ABSTRACT
Introduction
Although psychoactive medicines (PMed) are needed in several psychiatric conditions, their use and misuse bear risks. We aimed at estimating the prevalence of PMed use and misuse.
Methods
Data on all PMed prescribed in 2017 and dispensed in community pharmacies of the Lisbon and Tagus Valley region of Portugal (ARSLVT) were extracted from ARSLVT medicines’ dispensing database. For 21 PMed among prescription opioids, benzodiazepines and z-drugs (BZDR), antidepressants (AD) and anticonvulsants (AC), we estimated the number of users of each PMed, and assessed PMed misuse by a set of proxy indicators for studying this practice: chronic use (use of ≥180 DDD during the study period) of PMed intended for short-term treatments, concomitant use of several PMed, in particular if involving long-term (≥ 30 days) opioid analgesic (OA) use, and doctor shopping (patients consulting several physicians in order to have access to a quantity higher than intended by each prescriber). Data were analysed using descriptive statistics and hypothesis testing, and multivariate logistic regression was used to explore potential factors affecting long-term concomitant treatment of chronic OA with other PMed.
Results
PMed use prevalence was 21.7%: 6.6% for OA, 12.7% for benzodiazepines (BZD), 5.3% for AD and 2.8% for AC. BZDR were mainly prescribed in primary care and OA in hospital outpatients. Chronic use of PMed was observed in 25%, especially with sertraline and buprenorphine for opioid use disorder (long-term treatment), and lorazepam (short-term treatment). About 56.6% of OA chronic users were long-term concurrent users with other PMed, mainly BZDR. Risk of abuse was low for BZDR, whilst four opioids had meaningful doctor shopping indicators – fentanyl, opioid use disorder buprenorphine, morphine and hydromorphone.
Conclusions
BZD are the main PMed used in ARSLVT, often chronically, especially lorazepam. Prevalence of OA use is low, although with higher risk of misuse than BZDR. Concomitant use of several PMed is frequent.
Introduction
Psychoactive drugs with therapeutic use – psychoactive medicines (PMed) – play an important role in the treatment and symptomatic relief of many mental health disorders like depression, psychosis, and anxiety, as well as in epilepsy and pain. However, despite their clinical benefits, PMed constitute a unique group of medicinal products given their high risk of misuse. The concept of medicines’ misuse varies in the literature (Barrett et al., Citation2008), namely as non-medical use (consumption of a medication not prescribed, or in a manner not intended by the prescriber [Araújo et al., Citation2022, Citation2023; Novak et al., Citation2016; Smith et al., Citation2017), abuse (intentional excessive use accompanied by harmful physical or psychological effects [European Medicines Agency, Citation2017]) and doctor shopping (intentional use of a dose higher than prescribed by seeking multiple clinicians to obtain several prescriptions [Biernikiewicz et al., Citation2019]). Chronic use of medicines intended for short-term treatments was also considered in our definition of misuse.
Misuse of PMed is a recognised public health problem (Motta-Ochoa et al., Citation2017; Worley & Thomas, Citation2014), namely in the United States of America (USA) (Wood & Dargan, Citation2021), where there is growing concern about the opioid crisis (Friedman & Shover, Citation2023). In addition to prescription and synthetic illegal opioids, other central-nervous system (CNS) medications like benzodiazepines (BZD) and anticonvulsants (Haukka et al., Citation2018; Simonsen et al., Citation2020) are also misused. The United Nations Office on Drugs and Crime has alerted about the increased risk of prescription opioid deaths by polydrug use of opioids and other CNS-acting drugs (Hockenhull et al., Citation2021; United Nations Office on Drugs and Crime, Citation2017b). Antidepressants like bupropion (Schifano et al., Citation2018), venlafaxine (Schifano et al., Citation2018) and paroxetine, also seem to have relevant withdrawal and dependence potential (Chiappini et al., Citation2022), although evidence is limited. Clonazepam is frequently detected in fatal poisonings (Haukka et al., Citation2018), and gabapentinoids – pregabalin and gabapentin – are reported to have significant misuse potential (Hägg et al., Citation2020).
In Europe, literature on PMed misuse is more limited (Araújo et al., Citation2022, Citation2023; Bramness & Person, Citation2014; Casati et al., Citation2012; van Amsterdam & van den Brink, Citation2015) and differences between national guidelines, prescribing practices, health systems’ organisation and availability of PMed hamper its evaluation (Araújo et al., Citation2023). Data available in Portugal essentially refer to consumption, pointing to high levels of antidepressant and especially BZD use (Conselho Nacional de Saúde Citation2019; Coordenação Nacional da Estratégia do Medicamento e dos Produtos de Saúde, Citation2017; INFARMED, Citation2017, Citation2020; OECD, Citation2020, Citation2023; Faria Vaz et al., Citation2017a). Recognising this problem, the National Health Plan has included a primary care monitoring indicator to tackle BZD excessive prescribing in the elderly (Administração Central dos Sistemas de Saúde, Citation2012), in most cases considered inappropriate (2023 American Geriatrics Society Beers Criteria® Update Expert Panel, Citation2023; O’Mahony et al., Citation2023) because their harmful consequences are more likely to occur in this age group. Nevertheless, BZD use is frequent also in younger individuals (INFARMED, Citation2017), including in substance users that may use BZD to self-medicate, for example for anxiety, or to provide relief from opioid withdrawal symptoms or adverse effects from alcohol or cocaine use (EMCDDA, Citation2023).
Guidelines aiming at reducing prescribing of BZD and therapy duration have been issued (Direção Geral da Saúde, Citation2015). A study looking at BZD consumption in the Lisbon and Tagus Valley region of Portugal (ARSLVT) (Gomes et al., Citation2023), has shown a decrease in BZD use between 2013 and 2020, aside with switching to other PMed, like antidepressants and gabapentinoids (pregabalin and gabapentin).
Prescription or reimbursement databases, containing data on prescribed drugs over a period of time allow the assessment of consumption patterns in real-life dispensing conditions. The magnitude of medicines’ misuse is generally related to their consumption level (Rossow & Bramness, Citation2015; Roussin et al., Citation2016), and some EU studies have addressed the use, misuse and consequences of PMed in the general population (Chenaf et al., Citation2019; Driot et al., Citation2019; Haukka et al., Citation2018; Hedenmalm et al., Citation2019; Kalkman et al., Citation2019; Kostnapfel et al., Citation2022; Pierce et al., Citation2021; Ponté et al., Citation2018; Public Health England, Citation2019; Rossow & Bramness, Citation2015; Schjerning et al., Citation2016). However, to our knowledge no such studies exist at national level.
We therefore aimed with this study to characterise the use of 21 PMed prescribed in a densely populated region of Portugal (ARSLVT), and to assess their misuse using several methodologies, contributing to real-world evidence on this topic in our country, important not only at national, but also at EU level given the paucity of published research.
Material and methods
Design, data source and setting
In this descriptive cross-sectional study, reported following the RECORD-PE (REporting of studies Conducted using Observational Routinely collected health Data statement for pharmacoepidemiology) guidelines (Langan et al., Citation2018), data were extracted from the information system database of ARSLVT (SIARS). This regional administrative branch of the Portuguese National Health Service covers 3.65 million inhabitants, 37.3% of the total population of Portugal mainland. All reimbursed drugs prescribed in ARSLVT and dispensed in community pharmacies, irrespective of prescription type (public or private) and dispensing location, are registered in SIARS, where information on patients’ diagnoses, as well as demographic and administrative data regarding patients and prescribers, is collected.
Inclusion criteria
All patients who were prescribed at least one reimbursed package of any of the 21 medications considered of interest to the study in ARSLVT in 2017, which were dispensed between 1st January 2017 and 30th June 2018, were included. The additional 6-month period in 2018 was added to cover dispensing of renewable prescriptions (validity: 6 months), issued in 2017 but dispensed only in 2018.
Medications studied
All analgesic opioids with sales data in Portugal were included in the analysis. The other PMed studied were defined based on a preliminary analysis of 5-year (2014–2018) nationwide sales data from Health Market Research Portugal on prescription-only reimbursed PMed, together with morbimortality data from the Portuguese Poison Control Centre (CIAV) and from the National Institute of Legal Medicine and Forensic Sciences (INMLCF, I.P.) on reports of poisonings and deaths involving PMed with sales data in Portugal. The PMed with higher sales data and most frequently involved in CIAV and INMLCF reports, were selected for the present study.
All PMed of interest, identified by their International Non-proprietary Name (INN), were classified in therapeutic groups according to the WHO ATC/DDD classification system,Footnote1 version 2022 (WHO Collaborating Centre for Drugs Statistics and Methodology, Citation2022a). The 21 medications and their therapeutic group are displayed in .
Table 1. Studied PMed and their therapeutic groups.
