ABSTRACT
Introduction
Healthcare systems in developing countries faced significant challenges during COVID-19, grappling with limited resources and staffing shortages. Assessment of the impact of pharmaceutical care expertise, particularly in critical care units during the pandemics, in developing countries remains poorly explored. The principal aim of our study was to assess the impact of the Drug and Therapeutics Committee (DTC), comprising clinical pharmacists, on the incidence, types, and severity of medication errors and associated costs in using COVID-19 medications, especially antibiotics.
Methods
An interventional pre–post study was carried out at a public isolation hospital in Egypt over 6 months.
Results
Out of 499 medication orders, 238 (47.7%) had medication errors, averaging 2.38 errors per patient. The most frequent were prescribing errors (44.9%), specifically incorrect drug choice (57.9%), excessive dosage (29.9%), treatment duplication (4.5%), inadequate dosage (4.5%), and overlooked indications (3.6%). Linezolid and Remdesivir were the most common medications associated with prescribing errors. Pharmacists intervened 315 times, primarily discontinuing medications, reducing doses, introducing new medications, and increasing doses. These actions led to statistically significant cost reductions (p < 0.05) and better clinical outcomes; improved oxygen saturation, decreased fever, stabilised respiratory rates, and normalised white blood cell counts. So, clinical pharmacist interventions made a notable clinical and economic difference (66.34% reduction of the expenses) in antibiotics usage specifically and other medications used in COVID-19 management during the pandemic.
Conclusion
Crucially, educational initiatives targeting clinical pharmacists can foster judicious prescribing habits.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused a dramatic humanistic and economic burden globally (Alenzi et al., Citation2022; Lui et al., Citation2021; Peramo-Álvarez et al., Citation2021; Smith Jervelund & Eikemo, Citation2021). It posed unprecedented challenges to clinicians and healthcare systems aiming for optimal disease management, especially in low to middle-income countries (LMICs) and low-resource settings due to insufficient staffing, poor communication, and inadequate resources (Al Meslamani et al., Citation2021; Elhadi et al., Citation2020; Kajal et al., Citation2020; Kassem et al., Citation2021). Critically ill patients usually receive more medications than patients in other units and most of these medications are high-risk and/or intravenous, with frequent changes in medication regimens (Fair et al., Citation2023; Wang et al., Citation2020). Those critically ill patients are more vulnerable to medication errors, which may result in serious adverse events, threaten their safety, and jeopardise the quality of care they receive (Di Simone et al., Citation2016; Suclupe et al., Citation2020).
Amid the COVID-19 pandemic, another silent pandemic, that of antibiotic resistance due to antibiotic overuse and misuse, was threatening the globe (Elsayed et al., Citation2021; Founou et al., Citation2021; Garg, Citation2021). Egypt is in the middle of the list of many countries in terms of antibiotic consumption expressed in defined daily doses (DDD)/1000 inhabitants/day (Klein et al., Citation2018). The misuse of antimicrobials is common in critical care units among LMICs, including Egypt, which is associated with the emergence of antimicrobial resistance (Elsorady et al., Citation2022; Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report, Citation2021). Antibiotics are sometimes sold by community pharmacies without a prescription, which further complicates the problem of antimicrobial resistance (Elsayed et al., Citation2021; Jamshed et al., Citation2018; Lambert et al., Citation2022). The insufficient health literacy levels of the Egyptian population can even make the problem worse (Almaleh et al., Citation2017; Anwar et al., Citation2020; Essam et al., Citation2022; Moustafa & Kassem, Citation2023). Therefore, applying strict institutional Antimicrobial Stewardship Program is urgently needed to enhance rational antibiotic use (AWaRe Classification, Citationn.d.; Barlam et al., Citation2016; Cunha, Citation2018).
