HCT patients unable to orally consume food, thus underscoring its therapeutic significance (Mousavi et al., Citation2013).
Pharmacists, when integrated into the Nutrition Support Team, contribute to individualised TPN support for HCT patients based on standardised protocols. The NST formulates tailored TPN prescriptions for each patient, encompassing appropriate non-protein energy content, amino acid dosages and types, as well as judicious carbohydrate/lipid and NPE/nitrogen ratios. The results of our study highlight that pharmacist-joint TPN services lead to significant enhancements in the nutritional status and clinical outcomes of HCT patients compared to those without pharmacist involvement.
In this study, we measured serum albumin levels, prealbumin levels, and body weight as key indicators of nutritional status. Serum albumin, widely used as a predictor of disease outcomes, holds particular relevance in HCT patients (Brock et al., Citation2016; Fang et al., Citation2020; Horsley et al., Citation2005; Yu et al., Citation2021). Prealbumin, characterised by a shorter half-life compared to albumin, offers superior sensitivity in assessing short-term nutritional changes (Mkhize et al., Citation2018; Ranasinghe et al., Citation2022). Our findings indicate that the average albumin and prealbumin levels were higher in the pharmacist-joint group after 7 days of TPN support, underscoring the ability of pharmacist involvement to improve the nutritional status of HCT patients.
Weight loss is of prognostic significance in cancer patients, with survival often compromised in those experiencing pre-treatment weight loss (Bekhit et al., Citation2021). Weight loss in HCT patients may be attributed to the gastrointestinal toxicity of chemotherapy, GVHD post-transplantation, and the use of immunosuppressive agents (Kamiya et al., Citation2019). The previous reports showed that body weight loss before HCT is associated with survival outcomes (Tamaki et al., Citation2021). Our study showed that patients in the pharmacist-joint TPN group exhibited a significant increase in body weight after 7 days of TPN support compared to the control group. Hence, the data suggested that the positive impact of pharmacist involved in patient prognosis.
5. Limitations
This study has certain limitations. Firstly, it represents a single-center retrospective study, rather than a prospective one. Secondly, the sample size is relatively small compared to prospective studies. Therefore, future studies should focus on elaborately designed, multi-center, and prospective investigations with larger sample sizes. Such refined management is required to substantiate the role of pharmacists in TPN support therapy for HCT patients at nutritional risk within the framework of DRGs.
Author contributions
Le Yang accomplished the data analysis and drafted the manuscript. Lu-lu Qiu designed the study process and refined the language. Hui-yi Lv provided suggestions and evaluated the significance. Miao Li devoted to the writing and completed the final revision of the manuscript. All authors approved the final manuscript to be published.
Ethical approval
The utilisation of human specimens and associated data for research purposes received approval from the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University (Approval No. [2024]23).
Supplemental Material
Download MS Word (19.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- August, D. A., & Huhmann, M. B. (2009). American Society for and D. Enteral Nutrition Board of. A.S.P.E.N. clinical guidelines: Nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. Journal of Parenteral and Enteral Nutrition, 5(33), 472–500. https://doi.org/10.1177/0148607109341804
- Bartholomew, R. R., Noble, B. N., Stanislaw, J. J., Viehmann, M., Herink, M. C., & Furuno, J. P. (2023). Frequency and clinical outcomes of pharmacist-driven switching from warfarin to direct oral anticoagulants in an underserved patient population: A retrospective cohort study. American Journal of Health-System Pharmacy, Suppl 3(80), S103–S110. https://doi.org/10.1093/ajhp/zxac375
- Bekhit, O. E., Yousef, R. M., Abdelrasol, H. A., & Mohammed, M. A. (2021). Serum albumin level as a predictor of outcome in patients admitted to pediatric intensive care units. Pediatric Emergency Care, 12(37), E855–E860. https://doi.org/10.1097/PEC.0000000000002567
- Boullata, J. I., Holcombe, B., Sacks, G., Gervasio, J., Adams, S. C., Christensen, M., Durfee, S., Ayers, P., Marshall, N., Guenter, P., & Nutr, A. S. P. E. (2016). Standardized Competencies for Parenteral Nutrition Order Review and Parenteral Nutrition Preparation, Including Compounding: The ASPEN Model. Nutrition in Clinical Practice, 4(31), 548–555. https://doi.org/10.1177/0884533616653833
- Brock, F., Bettinelli, L. A., Dobner, T., Stobbe, J. C., Pomatti, G., & Telles, C. T. (2016). Prevalence of hypoalbuminemia and nutritional issues in hospitalized elders. Revista Latino-Americana de Enfermagem, 24(0), e2736. https://doi.org/10.1590/1518-8345.0260.2736
- China Guidelines for Pharmacoeconomic Evaluations. (2020). Chinese Pharmaceutical Association.