Variables extracted
For each medicine, the following variables were extracted according to the study protocol submitted to ARSLVT and approved by ARSLVT Ethics Committee: anonymised ID, age, gender and the following International Classification of Primary Care diagnosis codes (ICPC-2) (World Health Organization, Citationn.d.); all ICPC-2 cancer codes and psychiatric codes P01 and P74 (anxiety), P03 and P76 (depression), P06 (sleep disturbance), P18 (medication abuse) and P19 (illegal drug abuse). Data were cleaned and validated to eliminate possible inconsistencies and to check for missing information. Prescribers’ anonymised ID and medical specialty were also collected, along with PMed dispensing dates and quantities – number of packages and defined daily doses (DDDFootnote2) (WHO Collaboration Centre for Drug Statistics Methodology, Citation2022b). Considering the analyses to be performed, new variables were created by grouping extracted data: for example, patients were divided in age groups, INN were gathered in therapeutic classes, similar diagnosis codes were grouped (P01 + P74 for anxiety, P03 + P76 for depression), and related prescriber specialties were assembled (e.g. orthopaedics and rheumatology, psychiatry and neurology, surgical specialties).
Data were validated by comparing the global number of packages and DDD dispensed by INN extracted from SIARS, with the corresponding data for the LVT region contained in the national reimbursement database, managed by the National Authority of Medicines and Health Products (INFARMED).
All personal data obtained were anonymised at source, both for patients and prescribers.
Classification of users
Users of each medicine were classified as chronic if they were dispensed at least 180 DDD of the medicine during the study period (T. Kurko et al., Citation2018; Luijendijk et al., Citation2008; Lunghi et al., Citation2020; Mellbye et al., Citation2016; Schonmann et al., Citation2018). With the aim of estimating the magnitude of long-term concomitant (LTC) use of OA with other CNS-acting medicines, known to carry increased risk of serious adverse consequences, chronic OA users were further classified, in terms of concomitant use with other therapeutic groups of the study, in non-concomitant users (no overlap in the days using the different medications), short-term concomitant (STC) users (from 1 to 29 consecutive days), and LTC users (for ≥30 consecutive days) (Wei et al., Citation2018). Treatment periods were defined as the interval between the dispensing date and the last day of supply covered by the prescription, assuming a daily dose of one DDD. Users that have multiple episodes of concomitant use were counted as many times in each combination as the number of episodes.
Data analysis
Analysis of consumption
Utilisation was assessed by estimating the prevalence of use of each PMed/therapeutic group based on population data from Statistics Portugal (INE), as well as the number and type of users, number of DDD consumed (DDD/1000 population/day and DDD/user/year, surrogates for point prevalence – therapeutic intensity), both at PMed and therapeutic group levels. Users of more than one of the studied PMed were counted as many times as the number of categories they belong to (e.g. a patient using a BZD and an antidepressant was counted in both groups – BZD and AD).
Analysis of misuse
We assessed misuse by investigating the pattern of use of the PMed included in the study. Concomitant use of several PMed, even if clinically recommended, increases their associated risks. Therefore, concomitant use, defined in our study as the overlapping of at least one day in the prescription periods of two or more PMed, was used as a proxy of misuse. Chronic use of PMed, that should be avoided in PMed mostly recommended for short-term treatments, such as BZDR, was also considered indicative of misuse.
Another misuse indicator defined in our study was long-term concomitant OA use with other PMed, considering the known harms associated with OA chronic treatment and their potential increase if OA are taken together with other PMed, which is common (Khan et al., Citation2021).
Estimation of doctor shopping parameters was also used as a proxy for medicines’ misuse, assessing the extent and risk of abuse of the studied PMed. Doctor shopping is a practice where patients obtain overlapping prescriptions from different prescribers, ultimately resulting in the access to a daily dose of medication that is higher than intended by each prescriber. The doctor shopping indicator is therefore a measure of the risk of abuse (i.e. excessive use) of a given medicine. Doctor shopping parameters were calculated and analysed according to the methodology described by several authors (Frauger et al., Citation2011, Citation2016; Micallef et al., Citation2015; Ponté et al., Citation2018; Pradel et al., Citation2004, Citation2009, Citation2010; Soeiro et al., Citation2023), both at INN and at therapeutic group level. A detailed description of the method, that considers the number of overlaps of prescriptions of a given medicine or therapeutic group issued by different prescribers, is provided in Supplemental Material 1.
For each dispensing of a given medicine/therapeutic group to a given patient, two variables were computed: the Quantity dispensed (Q) and the Doctor Shopping Quantity (DSQ): these were estimated considering the number of prescription periods overlapping at the date of dispensing and removing the proportion of medication obtained by overlapping prescriptions from repeated visits to different prescribers that is considered medically legitimate. Summing up these quantities for all users, the total dispensed Quantity (Qtot) and the total DSQ (DSQtot) for each PMed and each group were calculated, forming the basis of the Doctor Shopping Indicator (DSI), the proportion of the quantity doctor shopped among the total quantity dispensed of each PMed/therapeutic group, expressed as a percentage (DSQtot/Qtot*100). The DSI, standardising the quantity doctor-shopped according to the use level of the drug, reflects the risk of abuse, while the DSQtot indicates the extent of the abuse. For the medications and therapeutic groups for which a DSI higher than 1% (empiric threshold derived from previous published studies [Nordmann et al., Citation2013; Ponté et al., Citation2018; Rouby et al., Citation2012]) was found, a correction was performed in order to minimise the background noise of overlapping prescriptions common to all medicines irrespective of their abuse potential. As such, for PMed and therapeutic groups with DSI > 1%, the corrected DSI (DSIc: DSI minus 1%) and the corrected DSQ (DSQc = Qtot*DSIc) were estimated. Because the quantities involved in this study are low, results were expressed in DDD/100,000 inhabitants/day, instead of DDD/1000 inhabitants/day (Ponté et al., Citation2018; Soeiro et al., Citation2023).
Statistical analysis
Data were analysed using descriptive statistics, summarising discrete variables as absolute and relative frequencies. We analysed continuous variables using measures of central tendency and dispersion. Results were presented for all patients and stratified by INN, therapeutic group and OA subgroup (strong/weak).
Comparisons were made using Chi-square tests for discrete variables or Wilcoxon/Kruskal–Wallis tests for continuous data. We used multivariate logistic regression to assess the chance of STC and LTC use vs. non-concomitant use, and to explore potential factors affecting long-term concomitant treatment of chronic OA users with other PMed, more susceptible to have adverse consequences. OR were computed, adjusted for age, gender, prescription by general practitioner (GP), psychiatrist or neurologist, or presence of a diagnosis of cancer, anxiety, depression, sleep disturbance, medication abuse or drug abuse.
In the doctor shopping method, we used an interruption threshold (IT) of 30 + 7 days (30 days – the validity of most prescriptions in 2017, plus 7 days to account for delayed prescription fills).
All analyses adopted a confidence level α = 0.05 and were performed using SAS Enterprise Guide v7.15 (SAS Institute, Cary NC, USA) and R Statistical Software.
Ethics approval
Ethical approval was granted by the Ethics Committee of ARSLVT l (Opinion 9981/CES/2018), following assessment of the study protocol (Proc.100/CES/INV/2017).
Results
Prescribing and dispensing
More than 4.5 million packages of PMed were dispensed in ARSLVT in 2017, of which 49.4% concerned BZDR, and 23.6% OA. An important part of PMed prescribing in ARSLVT is performed in primary care (PC), especially BZDR (lorazepam and zolpidem standing out, both with almost half of dispensed packages prescribed in PC) and the three weak opioids (also approximately half of dispensed packages prescribed in PC), contrasting with strong opioids, mostly prescribed in the hospital outpatient setting (except tapentadol, essentially PC prescribing).
Prevalence of PMed use in ARSLVT was 21.7%, corresponding to 29.0% of female and 13.4% of the ARSLVT male populations. Female predominance – 70.8% of the total 778,772 ARSLVT PMed users – was observed for all PMed included in the study, except for OUD buprenorphine and BZD in young boys (≤14 years). Prevalence of strong OA use was 1.0%, and 6.0% for weak OA, and there were 2.9 times more women using strong OA than men (21,564 vs. 7483 users). Prevalence of any OA use was 6.6% (236,314 users, 71.3% females and 47.3% older than 65 years), 68.9% of the combination tramadol + paracetamol, with 17.4% of all ARSLVT older females (≥65 years) having been dispensed at least one package of this tramadol combination. Almost half (49.3%) of the users of this combination were elderly. Prevalence of BZD use was 12.7%, while AD were used by 5.3% of the ARSLVT population. About 11.5% and 8.0% of ARSLVT older females were alprazolam and sertraline users, respectively.
Looking at consumption expressed in DDD/1000 population/year, the highest PMed consumption in 2017 was of BZD (51.9), especially alprazolam (17.6) and lorazepam (10.3), and of antidepressants (26.6), particularly sertraline (21.2).