Antimicrobial time-out (ATO), previously advocated by The Centers for Disease Control and Prevention (CDC), is a sub-domain of anti-microbial stewardship that involves reviewing antimicrobial therapy after initiation and deciding to continue, adjust, or discontinue according to clinical and microbiologic data (Van Schooneveld et al., Citation2020). The advantage of ATO is that it can theoretically be implemented without additional resources, personnel, expertise, or outside input, which would be of great value in LMICs such as Egypt and in low-resource settings. However, published data on the efficacy of ATO strategy application is scarce (Richardson et al., Citation2019; Thom et al., Citation2019).
Pharmacists’ services have evolved from mere drug dispensing to more individualised and specialised patient care to align with institutional goals and best practices (Al Aqeel et al., Citation2018; Al Meslamani, Citation2023a, Citation2023b; Presley et al., Citation2019). They follow evidence-based guidelines, formulate local guidelines, design drug use policies, diminish medication errors, champion medication safety initiatives, and assist in treatment decisions (Kassem et al., Citation2021; Lee et al., Citation2017; Stasiak et al., Citation2014; Van der Linden et al., Citation2020). Their role in reducing medication errors in the vulnerable population of the ICU is expanding (Arredondo et al., Citation2021; Kharaba et al., Citation2022).
Clinical pharmacists are potential key players in emergencies and disasters (Erstad, Citation2021; Li et al., Citation2021; Perez et al., Citation2022). Their scope of practice during the pandemic involved providing prevention of infection, providing drug information for healthcare personnel and patients, and optimising the drug therapy (Al Meslamani et al., Citation2021; Arain et al., Citation2021; Li et al., Citation2021; Visacri et al., Citation2021). In addition, dedicated clinical pharmacists involvement in patient care improves healthcare outcomes and reduces costs (Coralic et al., Citation2014; Hyland et al., Citation2020; Morgan et al., Citation2018; Niznik et al., Citation2018).
Although most critical care physicians think the advice from a clinical pharmacist is helpful (Shendy et al., Citation2022), clinical pharmacy services are still not optimally implemented in most of the hospitals in LMICs such as Egypt (Nguyen et al., Citation2014; Said et al., Citation2020), especially in low-resource settings (Eldin et al., Citation2022; Nguyen et al., Citation2014). Therefore, training clinical pharmacists, especially those working in the ICU may have a positive impact on clinical and economic outcomes (Arredondo et al., Citation2021; Barlow et al., Citation2022; Johansen et al., Citation2016; Nguyen et al., Citation2014). They can act as an alternative to infectious diseases specialists or infectious diseases clinical pharmacists for antibiotic optimisation when resources are limited (Cunha, Citation2018; Moody et al., Citation2012). Moreover, the Society for Infectious Disease Pharmacists emphasises the role of pharmacists in antimicrobial stewardship, even if the pharmacist is not formally trained in infectious diseases (Heil et al., Citation2016).
The Drug and Therapeutics Committee (DTC) is the highest in the hierarchy of hospital pharmacy management that promotes the best practices in medicine use. It has a wide range of functions, including medication selection for the local formulary, drug information provision to medical staff, drug-use policies development and auditing, and applying standard treatment guidelines. It reduces and manages medication errors (Kabba et al., Citation2023).
Notably, there is a scarcity of research related to clinical pharmacy interventions or DTC activities to optimise treatment plans and save costs during COVID-19 in LMICs, especially in intensive care units (ICUs). Encouraging multidisciplinary collaboration of pharmacists within the health system by being involved in the DTC was previously encouraged (Arredondo et al., Citation2021; Cameron et al., Citation2022; Kabba et al., Citation2023). They can act proactively in light of the goals of this committee to reduce medication errors and expenditures. However, previously published data usually show the clinical effectiveness of pharmacist-led services provided after DTC implementation, while not analyzing the economic savings of the implementation of these services. Therefore, this study aims, not only to assess the clinical outcomes but also the direct medication cost-savings upon implementing clinical pharmacist-led interventions after the initiation and implementation of the DTC committee during COVID-19 in a low-resource setting in a LMIC. The clinical pharmacist’s interventions aimed to prevent medication errors and to optimise the use of drugs, especially antibiotics in an adult infectious disease ward and an adult ICU of a public COVID-19 isolation hospital.