- Draft statement on pharmaceutical care. (1993). Ashp council on professional affairs. American society of hospital pharmacists. American Journal of Hospital Pharmacy, 1(50), 126–128.
- Evans, J., Green, D., O’Connor, G., Lanigan, J., & Gibson, F. (2022). Nutrition support practices and opinions toward gastrostomy Use in pediatric bone marrow transplant centers: A national survey. Pediatric Blood & Cancer, 69, S206–S206.
- Evans, J. C., Hirani, S. P., & Needle, J. J. (2019). Nutritional and post-transplantation outcomes of enteral versus parenteral nutrition in pediatric hematopoietic stem cell transplantation: A systematic review of randomized and nonrandomized studies. Biology of Blood and Marrow Transplantation, 8(25), e252–e259. https://doi.org/10.1016/j.bbmt.2019.02.023
- Fang, Y., Liu, M. J., Zhang, W. W., Xie, C., & Liu, Z. Z. (2020). Nutrition support practices of hematopoietic stem cell transplantation centers in mainland China. Current Medical Science, 4(40), 691–698. https://doi.org/10.1007/s11596-020-2231-z
- Gurwitz, J. H., Kapoor, A., Garber, L., Mazor, K. M., Wagner, J., Cutrona, S. L., Singh, S., Kanaan, A. O., Donovan, J. L., Crawford, S., Anzuoni, K., Konola, T. J., Zhou, Y., & Field, T. S. (2021). Effect of a Multifaceted Clinical Pharmacist Intervention on Medication Safety After Hospitalization in Persons Prescribed High-risk Medications: A Randomized Clinical Trial. JAMA Internal Medicine, 5(181), 610–618. https://doi.org/10.1001/jamainternmed.2020.9285
- Horsley, P., Bauer, J., & Gallagher, B. (2005). Poor nutritional status prior to peripheral blood stem cell transplantation is associated with increased length of hospital stay. Bone Marrow Transplantation, 11(35), 1113–1116. https://doi.org/10.1038/sj.bmt.1704963
- Kamiya, T., Hira, D., Hoshino, N., Kurihara, M., Nakagawa, M., Sasaki, M., & Terada, T. (2019). Low Body Mass Index and Myeloablative Conditioning Regimen Prolong the Duration of Parenteral Nutrition During Hematopoietic Stem Cell Transplantation. Annals of Nutrition & Metabolism, 2(74), 107–114. https://doi.org/10.1159/000496457
- Katoue, M. G., & Al-Taweel, D. (2016). Role of the pharmacist in parenteral nutrition therapy: challenges and opportunities to implement pharmaceutical care in Kuwait. Pharmacy Practice, 2(14), 680. https://doi.org/10.18549/PharmPract.2016.02.680
- Kooda, K., Canterbury, E., & Bellolio, F. (2022). Impact of pharmacist-led antimicrobial stewardship on appropriate antibiotic prescribing in the emergency department: A systematic review and meta-analysis. Annals of Emergency Medicine, 4(79), 374–387. https://doi.org/10.1016/j.annemergmed.2021.11.031
- Lang, X., Guo, J., Li, Y., Yang, F., & Feng, X. (2023). A Bibliometric Analysis of Diagnosis Related Groups from 2013 to 2022. Risk Management and Healthcare Policy, 16, 1215–1228. https://doi.org/10.2147/RMHP.S417672
- LeCompte, M. C., & Brawley, O. W. (2023). The Cause of Death in Patients with Cancer. Jacc: Cardiooncology, 1(5), 67–69. https://doi.org/10.1016/j.jaccao.2023.01.004
- Mathauer, I., & Wittenbecher, F. (2013). Hospital payment systems based on diagnosis-related groups: experiences in low- and middle-income countries. Bulletin of the World Health Organization, 10(91), 746–756A. https://doi.org/10.2471/BLT.12.115931
- McClave, S. A., DiBaise, J. K., Mullin, G. E., & Martindale, R. G. (2016). Acg clinical guideline: Nutrition therapy in the adult hospitalized patient. American Journal of Gastroenterology, 3(111), 315–334. https://doi.org/10.1038/ajg.2016.28
- Mizutani, Y., Kawamoto, S., Takahashi, M., Doi, H., Wakida, K., Tabuchi, S., Tanda, M., Soga, A., Chijiki, R., Takakura, H., Kawaguchi, K., Higashime, A., Watanabe, M., Ichikawa, H., Matsumoto, S., Sakai, R., Goto, H., Kurata, K., Kakiuchi, S., … Minami, H. (2023). Efficacy and safety of synbiotics in patients undergoing autologous hematopoietic stem cell transplantation: A randomized, double-blinded, placebo-controlled pilot study. Internal Medicine, 20(62), 2949–2958. https://doi.org/10.2169/internalmedicine.1314-22
- Mkhize, B. T., Mabaso, M. H. L., Madurai, S., & Mkhize-Kwitshana, Z. L. (2018). The investigation of the use of prealbumin as a tool for nutritional assessment in adults coinfected with HIV and intestinal helminth parasites in KwaZulu-Natal, South Africa. Biomed Research International, 1–8. doi:Artn 780585710.11552018/7805857.