Nearly 13.6% of OA users had a neoplastic diagnosis, 25.1% for strong and 13.4% for weak OA users, with a cancer diagnosis present for 38.1% of morphine, 33.3% of fentanyl and 15.2% of tapentadol users. Only 19.0% of patients treated with BZD had been diagnosed with anxiety, and a scarce 15.3% of zolpidem users had a sleeping disorder diagnosis (the only approved therapeutic indication for zolpidem in Portugal), while 37.7% of patients treated with AD had a diagnosis of depression (, , and ).
Table 2a. Characteristics of psychoactive medicine users, consumption and prescription, by therapeutic group.
Table 2b. Characteristics of users of opioid psychoactive medicines, consumption and prescription, by INN.
Table 2c. Characteristics of users of non-opioid psychoactive medicines, consumption and prescription, by INN.
In 2017, 17.6% of PMed prescribers were GP. These were the main prescribers of OUD buprenorphine (41.0% of total OUD buprenorphine prescribers), as well as of OA (20.5% of total OA prescribers) and BZD (17.0% of total BZD prescribers). About 94.3% of oncologists prescribing OA prescribed strong OA, contrasting with dentists for which this proportion was much lower – 5.7%. Only 7.0% of AD prescribers were psychiatrists or neurologists.
Misuse
Chronic PMed use
About 24.5% of users of at least one PMed were chronic, summing up a total of 8455 chronic users of OA (78% of which females), 111,176 of BZD (73% females), 15,978 of the z-drug zolpidem (75% females), 6981 of AC (67% females), and 69,518 of AD (75% females), corresponding to 3.6%, 24.4%, 20.5%, 7.0% and 36.7%, respectively, of total users of each therapeutic group ().
Table 3. High-dose PMed use and demographics of chronic users.
About 13.1% of strong OA users were chronic, contrasting with only 2.9% of weak opioid users. With the highest proportion of chronic users were AD (36.7%), followed by OUD buprenorphine (35.7%) and by anxiolytics (24.4%). At medicine level, 56.2% of sertraline, 38.8% of lorazepam (67.4% of which older than 65y), 35.7% of OUD buprenorphine and 27.5% of alprazolam (52.4% of which elderly) users were chronic. Significant differences were found between age of chronic and non-chronic users for all PMed, except for diazepam, morphine, oxycodone and oxycodone + naloxone, with the highest differences observed for OUD buprenorphine (mean age chronic users 46.0y, SD = 8.0; non-chronic users 61.3, SD = 17.5) and clonazepam (mean age chronic users 49.8y, SD = 13.2; non-chronic users 61.3, SD = 17.5). The oldest PMed chronic users were pain buprenorphine (mean 73.6y, SD = 13.4), tramadol + paracetamol (71.5y, SD = 13.6) and lorazepam (70.6y, SD = 13.7) users.
Concomitant use of two or more PMed
About 34.6% of ARSLVT PMed users were concomitant users of two or more PMed (). BZDR were frequently consumed in association with other therapeutic groups: in a rate of 2558 users/100,000 ARSLVT inhabitants combined with AD, and in 2310 users/100,000 ARSLVT inhabitants, the concomitant use was with OA (in both cases, other PMed therapeutic groups could also be present). Almost half (48.5%, N = 91,939) of AD users concomitantly used BZDR and 35.1% of OA users were additionally being treated with BZDR. At substance level, the most frequent combinations found were alprazolam with sertraline (8430 patients – 7.8% of sertraline users and 5.2% of alprazolam users), diazepam with tramadol + paracetamol (7573 patients – 6.2% of diazepam users and 4.7% of tramadol + paracetamol users), alprazolam with trazodone (6999 patients – 7.2% of trazodone users and 4.3% of alprazolam users), and alprazolam with tramadol + paracetamol (6900 patients – 4.2% of both tramadol + paracetamol and alprazolam users).
Table 4. Concomitant psychoactive medicine use.
Long-term concomitant treatment of chronic OA users with other PMed
About 24.2% of OA chronic users had a diagnosis of cancer, 27.6% of depression, 14.3% of anxiety and 9.7% of sleeping disorders. More than half (N = 4403; 52.1%) of total OA chronic users were LTC users with at least another PMed of the study. About 72.6% (N = 3196) of total LTC chronic OA users were long-term concomitant users with BZDR, 81.7% of which females and 61.0% aged ≥65y. Nearly three quarters (74.1%) of these LTC OA-BZDR users received at least one prescription from a GP. LTC chronic OA use with AD is less common (N = 1427; 32.4% of total OA chronic users), with a slightly higher female (83.3%) and older age (63.8% aged ≥65y) predominance. Less than a quarter of LTC OA-BZDR, OA-AD and OA-AE users (22.8%, 22.4% and 22.1%, respectively) had a cancer diagnosis ().
Table 5. Characteristics of long-term concomitant chronic OA use with BZDR, AD, AC, BZDR + AD, BZDR + AC, AD + AC, BZDR + AD + AC.
The results of the multivariate logistic regression used to explore potential factors affecting long-term concomitant treatment of chronic OA users with other PMed have shown that age (aOR = 0.996, p = 0.0210), and gender (aOR = 1.388, p < 0.0001), influence the risk of LTC OA-BZDR use, with female OA chronic users having higher odds of LTC use with BZDR – . The existence of a depression or anxiety diagnosis in OA chronic users was also identified as a risk factor for LTC OA-BZDR use (aOR = 1.563 and aOR = 1.432, p < 0.0001) and LTC OA-AD use (aOR = 2.593, p < 0.0001 and aOR = 1.200, p = 0.0267, respectively). In addition, OA chronic users that had a prescription from a psychiatrist or neurologist had a higher odds of LTC use with BZDR, AD or AC (aOR = 1.556, 1.627 and 1.832, p < 0.0001). Having a medication abuse diagnosis was identified as a risk factor for LTC OA-AD use (aOR = 1.940, p = 0.0383). Younger age and a depression diagnosis were also identified as risk factors for LTC OA-AC use (aOR = 0.975 and aOR = 1.411, respectively, p < 0.0001).
Table 6. Factors influencing LTC OA use with other PMed.
Doctor shopping of PMed
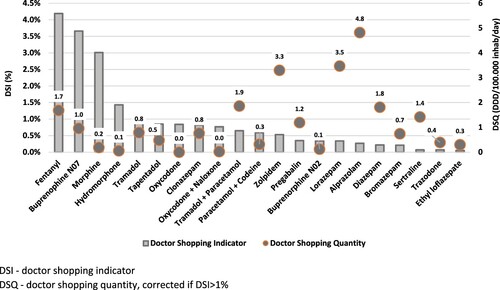
The detailed results, included in and , show that several strong opioids had DSI higher than 1%, therefore possessing a relevant risk of abuse: fentanyl (4.2%), OUD buprenorphine (3.7%) – the only opioid for OUD sold in community pharmacies in Portugal – morphine (3.0%) and hydromorphone (1.4%). For weak opioids, widely used in ARSLVT, the risk of abuse was not meaningful (DSI = 0.8%), with the three studied medicines showing a DSI below 1% (tramadol: 0.9%; tramadol + paracetamol: 0.7%; paracetamol + codeine: 0.6%).
Table 7. Doctor shopping parameters, by therapeutic group and opioid analgesic subgroup.
OA as a whole had a DSI of 1.6%. AD, BZD and z-drug zolpidem seem to pose no risk of significant abuse, with DSI of 0.1%, 0.3% and 0.5%, respectively ().
Regarding DSQ, that provides an estimate of the extent of medicine abuse, data have shown that, although the DSQ for BZD is almost double than for OA (14.0 vs. 9.2 DDD/100,000 inhabitants/day), their doctor shopping indicator is one fifth that of OA (0.3% vs 1.6%).
Discussion
Main findings and implications
Our study results have shown that psychoactive medicine users are more likely to be females, confirming previously published data (Carmona Araújo et al., Citation2023; Conselho Nacional de Saúde, Citation2019; Coordenação Nacional da Estratégia do Medicamento e dos Produtos de Saúde, Citation2017; INFARMED, Citation2017, Citation2020; Faria Vaz et al., Citation2017a). Existing evidence points to a higher prevalence of pain (Campbell et al., Citation2010; United Nations Office on Drug and Crime, Citation2017a), depression (European Medicines Agency, Citation2023) and anxiety in females (Conselho Nacional de Saúde, Citation2019), which combined with a greater general medicine consumption in the female gender (Boyd et al., Citation2015; Campbell et al., Citation2010; Carmona Araújo et al., Citation2023; Cartagena et al., Citation2017; Delaš Aždajić et al., Citation2019; Hedenmalm et al., Citation2019; Hockenhull et al., Citation2021; Madeira et al., Citation2023; Muller et al., Citation2019; Schjerning et al., Citation2016), contributes to the clear female predominance in psychoactive medicine consumption. This gender gap in Portugal is reported to be the widest across the EU in what concerns depression (OECD European Observatory on Health Systems and Policies, Citation2023). In our study, this was reflected in the striking difference in AD consumption (triple in women compared to men), in line with previous research (Madeira et al., Citation2023). It is also acknowledged that women, as well as the elderly, have an increased risk of misusing medicines (Araújo et al., Citation2023; Casati et al., Citation2012), resulting in a higher probability of adverse consequences in older females. A recent OECD report (OECD European Observatory on Health Systems and Policies, Citation2023) emphasises the high prevalence of mental health problems in Portugal (22.0% of the population, higher than the EU average of 16.7%), driven mainly by anxiety and depressive disorders (9% and 6% of the population in 2019, respectively).