2. Patients and Methods
2.1. Study design and setting
2.1.1. Study design
A prospective, comparative pre–post interventional study.
2.1.2. Setting
An infectious disease ward (150 beds) and an intensive care unit (ICU) (50 beds) of a general medium-sized isolation hospital in Egypt.
2.1.3. Duration
From June 2021 to December 2021.
2.1.4. Inclusion criteria
All adults aged 18 years or older, with laboratory-confirmed COVID-19 through polymerase chain reaction (PCR) test of nasopharyngeal swaps and an oxygen saturation of less than 95%, admitted to the isolation ward in the general hospital in Egypt, with an indication of at least one antibiotic, were considered eligible for the Drug and Therapeutics Committee (DTC) interventions.
2.1.5. Exclusion criteria
Ambulatory patients, individuals with human immunodeficiency virus (HIV) infections, pregnant women, those with oxygen saturation of 95% or higher, and those who declined participation were excluded from the study.
2.2. Phases of the study
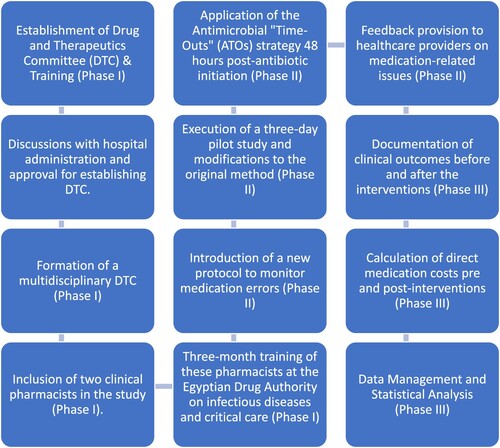
The study flow is illustrated in .
2.2.1. Phase I
Establishment of the DTC and training of its members:
2.2.1.1. Committee members
Multidisciplinary, comprised of physicians, clinical pharmacists, nurses, and other various stakeholders.
2.2.1.2. Training program
Two of the DTC clinical pharmacists received their first formal infectious diseases training and critical care training at the Egyptian Drug Authority for three months. Those two trained clinical pharmacists participated in this study. Infectious diseases training aimed at establishing an enhanced knowledge base regarding antimicrobials. It focused on various aspects of antimicrobial therapy including the stewardship program, selective infections treating algorithms, how to adjust drug doses according to renal and hepatic function, intravenous to oral switching, pharmacokinetic and pharmacodynamic-based dosing, and antibiotic resistance potential. It is worth to mention that there was no infectious disease physician consultant in the selected hospital.
2.2.2. Phase II
DTC-trained clinical pharmacists’ clinical interventions
2.2.2.1. A new protocol establishment
The principal objective of the newly established protocol is to detect and control the rate of medication errors, especially those related to antimicrobial agents.
2.2.2.2. A small-scale pilot study
A pilot study was conducted for three days to evaluate the feasibility and practicality before conducting the final study. As a result of this preliminary study, minor modifications to the original method were made.
2.2.2.3. Interventions
Trained clinical pharmacists in the DTC independently accessed all medical records for clinical information. They reported demographic data (age, gender), care unit (ward or ICU), comorbidities, and pre-intervention prescribed medications. They provided comprehensive and individualised pharmaceutical care to the patients, based on the latest guidelines and protocols. They reviewed the prescribing and dispensing of the medication in 100 medication records against the patients’ clinical data to detect, report, and prevent medication errors. One medication record can contain more than one medication error. Interventions performed by the clinical pharmacist included stopping, starting, or changing the drug dosage. They also aimed at improving the quality of dispensing, as well. They recorded prescribers’ acceptance of the teams’ interventions.
Optimising antimicrobial therapy was done via antimicrobial ‘time-outs' (ATOs) strategy. DTC-trained clinical pharmacists applied the ATOs strategy 48-hours post-antibiotic initiation, after thoroughly assessing the patient characteristics, and reviewing their medical records, in line with a previous study (Van Schooneveld et al., Citation2020). Details of medication errors were verified by two researchers of clinical pharmacy and pharmacy practice, who reviewed the clinical situations, the medication errors, and the interventions and compared the database with the original data sheets and notes written by the team members.