- Mousavi, M., Hayatshahi, A., Sarayani, A., Hadjibabaie, M., Javadi, M., Torkamandi, H., Gholami, K., & Ghavamzadeh, A. (2013). Impact of clinical pharmacist-based parenteral nutrition service for bone marrow transplantation patients: A randomized clinical trial. Supportive Care in Cancer, 12(21), 3441–3448. https://doi.org/10.1007/s00520-013-1920-6
- Muskens, K. F., Lindemans, C. A., Dandis, R., Nierkens, S., & Belderbos, M. E. (2023). Definitions, incidence and outcome of poor graft function after hematopoietic cell transplantation: A systematic review and meta-analysis. Blood Reviews, 60, 101076. https://doi.org/10.1016/j.blre.2023.101076
- Navin, S. F., Nardolillo, J., Stambaugh, A., Young, C., Nguyen, P., & Apodaca, M. (2022). Pharmacist monitoring of direct oral anticoagulants for American Indians and Alaska Natives in the outpatient setting. Journal of the American Pharmacists Association, 2(62), 598–603. https://doi.org/10.1016/j.japh.2021.10.009
- Nieto-Gomez, P., Moron Romero, R., Planells Del Pozo, E., Cabeza-Barrera, J., & Colmenero Ruiz, M. (2021). Evaluation of quality indicators for nutrition and metabolism in critically ill patients: role of the pharmacist. European Journal of Hospital Pharmacy, Suppl 2(28), e62–e65. https://doi.org/10.1136/ejhpharm-2019-002195
- Pinet, E., Sabatier, P., Fernandez-Gerlinger, M. P., Jannot, A. S., Mainardi, J. L., Sabatier, B., & Caruba, T. (2022). Impact of medical and pharmaceutical interventions on anti-infective prescriptions: an observational study. European Journal of Clinical Microbiology & Infectious Diseases, 7(41), 1077–1086. https://doi.org/10.1007/s10096-022-04465-w
- Ranasinghe, R. N. K., Biswas, M., & Vincent, R. P. (2022). Prealbumin: The clinical utility and analytical methodologies. Annals of Clinical Biochemistry: International Journal of Laboratory Medicine, 1(59), 7–14. https://doi.org/10.1177/0004563220931885
- Shi, H. Y., Tharnpanich, T., & Yu, B. (2021). Highlights of pharmacist roles in hematopoietic cell transplantation and cellular therapy. BLOOD CELL THERAPY/The Official Journal of APBMT, 4, S8–S13. https://doi.org/10.31547/bct-2021-016
- Shrestha, S., Shrestha, R., Ahmed, A., Sapkota, B., Khatiwada, A. P., Christopher, C. M., Thapa, P., Kc, B., Blebil, A. Q., Khanal, S., & Paudyal, V. (2022). Impact of pharmacist services on economic, clinical, and humanistic outcome (ECHO) of South Asian patients: a systematic review. Journal of Pharmaceutical Policy and Practice, 1(15), 37. https://doi.org/10.1186/s40545-022-00431-1
- Sun, L. C. Y., Zhang, Z. H., Ji, W. Y., Zhang, Q. H., & Jiang, D. C. (2023). Exploration of Rationality and economy of drug use by pharmacists based on diagnosis related group rules. Evaluation and Analysis of Drug-Use in Hospitals of China, 23(05), 624–627.