Relevant proportions of chronic users of BZDR, a therapeutic class whose included PMed are recommended to be administered for short periods, were observed in our study: 38.8% of lorazepam, 27.5% of alprazolam and 20.5% of zolpidem users were chronic. Lorazepam, mainly prescribed in primary care, is an intermediate-acting, high potency BZD reported to be a significant predictor of long-term BZD use, dose escalation or heavy use (Kurko et al., Citation2015). Considering that lorazepam is included in the EU(7)-PIM list adapted to Portugal, and also in the most recent updates of both Beers and STOPP and START criteria (2023 American Geriatrics Society Beers Criteria® Update Expert Panel, Citation2023; O’Mahony et al., Citation2023), identifying potentially inappropriate medicines in older patients (Rodrigues et al., Citation2020) who are more susceptible to suffer from BZDR adverse effects (Gomes et al., Citation2023; Madeira et al., Citation2023; Prazeres, Citation2023), and that most ARSLVT lorazepam users are older patients – 53.7% of our chronic lorazepam users were aged 65 or more – the lorazepam chronic use found in our study is a cause for concern. Besides lorazepam, our results have also shown that analgesic buprenorphine and tramadol + paracetamol users are the oldest PMed users (mean 73.6 and 71.5 years, respectively). Considering that opioids are included in the Ghent Older People’s Prescriptions community Pharmacy Screening (GheOP3S)-tool (Ghent University – Faculty of Pharmaceutical Sciences, Citation2023) as potentially inappropriate medication for older people, according to which tramadol should be especially avoided because of increased risk of hypoglycaemia, hyponatremia and serotonin syndrome due to drug–drug interactions with other serotonergic medicines, our findings on chronic PMed use in the elderly deserve special attention.
Female gender predominance in PMed use (70.8% of PMed ARSLVT users), together with its identification as a risk factor for both LTC OA-BZDR and LTC OA-AD use in our study, reinforces the need of intervention programmes to increase awareness of the risks of PMed utilisation with a particular focus on the most vulnerable groups. Medication abuse diagnosis was also identified as a risk factor for LTC OA-AD use, and females have an increased risk of misusing medicines (Araújo et al., Citation2023; Casati et al., Citation2012). Therefore, such intervention programmes should aim at reducing PMed consumption, especially BZDR, and minimising their misuse, targeting most frequent users – especially older females (Lombardi et al., Citation2020) – and the healthcare workforce that has a role in prescribing and dispensing – clinicians and pharmacists.
Off-label use, defined as the use of a medicine outside its approved therapeutic indications, is a relatively common practice worldwide, and Portugal is no exception (Conselho Nacional de Ética para as Ciências da Vida, Citation2023). In our study, we assumed diagnoses as a proxy for the drug’s main therapeutic indication. Therefore, the use of a medicine in a patient not having the diagnosis of its main indication was considered off-label use. It is true that the use of a medicine in a secondary indication is not off-label use, and that missing diagnoses in the database may not necessarily mean that patients do not have those conditions, but only that records are incomplete, both leading to an overestimation of off-label use. Nevertheless, it was evident in our results that this phenomenon has a relevant expression in ARSLVT: a scarce 15.3% of zolpidem users had a sleeping disorder diagnosis, the only therapeutic indication for zolpidem approved in Portugal; only 37.7% of antidepressant users had a depression diagnosis, and only 28.2% of OUD buprenorphine users had a diagnosis of illicit drug use included in SIARS.
Most OA are not exclusively intended to treat cancer pain, but controversy currently exists on the effectiveness of OA in non-cancer pain treatment. Only about 13.5% of OA users had a neoplastic diagnosis, reaching 25.1% for strong OA users and 13.4% for weak OA users, with a cancer diagnosis present for 38.1% of morphine, 33.3% of fentanyl and 15.2% of tapentadol users. OA are only used in pain treatment; not having data on pain diagnoses, we assumed that all OA use with no neoplastic diagnosis was for the treatment of acute or chronic non-cancer pain (CNCP). Given the low proportions of neoplastic diagnoses in ARSLVT OA users (13.5% any OA, 21.5% strong and 13.4% weak OA) and that for chronic use only 24.2% of OA chronic users had a cancer diagnosis, it is reasonable to assume that most OA use in ARSLVT is in CNCP treatment, which is line with several other studies (Bedson et al., Citation2016; Hider-Mlynarz et al., Citation2018; Kalkman et al., Citation2019; Zin et al., Citation2014).
OA, used in chronic cancer and non-cancer pain, should be prescribed with caution, not only because they can pose serious health risks, that appear to be dose-dependent (Chou et al., Citation2015), but also due to limited evidence of long-term benefit (Campbell et al., Citation2010; Chou et al., Citation2015; Keto et al., Citation2022). Our study has shown that the prevalence of OA use in ARSLVT is low – 6.6% – when compared with data from other countries, with the exception of Germany (long-term opioid therapy – 1.3%) (Marschall et al., Citation2016) and the Netherlands (6.0%) (Bedene et al., Citation2019). In fact, in France the prevalence of OA use was 14% in one study (Ponté et al., Citation2018) and 17.5% in another study (Chenaf et al., Citation2019), in Italy 12.2% (OsMed, Citationn.d.), in Slovenia 12.6% (Kostnapfel et al., Citation2022), in Finland 7% (Keto et al., Citation2022), in Spain 6.7% (Regueras & López Guzmán, Citation2021) and 4.9% in young adults (Carrasco-Garrido et al., Citation2022), and in the UK 13% (Public Health England, Citation2019). In Norway, Denmark and Sweden, higher prevalences in women compared to men, have been observed (12.1%, 8.9% and 8.4% vs 9.2%, 6.6% and 6.3%, respectively) (Muller et al., Citation2019). In addition, our data has shown that, except for tapentadol, most strong OA are prescribed in hospitals, thereby ensuring closer monitoring of opioid therapy, probably in the context of pain management consultations.
However, the scenario regarding BZD use is quite different, and our results, pointing to chronic BZDR use, especially by the elderly, are in line with OECD data: in 2017, Portugal ranked third regarding chronic BZD use in people aged 65 and over, with 65.5 DDD/1000 inhabitants/day (OECD, Citation2020). Recognising this problem, in the last years several interventions have been implemented in our country, both at national and regional level, to encourage BZD discontinuation in all age groups (Faria Vaz et al., Citation2017a; Fernandes et al., Citation2022; Gomes et al., Citation2023; Oliveira et al., Citation2019; Vaz, et al., Citation2017b). However, although some improvement has been observed in the use of anxiolytics (ATC code N05B) (Fernandes et al., Citation2022; Gomes et al., Citation2023; Oliveira et al., Citation2019), decreasing consumption from 93.9 DDD/1000 inhabitants/day in 2017, to 85.0 DDD/1000 inhabitants/day in 2022, a decrease has been observed also in other OECD countries. This implies that despite the decrease in absolute figures, our country is still on the top of the BZD use ranking in relative terms (OECD, Citation2023).
Concomitant use of several PMed, reported in other studies (Torrance et al., Citation2018), and especially long-term concomitant use of OA with BZDR or other CNS depressants bears serious health risks (Araújo et al., Citation2023), including fall-related injury, hospitalisations and emergency department visits, fatal and non-fatal opioid overdoses that can ultimately result in respiratory depression, coma and death (FDA, Citation2016). Despite the relatively low OA prevalence of use in ARSLVT, more than half (52.1%) of OA chronic users were LTC users with other PMed, especially with BZDR, reinforcing the need to raise awareness, both of prescribers and patients, on the possible harms of LTC use of OA concomitantly with other PMed.