2.2.2.4. Definitions:
Medication error is defined as ‘any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer' ([email protected], Citation2014).
A prescription error is defined as any failure in writing the prescription that leads to a wrong instruction (Aronson, Citation2009).
A dispensing error is defined as the inconsistency between the prescription and the dispensing process (Aronson, Citation2009; Maharaj et al., Citation2020).
Prescribing a wrong drug is defined as any irrational, inappropriate prescribing according to guidelines/formulary or prescribing a contraindicated drug (Aronson, Citation2009; Deawjaroen et al., Citation2022).
An untreated indication or drug omission means the omission of a potentially beneficial drug that may cause therapeutic inertia (Deawjaroen et al., Citation2022; Guignard et al., Citation2015).
Treatment duplication is defined as double prescribing of the same therapeutic group or active ingredient (Deawjaroen et al., Citation2022).
Low dose is defined as a dose that is lower than the minimal recommended dose for a given indication (Deawjaroen et al., Citation2022; Guignard et al., Citation2015).
High dose is defined as a dose higher than the maximal recommended dose for a given indication (Deawjaroen et al., Citation2022; Guignard et al., Citation2015).
Recommendations delivery method: DTC-trained clinical pharmacists gave feedback to the treating healthcare provider on guidelines-compliant prescribing and medication-related issues via a notification placed in the patient’s file or verbally, referenced by relevant up-to-date guidelines.
2.2.3. Phase III
Effect of pharmacist-led interventions on clinical outcomes and direct medication cost savings to the institution:
2.2.3.1. Clinical outcomes
Patients were followed up from admission to either death or discharge. Also, glucose level, oxygen saturation, platelet count, white Blood cell counts (WBCs), hemoglobin, temperature, respiratory rate, and overall hospital length of stay were documented before and after the intervention.
2.2.3.2. Direct cost saving
Direct medication cost of treating patients admitted during the study period (June–December 2021) was estimated pre- and post-interventions. Cost savings due to interventions were calculated as the cost of therapy pre-intervention minus the sum of the cost of therapy after intervention and the pharmacists’ services cost.
2.2.3.3. Data management
The DTC-trained clinical pharmacists’ team was responsible for collecting data sheets and building the final database. To determine the appropriate sample size for our study, we used the G*Power software, which allowed for a precise estimation based on the expected effect size, power, and significance level. Assuming an effect size of 0.3, a power of 90%, and an alpha level of 0.05, the software recommended a minimum sample size of 88 patients. To account for potential dropouts and ensure robust statistical power, we decided to enroll 100 patients in the study.
2.2.4. Statistical analysis
Our study was designed with specific hypotheses in mind, aiming to evaluate the impact of pharmacist interventions on various outcomes. The primary hypothesis was that these interventions would lead to significant changes in clinical outcomes, cost, and medication errors. To test this, SPSS version 26 was used. For categorical variables, differences were assessed using the Chi-square test or Fisher’s exact test as appropriate. The data were not normally distributed, as indicated by the results of the Shapiro–Wilk test, which showed that the data significantly deviated from a normal distribution (p < 0.05). To evaluate changes in median values before and after pharmacist interventions, the Wilcoxon signed-rank test was utilised. This non-parametric test is appropriate for our before-and-after study design, as it compares paired observations and is robust against non-normal distributions of the data.
Furthermore, to visualise differences in median costs before and after interventions, an error bar chart was used. We acknowledged the potential for regression to the mean, a phenomenon where extreme observations tend to move towards the average on subsequent measurements. This effect was considered in our data interpretation, particularly in assessing whether observed changes could be attributed solely to the interventions or partly to this statistical phenomenon. A p-value of less than 0.05 was considered statistically significant. However, we recognise that statistical significance does not always equate to clinical significance, and hence, our discussion also considers the practical implications of our findings.