- Tamaki, M., Nakasone, H., Nakamura, Y., Kawamura, M., Kawamura, S., Takeshita, J., Yoshino, N., Misaki, Y., Yoshimura, K., Matsumi, S., Gomyo, A., Tanihara, A., Kusuda, M., Kameda, K., Akahoshi, Y., Kimura, S. I., Kako, S., & Kanda, Y. (2021). Body weight loss before allogeneic hematopoietic stem cell transplantation predicts survival outcomes in acute leukemia patients. Transplant cell ther, 27(4), 340.e1–340.e6. https://doi.org/10.1016/j.jtct.2021.01.006. Epub 2021 Jan 9. PMID: 33836885
- Tong, Y., Sun, J., Xin, W., Chen, L., Kong, S., Mi, X., Feng, Y., Jin, W., Wu, Y., Ding, H., & Fang, L. (2022). Pharmacist-led standardization of total parenteral nutrition improves postoperative nutritional status in colorectal cancer patients. Annals of Translational Medicine, 6(10), 339. https://doi.org/10.21037/atm-22-1172
- Trissel, L. A. (2012). Institute for safe medication practices lifetime achievement award 2011. International Journal of Pharmaceutical Compounding, 1(16), 54–56.
- Vlug, L. E., Nagelkerke, S. C. J., Jonkers-Schuitema, C. F., Rings, E., & Tabbers, M. M. (2020). The role of a nutrition support team in the management of intestinal failure patients. Nutrients, 1(12), 1–14. https://doi.org/10.3390/nu12010172.
- Wang, Y., Dai, Y., Yang, J., Zhou, H., Chen, Z., & Li, G. (2020). A survey of Chinese pharmacists participating in anti-infective therapy and its related information technology support. Journal of Clinical Pharmacy and Therapeutics, 4(45), 707–714. https://doi.org/10.1111/jcpt.13152
- Wang, Z., Peng, Y., Cai, X., Cao, Y., Yang, G., & Huang, P. (2019). Impact of total parenteral nutrition standardization led by pharmacist on quality in postoperative patients with colorectal cancer. European Journal of Clinical Nutrition, 2(73), 243–249. https://doi.org/10.1038/s41430-018-0281-0
- Wong, S. L., Sulaiman, N., Ng, K. M., & Lee, Z. Y. (2021). Pharmacist-structured review of proton pump inhibitor utilisation in primary care: A nonrandomised control study. Malaysian Family Physician, 3(16), 87–96. https://doi.org/10.51866/oa1153
- Wu, Y. L., Xian, Q. W., Li, C., Chen, S. G., Hou, M., Luo, X. F., & Liu, Y. (2022). Practice and exploration of clinical pharmacists participating in refined pharmaceutical management of oncology center from the perspective of DRG. China Pharmacy, 33(22), 2801–2806.
- Yailian, A. L., Huet, E., Charpiat, B., Conort, O., Juste, M., Roubille, R., Bourdelin, M., Gravoulet, J., Mongaret, C., Vermorel, C., Bedouch, P., & Janoly-Dumenil, A. (2022). Characteristics of pharmacists’ interventions related to proton-pump inhibitors in French hospitals: An observational study. International Journal of Clinical Practice, 9619699. https://doi.org/10.1155/2022/9619699
- Yu, K. N., Ozer, M., Cockrum, P., Surinach, A., Wang, S., & Chu, B. C. (2021). Real-world prognostic factors for survival among treated patients with metastatic pancreatic ductal adenocarcinoma. Cancer Medicine, 24(10), 8934–8943. https://doi.org/10.1002/cam4.4415
- Zang, Y., Xu, W., Qiu, Y., Gong, D., & Fan, Y. (2023). Association between risk of malnutrition defined by the nutritional risk screening 2002 and postoperative complications and overall survival in patients with cancer: A meta-analysis. Nutrition and Cancer, 8(75), 1600–1609. https://doi.org/10.1080/01635581.2023.2227402
- Zhang, Y., Yang, H., Kong, J., Liu, L., Ran, L., Zhang, X., Yun, J., & Gu, Q. (2021). Impact of interventions targeting the inappropriate use of proton-pump inhibitors by clinical pharmacists in a hepatobiliary surgery department. Journal of Clinical Pharmacy and Therapeutics, 1(46), 149–157. https://doi.org/10.1111/jcpt.13273
- Zhou, X., Qiu, F., Wan, D., Sun, S., Yao, G., Liu, Y., & Li, J. (2019). Nutrition support for critically ill patients in China: role of the pharmacist. Asia Pacific Journal of Clinical Nutrition, 2(28), 246–251. https://doi.org/10.6133/apjcn.201906_28(2).0006
- Zou, K., Li, H. Y., Zhou, D., & Liao, Z. J. (2020). The effects of diagnosis-related groups payment on hospital healthcare in China: A systematic review. Bmc Health Services Research, 1(20), 1–11. doi:ARTN 11210.1186/s12913-020-4957-5.