The doctor shopping analysis performed in our study has shown that this does not seem to be a cause for concern in ARSLVT. In France the scenario is different, with several studies (Ponté et al., Citation2018; Pradel et al., Citation2010; Soeiro et al., Citation2023) pointing to higher DSI, and for more PMed, than in ARSLVT. In our results, although the DSQ for BZD (14.0 DDD/100,000 inhabitants/day) was almost 2-fold higher than for OA (corrected DSQ = 8.6 DDD/100,000 inhabitants/day), their doctor shopping indicator (0.3%) was one fifth that of OA (1.5%). This apparent contradiction of a higher DSQ for BZD and a lower DSI compared to OA, highlights the fact that the extent of psychoactive medicine abuse is a combination of its abuse potential (generally lower for BZD) and the availability of the medicine (higher for BZD). In fact, in Portugal BZD are widely prescribed, whereas physicians refrain from prescribing opioids, especially strong, which are all controlled substances in our country, in line with the United Nations Single Convention on Narcotic Drugs (Transnational Institute, Citation2015). Nevertheless, the first four positions of the ARSLVT DSI ranking are occupied by strong opioids, all shown to possess a meaningful risk of abuse, as opposed to BZD and AD, which are in the bottom of the DSI ranking. Fentanyl is the PMed with the highest DSI – 4.2% – which together with its relevant DSQ (1.7 DDD/100,000 inhabitants/day, sixth position in the DSQ ranking) found in our study, points to the need to closely monitor the use of this opioid. Due to its high lipophilicity, fentanyl has a fast transition through the blood – brain barrier and consequently a rapid onset of action, thereby possessing a high abuse liability. Transdermal formulations are expected to have lower abuse liability, nonetheless fentanyl is also available in Portugal in immediate release transmucosal formulations that, bypassing first-pass metabolism, provide fast analgesia and are for that reason indicated for breakthrough cancer pain. These transmucosal formulations, being undeniably important in the management of intense pain in cancer patients, are also more prone to be misused. As such, it would be important to further develop the doctor shopping analysis stratifying by formulation (transdermal vs. transmucosal), in order to distinguish their specific abuse risk. Yet it should be emphasised that, given the above mentioned relatively low prevalence of OA use in ARSLVT, and in particular the reduced number of fentanyl users (3% of total OA users) when compared to other opioids, the possibly higher risk of transmucosal fentanyl is not expected to have a strong impact from a public health protection perspective.
An important next step of our work would be to study, at national level, the use and misuse of PMed, especially the PMed highlighted in our research as requiring particular attention, namely by analysing the morbimortality consequences (e.g. hospitalisations, poisonings and deaths) associated with their use, allowing a detailed assessment of the risks associated with PMed use and misuse in Portugal.
Strengths and limitations
This study is, to our knowledge, the first in Portugal combining the analysis of consumption of PMed with information on their potential misuse. Using patient-level data, we describe PMed consumption patterns in the ARSLVT region of Portugal while also analysing their possible misuse, based on the type of use (chronic use of the studied medicines that are recommended for short-term treatments, as well as long-term concomitant use of opioids with other PMed and their risk factors) and estimation of doctor shopping indicators. The main strength of the study is the use of a large population-based cohort containing detailed prescription and dispensing data that also includes patient diagnoses (used as proxies for indications of use), as well as information on prescribers and prescription setting. Further, the use of data on prescriptions actually dispensed, instead of issued prescriptions, ensures that the analysis is closer to actual medicine consumption.
However, our study also has several limitations, the first of which is related to the potential limited representativeness of the population-based cohort studied, which cannot be ascertained, hampering generalisability (external validity) of results to the whole Portuguese population. In addition, the use of DDD as a consumption measure has several limitations. In fact, in the situations where the actual daily dose is significantly different from the DDD, such as when evaluating drug use in older adults (in whom lower doses are frequently used), in indications other than the main therapeutic indication (indications are not included in SIARS), or when studying prescription opioid use (where doses are often titrated according to the patient’s response to obtain sufficient pain relief), the use of DDD leads to less precise consumption estimates (Nielsen et al., Citation2017). Consequently, classifying users as chronic based on the number of DDD consumed may not be totally accurate. Likewise, the calculation of the number of days of supply assumes the use of 1 DDD/day, leading to over or underestimation of this number when the dose actually used is significantly different from the DDD.
Another limitation is the fact that SIARS only contains information on reimbursed medicines; as such, medicines not reimbursed or dispensed without a medical prescription, or obtained through illicit sources, as well as consumption by hospitalised patients, are not covered by our data. Besides, we acknowledge that our off-label estimates may be overestimated due to missing diagnosis codes in SIARS. We also assumed that diagnoses were present at the time the medicine was dispensed, which may not be the case as we had no information on the date the patient was diagnosed with the medical condition.
In the concomitant use analysis and in the doctor shopping method, we considered patients to be continuously exposed to the medicines based on dispensing dates and days’ supply, not being able to ascertain whether patients actually consumed these medications continuously over the study period. In addition, our measure on treatment interruption was based on the validity of most prescriptions in 2017 (30 days), not taking into consideration the higher validity (180 days) of renewable prescriptions. However, considering that most studied medicines are intended for short-term treatments, this limitation is expected to have low impact on the results. We also assumed that overlapping dispensing periods of two or more medicines meant their concomitant use, which we cannot be certain as we are not sure if they both were actually consumed during the same treatment period. Finally, the different PMed dosages available in the Portuguese market, which have not been taken into account in our study, could influence the doctor shopping results, as higher dosages are expected to have higher DSI (Pradel et al., Citation2010).
Conclusions
The PMed with higher prevalence of use are BZD, followed by OA and AD. Moreover, there is a high proportion of chronic users of BZDR, especially of lorazepam and alprazolam. OA are mainly used for conditions other than chronic cancer pain, and long-term concomitant use of OA with other PMed is frequent, particularly with BZDR. The female gender is a risk factor for long-term concomitant use of OA with AD and with BZDR. This female predominance in PMed consumption resulting in a higher probability of adverse consequences in females, especially older, prompts the need to develop specific policies to more effectively address excessive PMed consumption, especially chronic. BZDR have smaller doctor shopping indicators but more expressive doctor shopping quantities than OA, reflecting their lower risk of abuse but higher accessibility, also suggesting that availability is an issue that needs further analysis.
A comprehensive and real-world-based characterisation of PMed use and misuse at national level, including of their morbimortality consequences, should be performed, ideally resorting to data linkage between national databases, a common practice in Northern European countries.
Supplemental Material
Download MS Word (76.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes
1 WHO ATC/DDD Index – Anatomical, Therapeutical and Chemical classification of the WHO Collaborating Centre for Drug Statistics Methodology.
2 DDD – technical unit of measurement of medicine use, defined as the assumed average maintenance dose per day for a medicine used for its main indication in adults. It is assigned by the WHO Collaborating Centre for Drug Statistics Methodology, according to the DDD-ATC methodology.
References
- Administração Central dos Sistemas de Saúde. (2012). Bilhete de identidade dos indicadores utilizados na contratualização dos ACES, USF e UCSP. Ministério Da Saúde.
- American Geriatrics Society Beers Criteria® Update Expert Panel. (2023). American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 71(7), 2052–2081. https://doi.org/10.1111/jgs.18372
- Araújo, A. C., Casal, R. J., Goulão, J., & Martins, A. P. (2022). Protocol for a scoping review on misuse of psychoactive medicines and its consequences. BMJ Open, 12(10), e060519. https://doi.org/10.1136/bmjopen-2021-060519
- Araújo, A. C., Casal, R. J., Goulão, J., & Martins, A. P. (2023). Misuse of psychoactive medicines and its consequences in the European Union – A scoping review. Journal of Substance Use, 00(00), 1–12. https://doi.org/10.1080/14659891.2023.2213325
- Barrett, S. P., & Meisner, J., & Stewart S. H. (2008). What constitutes prescription drug misuse? Problems and pitfalls of current conceptualizations. Current Drug Abuse Reviews, 1(3), 255–262. https://doi.org/10.2174/1874473710801030255
- Bedene, A., Lijfering, W. M., Niesters, M., van Velzen, M., Rosendaal, F. R., Bouvy, M. L., Dahan, A., & Van Dorp, E. L. A. (2019). Opioid prescription patterns and risk factors associated with opioid use in the Netherlands. In JAMA network open (Vol. 2, Issue 8). American Medical Association.