2.2.5. Ethical approval
The study received approval from the Institutional Review Board of Damanhur University (Project No.1023PP69). It was conducted in adherence to the ethical standards of the Declaration of Helsinki (2013). Informed consent was secured from all patients or their substitute legal guardians before including them in the study. The hospital approval was obtained in June 2021 for establishing a DTC for reviewing patients’ files, especially those with prescriptions for antibiotics.
3. Results
3.1. Demographic data.
During this prospective interventional study, pharmacists monitored 100 patients’ medical records and detected, reported, and prevented medication errors in the infectious disease ward and intensive care unit (ICU) of a general hospital in Egypt through the period from July 2021 to December 2021. The total number of patients included in this study was 100, of which 56 (56.0%) were females, 58 (58.0%) were in the ICU because of complications, such as acute respiratory distress syndrome, shock, or arrhythmia, and 21 (21.0%) had at least 3 comorbidities (). Additionally, the median age for all patients was 69 years with an IQR of 56.3–75.0 years. Among the patients, 91 (91.0%) had improved clinical outcomes after pharmacists’ interventions. Statistically significant differences in clinical outcomes were observed based on the clinical setting (p = 0.009) and the requirement for ventilation (p = 0.001). Specifically, among patients treated outside of the ICU (n = 42) and those who did not necessitate ventilation (n = 52), 100% of them demonstrated improved clinical outcomes ().
Table 1. Basic characteristics of patients (N = 100).
Table 2. Distribution of improved and non-improved cases.
The median hospital LOS for improved cases was significantly shorter at 7 days (IQR 5–10 days) compared to 9 days (IQR 7–12 days) for non-improved cases (p = 0.034). Similarly, the ICU LOS median was shorter for the improved group at 3 days (IQR 2–5 days) versus 5 days (IQR 3–7 days) for the non-improved group (p = 0.048). Mortality was observed in 2% of improved cases compared to 10% in non-improved cases (p = 0.022). Antibiotic consumption was reduced in the improved group, showing a decrease in defined daily doses (DDDs) per 100 bed days from 120 to 85 (p = 0.015), indicating more judicious use of antibiotics following pharmacist interventions.
3.2. Medication errors reporting
A total of 499 medication orders were documented, of which 238 (47.7%) medication errors were identified, equating to an average of 2.38 errors per patient (). The most common erroneous antibiotic medications in prescribing included ceftriaxone (n = 68; 30.4%), levofloxacin (n = 32; 14.3%), and linezolid (n = 23; 10.3%) (as shown in ). Ceftriaxone (n = 6; 42.9%) was the most common erroneous antibiotic medication included in dispensing errors of antibiotics. Prescribing errors constituted a significant segment, with 224 (44.9%) errors observed. The most common prescribing errors encompassed wrong drug (n = 129, 57.9%), prescribing an excessively high dose (n = 67, 29.9%), treatment duplication (n = 10, 4.5%), prescribing an insufficient dose (n = 10, 4.5%), and overlooking untreated indications (n = 8, 3.6%).
Table 3. Medication errors identified by pharmacists and pharmacist interventions.
Table 4. Types of erroneous medications.
Dispensing errors, on the other hand, were comparatively less frequent with 14 identified, accounting for 2.8% of the total. These predominantly pertained to the administration of incorrect doses (n = 12, 85.7%), followed by dispensing the wrong drugs (n = 2, 14.3%).
3.3. Pharmacists’ interventions
A total of 315 pharmacist interventions were recorded. These primarily involved ceasing medications (n = 141, 44.8%), dose reductions (n = 91, 28.9%), introducing new medications (n = 77, 24.4%), and, less frequently, increasing doses (n = 6, 1.9%). Examples of clinical scenarios are described in .
Table 5. Clinical scenarios.
3.4. Pre-intervention versus post-intervention parameters
Following the intervention, several significant changes were observed in the evaluated parameters. The number of prescribed medications pre- and post-intervention remained relatively consistent: 5 (IQR: 4–6) compared to 5 (IQR: 3.25–6); this difference was not statistically significant (p = 0.157).