- Bedson, J., Chen, Y., Hayward, R. A., Ashworth, J., Walters, K., Dunn, K. M., & Jordan, K. P. (2016). Trends in long-term opioid prescribing in primary care patients with musculoskeletal conditions: An observational database study. Pain, 157(7), 1525–1531. https://doi.org/10.1097/j.pain.0000000000000557
- Biernikiewicz, M., Taieb, V., & Toumi, M. (2019). Characteristics of doctor-shoppers: A systematic literature review. Journal of Market Access & Health Policy, 7(1), 1595953. https://doi.org/10.1080/20016689.2019.1595953
- Boyd, A., Van de Velde, S., Pivette, M., ten Have, M., Florescu, S., O’Neill, S., Caldas-de-Almeida, J. M., Vilagut, G., Haro, J. M., Alonso, J., & Kovess-Masféty, V. (2015). Gender differences in psychotropic use across Europe: Results from a large cross-sectional, population-based study. European Psychiatry, 30(6), 778–788. https://doi.org/10.1016/j.eurpsy.2015.05.001
- Bramness, J. G., & Person, O. (2014). A bibliometric analysis of European versus USA research in the field of addiction. Research on alcohol, narcotics, prescription drug abuse, tobacco and steroids 2001-2011. European Addiction Research, 20(1), 16–22. https://doi.org/10.1159/000348260
- Campbell, C. I., Weisner, C., LeResche, L., Ray, G. T., Saunders, K., Sullivan, M. D., Banta-Green, C. J., Merrill, J. O., Silverberg, M. J., Boudreau, D., Satre, D. D., & Von Korff, M. (2010). Age and gender trends in long-term opioid analgesic use for noncancer pain. American Journal of Public Health, 100(12), 2541–2547. https://doi.org/10.2105/AJPH.2009.180646
- Carmona Araújo, A., Fernandes, E., Franco Ruivo, I., do Céu Machado, M., Faria Vaz, A., & Furtado, C. (2023). Prevalência da Dispensa de Medicamentos em Ambulatório na População Idosa em Portugal: Um Estudo Transversal. Acta Médica Portuguesa, 36(12), 792–801. https://doi.org/10.20344/amp.19254
- Carrasco-Garrido, P., Gallardo-Pino, C., Jiménez-Trujillo, I., Hernández-Barrera, V., García-Gómez-Heras, S., Lima Florencio, L., & Palacios-Ceña, D. (2022). Nationwide population-based study about patterns of prescription opioid use and misuse among young adults in Spain. International Journal of Public Health, 67(August), 1–10. https://doi.org/10.3389/ijph.2022.1604755
- Cartagena, F. J., Porter, L., McManus, S., Strang, J., Hickman, M., Reed, K., & Smith, N. (2017). Prescribing patterns in dependence forming medicines. http://phrc.lshtm.ac.uk/
- Casati, A., Sedefov, R., & Pfeiffer-Gerschel, T. (2012). Misuse of medicines in the European union: A systematic review of the literature. European Addiction Research, 18(5), 228–245. https://doi.org/10.1159/000337028
- Chenaf, C., Kaboré, J. L., Delorme, J., Pereira, B., Mulliez, A., Zenut, M., Delage, N., Ardid, D., Eschalier, A., & Authier, N. (2019). Prescription opioid analgesic use in France: Trends and impact on morbidity–mortality. European Journal of Pain, 23(1), 124–134. https://doi.org/10.1002/ejp.1291
- Chiappini, S., Vickers-Smith, R., Guirguis, A., Corkery, J. M., Martinotti, G., & Schifano, F. (2022). A focus on abuse/misuse and withdrawal issues with selective serotonin reuptake inhibitors (SSRIs): Analysis of both the European EMA and the US FAERS pharmacovigilance databases. Pharmaceuticals, 15(5), 1–17. https://doi.org/10.3390/ph15050565
- Chou, R., Turner, J. A., Devine, E. B., Hansen, R. N., Sullivan, S. D., Blazina, I., Dana, T., Bougatsos, C., & Deyo, R. A. (2015). The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a national institutes of health pathways to prevention workshop. Annals of Internal Medicine, 162(4), 276–286. https://doi.org/10.7326/M14-2559
- Conselho Nacional de Saúde. (2019). Sem mais tempo a perder – Saúde mental em Portugal: um desafio para a próxima década. CNS, 1–130. https://fronteirasxxi.pt/wp-content/uploads/2020/02/“Sem-mais-tempo-a-perder”-CNS-2019.pdf
- Conselho Nacional de Ética para as Ciências da Vida. (2023). Parecer 123/CNECV/2023 sobre o uso off-label de medicamentos – Implicações éticas.
- Coordenação Nacional da Estratégia do Medicamento e dos Produtos de Saúde. (2017). Sobreutilização das Benzodiazepinas e dos Z-Hipnóticos na Ansiedade e na Insónia. Ministério Da Saúde. https://www.ordemfarmaceuticos.pt
- Delaš Aždajić, M., Likić, R., Aždajić, S., Šitum, M., Lovrić, I., & Štimac Grbić, D. (2019). Outpatient benzodiazepine utilization in Croatia: Drug use or misuse. International Journal of Clinical Pharmacy, 41(6), 1526–1535. https://doi.org/10.1007/s11096-019-00915-2
- Direção Geral da Saúde. (2015). Tratamento sintomático da ansiedade e insónia com benzodiazepinas e fármacos análogos. Normas de Orientação Clínica, 55/2011, 1–10.
- Driot, D., Jouanjus, E., Oustric, S., Dupouy, J., & Lapeyre-Mestre, M. (2019). Patterns of gabapentin and pregabalin use and misuse: Results of a population-based cohort study in France. British Journal of Clinical Pharmacology, 85(6), 1260–1269. https://doi.org/10.1111/bcp.13892
- EMCDDA. (2023). Spotlight on … Non-medical use of benzodiazepines. 2023. https://www.emcdda.europa.eu/spotlights/non-medical-use-benzodiazepines_en
- European Medicines Agency. (2017). Good pharmacovigilance practices – Annex 1 definitions (Rev.4). Good Pharmacovigilance Practices. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices#final-gvp-annex-i—definitions-section
- European Medicines Agency. (2023). Guideline on clinical investigation of medicinal products in the treatment of depression. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-clinical-investigation-medicinal-products-treatment-depression-revision-3_en.pdf
- Faria Vaz, A., Magalhães, A. S., Lourenço, A., Costa, J., Guerreiro, M., & Ribeiro, N. (2017a). Estratégias para a descontinuação de benzodiazepinas. Boletim Terapêutico – ARS LVT, Anexo I, 1–9.
- Faria Vaz, A., Magalhães, A. S., Lourenço, A., Costa, J., Guerreiro, M., & Ribeiro, N. (2017b). Utilização de Benzodiazepinas: Um grave problema de saúde pública. Boletim Terapêutico – ARS LVT, 1, 1–6.
- FDA. (2016, August 31). FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use | FDA. FDA News Release. https://www.fda.gov/news-events/press-announcements/fda-requires-strong-warnings-opioid-analgesics-prescription-opioid-cough-products-and-benzodiazepine
- Fernandes, M., Neves, I., Oliveira, J., Santos, O., Aguiar, P., Atalaia, P., Matos, F., Freitas, C., Alvim, A., & Maria, V. (2022). Discontinuation of chronic benzodiazepine use in primary care: A nonrandomized intervention. Family Practice, 39(3), 563. https://doi.org/10.1093/fampra/cmac037
- Frauger, E., Amaslidou, D., Spadari, M., Allaria-Lapierre V., Braunstein D., Sciortino V., Thirion X., Djezzar S., & Micallef, J. (2016). Patterns of methylphenidate use and assessment of its abuse among the general population and individuals with drug dependence. European Addiction Research, 22(3), 119–126. https://doi.org/10.1159/000439273
- Frauger, E., Pauly, V., Pradel, V., Rouby, F., Arditti, J., Thirion, X., Lapeyre Mestre, M., & Micallef, J. (2011). Evidence of clonazepam abuse liability: Results of the tools developed by the French Centers for Evaluation and Information on Pharmacodependence (CEIP) network. Fundamental & Clinical Pharmacology, 25(5), 633–641. https://doi.org/10.1111/j.1472-8206.2010.00882.x
- Friedman, J., & Shover, C. L. (2023). Charting the fourth wave: Geographic, temporal, race/ethnicity and demographic trends in polysubstance fentanyl overdose deaths in the United States, 2010–2021. Addiction, 118(12), 2477–2485. http://doi.org/10.1111/add.v118.12
- Ghent University – Faculty of Pharmaceutical Sciences. (2023). Ghent Older People’s Prescriptions community Pharmacy Screening (GheOP3S)-tool – Version 2. 390. https://www.ugent.be/fw/nl/onderzoek/bioanalyse/farmzorg/tools/gheop3s-tool-versie-2/gheop3s-tool-update_eng/view
- Gomes, S., Broeiro-Gonçalves, P., Meireles, C., Caldeira, D., Costa, J., Guerreiro, M. P., Ribeiro, N., & Afonso, R. (2023). Prescrição de Benzodiazepinas e outros Sedativos na Administração Regional de Saúde de Lisboa e Vale do Tejo de 2013 a 2020: Um Estudo Retrospetivo. Acta Médica Portuguesa, 36(4), 264–274. https://doi.org/10.20344/amp.18680
- Hägg, S., Jönsson, A. K., & Ahlner, J. (2020). Current evidence on abuse and misuse of gabapentinoids. Drug Safety, 43(12), 1235–1254. https://doi.org/10.1007/s40264-020-00985-6
- Haukka, J., Kriikku, P., Mariottini, C., Partonen, T., & Ojanperä O. I. (2018). Non-medical use of psychoactive prescription drugs is associated with fatal poisoning. Addiction, 113(3), 464–472. https://doi.org/10.1111/add.14014
- Hedenmalm, K., Slattery, J., Skibicka-Stepien, I., Kurz, X., & Morales, D. (2019). Prescribing patterns of tramadol in adults in IMS® primary care databases in France and Germany between 1 January 2006 and 30 June 2016. European Journal of Clinical Pharmacology, 75(5), 707–716. https://doi.org/10.1007/s00228-018-02622-9
- Hider-Mlynarz, K., Cavalié, P., & Maison, P. (2018). Trends in analgesic consumption in France over the last 10 years and comparison of patterns across Europe. British Journal of Clinical Pharmacology, 84(6), 1324–1334. https://doi.org/10.1111/bcp.13564
- Hockenhull, J., Black, J. C., Haynes, C. M., Rockhill, K., Dargan, P. I., Dart, R. C., & Wood, D. M. (2021). Nonmedical use of benzodiazepines and Z-drugs in the UK. British Journal of Clinical Pharmacology, 87(4), 1676–1683. https://doi.org/10.1111/bcp.14397
- INFARMED. (2017). Benzodiazepinas e Análogos. Infarmed. http://www.infarmed.pt/documents/15786/2219894/Utlilização+de+Benzodiazepinas+e+análogos/adb100fa-4a77-4eb7-9e67-99229e13154f
- INFARMED. (2020). Psicofármacos na última década em Portugal. INFARMED Notícias, 71, 26–29. https://www.infarmed.pt/web/infarmed/institucional/documentacao_e_informacao/publicacoes/institucionais/infarmed_noticias/infarmed-noticias-arquivo
- Kalkman, G. A., Kramers, C., van Dongen, R. T., van den Brink, W., & Schellekens, A. (2019). Trends in use and misuse of opioids in the Netherlands: A retrospective, multi-source database study. The Lancet Public Health, 2667(19), E498-E505. https://doi.org/10.1016/S2468-2667(19)30128-8
- Keto, J., Heiskanen, T., Hamunen, K., Kalliomäki, M. L., & Linna, M. (2022). Opioid trends in Finland: A register-based nationwide follow-up study. Scientific Reports, 12(1), 1–9. doi:10.1038/s41598-022-10788-7
- Khan, N. F., Bykov, K., Glynn, R. J., Barnett, M. L., & Gagne J. J. (2021). Coprescription of opioids with other medications and risk of opioid overdose. Clinical Pharmacology & Therapeutics, 110(4), 1011–1017. https://doi.org/10.1002/cpt.2314
- Kostnapfel, T., Jandl, M., Hocevar Grom, A., Korosec, A., & Kastelic, A. (2022). Prescription opioid use and opioid-related mortality monitoring in Slovenia. Heroin Addiction and Related Clinical Problems, 24(4), 33–39.