Glucose levels displayed a notable reduction from a median of 407.5 (IQR: 90.7–548.7) to 130 (IQR: 110–179), though this change was not statistically significant (p = 0.109) ().
Table 6. The impact of pharmacist interventions on the number of prescribed medication and clinical parameters.
Oxygen saturation exhibited a significant increase from 88% (IQR: 83.5–89.5) pre-intervention to 96% (IQR: 94.5–96.5) post-intervention (p = 0.011). While the platelet count rose from 79,000 (IQR: 70,000–86,200) to 115,000 (IQR: 110,000–115,000), this change was not statistically significant (p = 0.180). Temperature demonstrated a marked reduction post-intervention, shifting from 39.5°C (IQR: 39–39.8) to 35.0°C (IQR: 30.0–36.0) (p = 0.001). The respiratory rate also saw a significant decrease, moving from 35 (IQR: 30–36) to 22 (IQR: 22–22) post-intervention (p = 0.002). White blood cell (WBC) counts declined substantially from 19800 (IQR: 18,000–24,000) pre-intervention to 8600 (IQR: 7200–9700) post-intervention. However, changes in hemoglobin levels did not achieve statistical significance (p = 0.317).
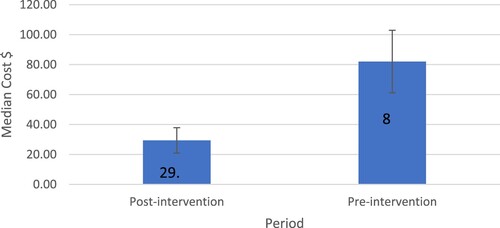
Antibiotic expenditure was retrieved from the hospital database. Without the intervention, the medication cost was supposed to be 12695.9 dollars. The medication cost after the intervention was 6195 dollars and the cost for pharmacist service in the intervention was 2483 dollars. The wage per work hour of a pharmacist was 2.6 dollars. The total length of stay of all patients was 955 days and each of the two clinical pharmacists worked for 30 minutes per case each day. Therefore, the total working hours dedicated to the service by the two clinical pharmacists were 955 hours, taking into account that the case workup will take approximately 30 minutes (including medication review, feedback to the physician, mind storming with the other clinical pharmacist, and documentation). About 4017.8 dollars were saved after subtracting the cost of pharmacists’ services. The findings showed a statistically significant decrease in the median cost of treatment after pharmacist interventions (82.0$ pre-intervention vs. 29.3$ post-intervention) ().
4. Discussion
Developing countries are in greater need of urgent healthcare plans due to their considerable health disparities (Al Meslamani et al., Citation2021; Gwatkin, Citation2017). Although clinical pharmacists can have a powerful impact on the optimisation of therapeutic regimens and saving medication costs, clinical pharmacy services in low-resource settings in developing countries are not actively implemented (Bronkhorst et al., Citation2020; Shrestha et al., Citation2019). Therefore, this study aimed to fill the gap of knowledge regarding the impact of clinical pharmacists’ interventions as members of a Drug and Therapeutics Committee (DTC) on improving clinical outcomes and reducing medication costs in an isolation governmental hospital during COVID-19, in a low-to-middle income country (LMIC).
In the current study, the DTC-trained clinical pharmacist interventions significantly improved patients’ clinical outcomes and reduced direct medication costs. Improved patients’ clinical outcomes reported included oxygen saturation, respiratory rate, fever, and WBC count in 91% of patients. Statistically significant differences in clinical outcomes were observed among patients treated outside of the intensive care unit (ICU) and those who did not require mechanical ventilation (100% of them demonstrated improved clinical outcomes). This may be attributed to the fact that ICU patients are usually more vulnerable than patients in the medical ward setting (Santhosh et al., Citation2019) and that the use of ventilator-associated events may be associated with worse clinical outcomes (Weinberger et al., Citation2021). It was reported previously that clinical pharmacist involvement in the ICU led to a significantly lower length of stay, medication costs, patient care costs, and risk of mortality (Khalili et al., Citation2013; Leguelinel-Blache et al., Citation2018; MacLaren & Bond, Citation2009).