- Kurko, T. A., Saastamoinen, L. K., Tähkäpää, S., Tuulio-Henriksson, A., Taiminen, T., Tiihonen, J., Airaksinen, M. S., & Hietala, J. (2015). Long-term use of benzodiazepines: Definitions, prevalence and usage patterns – A systematic review of register-based studies. European Psychiatry, 30(8), 1037–1047. https://doi.org/10.1016/j.eurpsy.2015.09.003
- Kurko, T., Saastamoinen, L. K., Tuulio-Henriksson, A., Taiminen, T., Tiihonen, J., Airaksinen, M., & Hietala, J. (2018). Trends in the long-term use of benzodiazepine anxiolytics and hypnotics: A national register study for 2006 to 2014. Pharmacoepidemiology and Drug Safety, 27(6), 674–682. https://doi.org/10.1002/pds.4551
- Langan, S. M., Schmidt, S. A., Wing, K., Ehrenstein, V., Nicholls, S. G., Filion, K. B., Klungel, O., Petersen, I., Sorensen, H. T., Dixon, W. G., Guttmann, A., Harron, K., Hemkens, L. G., Moher, D., Schneeweiss, S., Smeeth, L., Sturkenboom, M., von Elm, E., Wang, S. V., & Benchimol, E. I. (2018). The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ, 363, k3532. https://doi.org/10.1136/bmj.k3532
- Lombardi, N., Bettiol, A., Crescioli, G., Ravaldi, C., Bonaiuti, R., Venegoni, M., Vighi, G. D., Mugelli, A., Mannaioni, G., Vannacci, A., Aiezza, M. L., Bettoni, D., Blandizzi, C., Borsi, V., Capuano, A., Cecchi, E., Convertino, I., Lungo, M., Del Mauro, C., … Di Vighi, G. V. (2020). Risk of hospitalisation associated with benzodiazepines and z-drugs in Italy: A nationwide multicentre study in emergency departments. Internal and Emergency Medicine, 15(7), 1291–1302. https://doi.org/10.1007/s11739-020-02339-7
- Luijendijk, H. J., Tiemeier, H., Hofman, A., Heeringa, J., & Stricker, B. H. C. (2008). Determinants of chronic benzodiazepine use in the elderly: A longitudinal study. British Journal of Clinical Pharmacology, 65(4), 593–599. https://doi.org/10.1111/j.1365-2125.2007.03060.x
- Lunghi, C., Antonazzo, I. C., Burato, S., Raschi, E., Zoffoli, V., Forcesi, E., Sangiorgi, E., Menchetti, M., Roberge, P., & Poluzzi, E. (2020). Prevalence and determinants of long-term utilization of antidepressant drugs: A retrospective cohort study. Neuropsychiatric Disease and Treatment, 16, 1157–1170. https://doi.org/10.2147/NDT.S241780
- Madeira, L., Queiroz, G., & Henriques, R. (2023). Prepandemic psychotropic drug status in Portugal: A nationwide pharmacoepidemiological profile. Scientific Reports, 13(1), 1–13. doi:10.1038/s41598-023-33765-0
- Marschall, U., L’Hoest, H., Radbruch, L., & Häuser, W. (2016). Long-term opioid therapy for chronic non-cancer pain in Germany. European Journal of Pain, 20(5), 767–776. https://doi.org/10.1002/ejp.802
- Mellbye, A., Karlstad, Ø., Skurtveit, S., Borchgrevink, P. C., & Fredheim, O. M. (2016). The duration and course of opioid therapy in patients with chronic non-malignant pain. Acta Anaesthesiologica Scandinavica, 60(1), 128–137. https://doi.org/10.1111/aas.12594
- Micallef, J., Frauger, E., Palmaro, A., Boucherie, Q., & Lapeyre Mestre, M. (2015). Example of an investigation of an “emergent” phenomenon in addiction vigilance: The case of methylphenidate. Therapies, 70(2), 197–202. https://doi.org/10.2515/therapie/2015013
- Motta-Ochoa, R., Bertrand, K., Arruda, N., Jutras-Aswad, D., & Roy, É. (2017). “I love having benzos after my coke shot”: The use of psychotropic medication among cocaine users in downtown Montreal. International Journal of Drug Policy, 49, 15–23. https://doi.org/10.1016/j.drugpo.2017.07.012
- Muller, A. E., Clausen, T., Sjøgren, P., Odsbu, I., & Skurtveit, S. (2019). Prescribed opioid analgesic use developments in three Nordic countries, 2006-2017. Scandinavian Journal of Pain, 19(2), 345–353. https://doi.org/10.1515/sjpain-2018-0307
- Nielsen, S., Gisev, N., Bruno, R., Hall, W., Cohen, M., Larance, B., Campbell, G., Shanahan, M., Blyth, F., Lintzeris, N., Pearson, S., Mattick, R., & Degenhardt, L. (2017). Defined daily doses (DDD) do not accurately reflect opioid doses used in contemporary chronic pain treatment. Pharmacoepidemiology and Drug Safety, 26(5), 587–591. https://doi.org/10.1002/pds.4168
- Nordmann, S., Pradel, V., Lapeyre-Mestre, M., Frauger, E., Pauly, V., Thirion, X., Mallaret, M., Jouanjus, E., & Micallef, J. (2013). Doctor shopping reveals geographical variations in opioid abuse. Pain Physician, 16(1), 89–100.
- Novak, S. P., Håkansson, A., Martinez-Raga, J., Reimer, J., Krotki, K., & Varughese, S. (2016). Nonmedical use of prescription drugs in the European Union. BMC Psychiatry, 16(1), 1–12. doi:10.1186/s12888-016-0909-3
- OECD. (2020). Health at a Glance: Europe 2020. OECD Publishing.
- OECD. (2023). Pharmaceutical market | OECD Health Statistics | OECD iLibrary. https://www.oecd.org/els/health-systems/health-data.htm
- OECD European Observatory on Health Systems and Policies. (2023). Country health profile 2023, State of Health in the EU – Portugal.
- Oliveira, J., Neves, I., Fernandes, M., Santos, O., & Maria, V. (2019). Prescribing and facilitating withdrawal from benzodiazepines in primary health care. Revista Portuguesa de Clínica Geral, 35(4), 305–312. https://doi.org/10.32385/rpmgf.v35i4.12239
- O’Mahony, D., Cherubini, A., Guiteras, A. R., Denkinger, M., Beuscart, J. B., Onder, G., Gudmundsson, A., Cruz-Jentoft, A. J., Knol, W., Bahat, G., van der Velde, N., Petrovic, M., & Curtin, D. (2023). STOPP/START criteria for potentially inappropriate prescribing in older people: Version 3. European Geriatric Medicine, 14(4), 625–632. https://doi.org/10.1007/s41999-023-00777-y
- OsMed. (n.d.). National report on medicines use in Italy 2017. 2017. https://www.aifa.gov.it/documents/20142/241052/OsMed_2017_eng.pdf
- Pierce, M., van Amsterdam, J., Kalkman, G. A., Schellekens, A., & van den Brink, W. (2021). Is Europe facing an opioid crisis like the United States? An analysis of opioid use and related adverse effects in 19 European countries between 2010 and 2018. European Psychiatry, 64(1), 1–18. https://doi.org/10.1192/j.eurpsy.2021.2219
- Ponté, C., Lepelley, M., Boucherie, Q., Mallaret, M., Lapeyre Mestre, M., Pradel, V., & Micallef, J. (2018). Doctor shopping of opioid analgesics relative to benzodiazepines: A pharmacoepidemiological study among 11.7 million inhabitants in the French countries. Drug and Alcohol Dependence, 187, 88–94. https://doi.org/10.1016/j.drugalcdep.2018.01.036
- Pradel, V., Delga, C., Rouby, F., Micallef, J., & Lapeyre-Mestre, M. (2010). Assessment of abuse potential of benzodiazepines from a prescription database using ‘doctor shopping’ as an indicator. CNS Drugs, 24(7), 611–620. https://doi.org/10.2165/11531570-000000000-00000
- Pradel, V., Frauger, E., Thirion, X., Ronfle, E., Lapierre, V., Masut, A., Coudert, C., Blin, O., & Micallef, J. (2009). Impact of a prescription monitoring program on doctor-shopping for high dosage buprenorphine. Pharmacoepidemiology and Drug Safety, 18(1), 36–43. https://doi.org/10.1002/pds.1681
- Pradel, V., Thirion, X., Ronfle, E., Masut, A., Micallef, J., & Bégaud, B. (2004). Assessment of doctor-shopping for high dosage buprenorphine maintenance treatment in a French region: Development of a new method for prescription database. Pharmacoepidemiology and Drug Safety, 13(7), 473–481. https://doi.org/10.1002/pds.892
- Prazeres, F. (2023). Letter to the editor on the identification of potentially inappropriate medications among elderly patients in ambulatory care in Portugal. Acta Médica Portuguesa, 36(12), 850–851. https://doi.org/10.20344/amp.20389
- Public Health England. (2019). Dependence and withdrawal associated with some prescribed medicines – An evidence review. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/829777/PHE_PMR_report.pdf
- Regueras, E., & López Guzmán, J. (2021). Cómo es el uso de medicamentos opioides en España? Análisis de los datos de la encuesta EDADES 2017. Multidisciplinary Pain Journal, 1, 141–148. https://doi.org/10.20986/MPJ.2021.1005/2021
- Rodrigues, D. A., Herdeiro, M. T., Thurmann, P. A., Figueiras, A., Coutinho, P., & Roque, F. (2020). Operacionalização para Portugal da Lista EU(7)-PIM para Identificação de Medicamentos Potencialmente Inapropriados nos Idosos [Operationalisation for Portugal of the EU(7)-PIM list for identification of potentially inappropriate medicines in older adults]. Acta Médica Portuguesa, 33(13), 194–200. www.actamedicaportuguesa.com
- Rossow, I., & Bramness, J. G. (2015). The total sale of prescription drugs with an abuse potential predicts the number of excessive users: A national prescription database study Health behavior, health promotion and society. BMC Public Health, 15(1), 1–9. doi:10.1186/s12889-015-1615-7
- Rouby, F., Pradel, V., Frauger, E., Pauly, V., Natali, F., Reggio, P., Thirion, X., & Micallef, J. (2012). Assessment of abuse of tianeptine from a reimbursement database using ‘doctor-shopping’ as an indicator. Fundamental & Clinical Pharmacology, 26(2), 286–294. https://doi.org/10.1111/j.1472-8206.2010.00906.x
- Roussin, A., Palmaro, A., & Lapeyre-Mestre, M. (2016). Weak opioid analgesics abuse and addiction. In Neuropathology of drug addictions and substance misuse (pp. 375–391). Elsevier. https://doi.org/10.1016/B978-0-12-800634-4.00036-6
- Schifano, F., Chiappini, S., Corkery, J. M., & Guirguis A. (2018). Abuse of prescription drugs in the context of novel psychoactive substances (NPS): A systematic review. Brain Sciences, 8(4), 73, https://doi.org/10.3390/brainsci8040073
- Schjerning, O., Pottegård, A., Damkier, P., Rosenzweig, M., & Nielsen, J. (2016). Use of pregabalin – A nationwide pharmacoepidemiological drug utilization study with focus on abuse potential. Pharmacopsychiatry, 49(4), 155–161. https://doi.org/10.1055/s-0042-101868
- Schonmann, Y., Goren, O., Bareket, R., Comaneshter, D., Cohen, A. D., & Vinker, S. (2018). Chronic hypnotic use at 10 years – Does the brand matter? European Journal of Clinical Pharmacology, 74(12), 1623–1631. https://doi.org/10.1007/s00228-018-2531-4
- Simonsen, K. W., Kriikku, P., Thelander, G., Edvardsen, H. M. E., Thordardottir, S., Andersen, C. U., Jönsson, A. K., Frost, J., Christoffersen, D. J., Delaveris, G. J. M., & Ojanperä, I. (2020). Fatal poisoning in drug addicts in the Nordic countries in 2017. Forensic Science International, 313, 110343. https://doi.org/10.1016/j.forsciint.2020.110343
- Smith, S. M., Dart, R. C., Katz, N. P., Paillard. F., Adams, E. H., Comer, S. D., Degroot, A., Edwards, R. R., Haddox, D. J., Jaffe, J. H., Jones, C. M., Kleber, H. D., Kopecky, E. A., Markman, J. D., Montoya, I. D., O'Brien C., Roland C. L., Stanton, M., … Dworkin, R. H. (2017). Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain, 154(11), 2287–2296. https://doi.org/10.1016/j.pain.2013.05.053
- Soeiro, T., Micallef, J., & Pradel, V. (2023). Systematic assessment of non-medical use of prescription drugs using doctor-shopping indicators : A nation-wide, repeated cross-sectional study. Addiction, 118(10), 1984–1993. https://doi.org/10.1111/add.16261
- Torrance, N., Mansoor, R., Wang, H., Gilbert, S., Macfarlane, G. J., Serpell, M., Baldacchino, A., Hales, T. G., Donnan, P., Wyper, G., Smith, B. H., & Colvin, L. (2018). Association of opioid prescribing practices with chronic pain and benzodiazepine co-prescription: A primary care data linkage study. British Journal of Anaesthesia, 120(6), 1345–1355. https://doi.org/10.1016/j.bja.2018.02.022
- Transnational Institute. (2015). The UN drug control conventions. https://www.tni.org/en/publication/the-un-drug-control-conventions
- United Nations Office on Drug and Crime. (2017a). World Drug Report 2017 Booklet 2 – GLOBAL OVERVIEW OF DRUG DEMAND AND SUPPLY. Retrieved April 30, 2023, from www.unodc.org/wdr2017
- United Nations Office on Drugs and Crime. (2017b). Non-medical use of benzodiazepines: a growing threat to public health? Global SMART Update, 18, 1-12, www.unodc.org/unodc/en/
- van Amsterdam, J., & van den Brink, W. (2015). The misuse of prescription opioids: A threat for Europe? Current Drug Abuse Reviews, 8(1), 3–14. https://doi.org/10.2174/187447370801150611184218
- Wei, Y.-J. J., Zhu, Y., Liu, W., Bussing, R., & Winterstein, A. G. (2018). Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Network Open, 1(4), e181152. https://doi.org/10.1001/jamanetworkopen.2018.1152
- WHO Collaborating Centre for Drug Statistics and Methodology. (2022a). WHOCC – Structure and principles. Retrieved May 26, 2023, from https://www.whocc.no/atc/structure_and_principles/
- WHO Collaboration Centre for Drug Statistics Methodology. (2022b). Guidelines for ATC classification and DDD assignment. https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/
- Wood, D. M., & Dargan, P. I. (2021). Regional, national and international datasets: How they improve our understanding of the acute harms associated with prescription medicine misuse. British Journal of Clinical Pharmacology, 87(4), 1654–1659. https://doi.org/10.1111/bcp.14592
- World Health Organization. (n.d.). International classification of primary care (2nd ed.). ICPC-2. Retrieved May 12, 2023, from https://www.who.int/standards/classifications/other-classifications/international-classification-of-primary-care.
- Worley, J., & Thomas, S. P. (2014). Women who doctor shop for prescription drugs. Western Journal of Nursing Research, 36(4), 456–474. https://doi.org/10.1177/0193945913509692
- Zin, C. S., Chen, L. C., & Knaggs, R. D. (2014). Changes in trends and pattern of strong opioid prescribing in primary care. European Journal of Pain, 18(9), 1343–1351. https://doi.org/10.1002/j.1532-2149.2014.496.x