There was no significant statistical difference between the number of prescribed medications pre- and post-intervention, in line with a previous study (Liou et al., Citation2021). Expenditures were measured by comparing medication costs before and after the implementation of the intervention, in line with a previous study (Beardsley et al., Citation2012). Calculations were based on prescriptions and administrations instead of purchasing data, which was previously recommended (Barlam et al., Citation2016).
The adopted antimicrobial ‘time-outs' (ATOs) strategy, a sub-domain of the anti-microbial stewardship program, was feasible and accepted. Stewardship strategies can save antibiotic medication costs (Malani et al., Citation2013; Nowak et al., Citation2012), in line with our study. However, some previous studies failed to demonstrate a significant healthcare resources reduction (Al-Hashar et al., Citation2018; Cypes et al., Citation2021; Liou et al., Citation2021). Even with fewer resources and supports and no dedicated pharmacist time, our DTC-trained clinical pharmacists-led antimicrobial stewardship interventions have produced significant reductions in antimicrobial use and expenditures, in line with a previous study with limited resources (Malani et al., Citation2013). There may have been a misconception that limited resources settings are incapable of applying antimicrobial stewardship programs (Lim et al., Citation2014). The role of pharmacists in the optimisation of antimicrobial use in ICU should be emphasised in developing countries (Shendy et al., Citation2022), even if the pharmacist is not formally trained in infectious diseases (Heil et al., Citation2016).
DTC-trained clinical pharmacists reported prescribing errors constituted 44.9% of all reported errors. Most of the medication errors that occurred in critically ill patients happened during prescribing (Merino et al., Citation2013). Non-antimicrobial medications involved in medication errors included remdesivir, insulin, and anticoagulants (heparin enoxaparin, and fondaparinux) which were among the top medications implicated in errors or reported to need careful prescribing in previous studies (Patil et al., Citation2022; USP Drug Safety Review: Top Citation10 Drugs Involved in Medication Errors, Citation2023; Wittich et al., Citation2014). In addition, most patients with COVID-19 treated in ICU received antiviral therapy (10). Remdesivir use in patients with an estimated glomerular filtration rate of less than 30 mL per minute may increase the potential for hepatic and renal toxicity (Bhimraj Adarsh & Falck-Yetter, Citation2023). Its use is not recommended in severe liver and kidney diseases (Kale et al., Citation2023).
The most common prescribing errors encompassed the wrong medication. DTC-trained clinical pharmacists stopped these inappropriate medications, in line with a previous study (Martin et al., Citation2018). In a previous study conducted in a nephrology ward in Iran, the majority of the clinical pharmacists’ interventions aimed to correct a wrong medication (Vessal, Citation2010). Overlooking untreated indications also was reported, in line with a previous study (Klopotowska et al., Citation2010).
The prescriptions were written manually, which might contribute to a higher incidence of medication errors versus using electronic prescribing (Riaz et al., Citation2014; Volpe et al., Citation2016). Medication errors in the ICU were higher, in line with a previous study (Suclupe et al., Citation2020). Most of the patients in this work were elderly. Moreover, we found statistically significant differences in clinical outcomes among patients treated outside of the ICU versus those treated inside the ICU. Patients who require ICU care are more likely to be older and have underlying comorbidities, including hypertension, diabetes, cardiovascular disease, and cerebrovascular disease, compared with non-ICU patients (Tang & Wang, Citation2020; Wang et al., Citation2020); which makes them more vulnerable to medication errors (Fick & Semla, Citation2012).
The frequent empirical prescribing of broad-spectrum antibiotics in the ICU settings in Egypt contributes to antimicrobial resistance as reported by a previous study in which nearly half of critical care patients received ≥3 antibiotics (Elsorady et al., Citation2022). The hospital in which this study was performed represents a setting of increased need for stewardship because small, nonteaching hospitals have a high rate of antibiotic use (Baggs et al., Citation2016). Most patients with COVID-19 were treated with empirical broad-spectrum antibiotics because the laboratory diagnosis of COVID-19 takes time, and it could be difficult to distinguish the disease from other bacterial and viral pneumonia (Guan et al., Citation2020; Huang et al., Citation2020; Wang et al., Citation2020).
Antibiotics with the highest number of pharmacist-led interventions included linezolid, ceftriaxone, cefepime, and levofloxacin. Linezolid, levofloxacin, and ceftriaxone were among the most prescribed antibiotics in Egypt during the pandemic (Elsayed et al., Citation2021). The World Health Organization (WHO) listed linezolid as a reserve antibiotic (used as a last resort) (AWaRe Classification, Citationn.d.). It should be reserved for targeting multi-drug resistant gram-positive bacteria, including MRSA, and should not be used as the first line in the absence of contraindication to vancomycin (Elsorady et al., Citation2022; Matrat et al., Citation2020). It was among the medications that pharmacists intervened upon during a previous study (Al Meslamani et al., Citation2021). Clinical pharmacists recommended stopping linezolid because of the absence of evidence of Methicillin-resistant Staphylococcus aureus (MRSA) or Vancomycin-resistant Staphylococcus aureus (VRSA) infection, which was confirmed subsequently by cultures (; cases 2,3, and 4). Prolonged linezolid therapy is associated with resistance and it is recommended to use high-dose, low-resistance potential antibiotics for the shortest duration to achieve clinical elimination of the infection (Cunha & Opal, Citation2018).
Ceftriaxone, cefepime, and levofloxacin were listed by WHO as a watch (as they have a high potential to develop antimicrobial resistance) antibiotics (AWaRe Classification, Citationn.d.). This finding correlates with previous studies performed in Egyptian critical care units revealing that cephalosporins were among the most commonly consumed antibiotics (Elsorady et al., Citation2022; Shawki et al., Citation2022). In a previous study performed during COVID-19 in Egypt, some of the surveyed community pharmacists reported dispensing a non-appropriate dose of ceftriaxone (Elsayed et al., Citation2021). Levofloxacin and ceftriaxone wide and irrational use before and during the pandemic in both the inpatient and the outpatient settings could have contributed to the increased antimicrobial resistance (King et al., Citation2019; Medic et al., Citation2023). Physicians accepted all of the DTC-trained clinical pharmacists-led recommendations, in line with a previous study (Malani et al., Citation2013).
Clinical pharmacists’ interventions in the current study included cessation of medications. Deprescribing is a complex decision (Balsom et al., Citation2020; Martin et al., Citation2018), that is rarely discouraged by healthcare professionals (Martin et al., Citation2018; Tannenbaum et al., Citation2014). Physicians may have poor knowledge of medications’ pharmacodynamics and pharmacokinetic properties and poor adherence to the latest therapeutic guidelines (Beshyah et al., Citation2017; Solà et al., Citation2014). Pharmacists increased prescribers’ perceptions of best practices which increases doctors’ motivation, enables them to increase the standard of their prescribing practice, and improves their capabilities (McLellan et al., Citation2016). The clinical pharmacists’ discussions with physicians can significantly reduce medication error occurrence (Shaker et al., Citation2023).
We recommend that the government and policymakers in LMICs should help healthcare providers optimise the healthcare provision and promote rational drug use by enabling the establishment of DTC due to its vital role in this aspect, especially during disasters and pandemics (Alefan et al., Citation2019; Moustafa & Kassem, Citation2023).
5. Conclusions
Drug and Therapeutics Committee (DTC) implementation resulted in significant improvement in clinical outcomes and direct medication cost savings via reducing irrational medication use, especially in antibiotic use which can decrease antibiotic resistance. DTC-trained clinical pharmacists’ team successfully ensured the safe dispensing and the optimum prescribing of medications to patients which resulted in an improvement of the vital signs and laboratory findings of the critical care patients, in addition to reducing the unnecessary medication costs. This study adds new value to clinical pharmacists’ proactive capabilities in providing high-quality care during disasters in low-resource settings in developing countries. This DTC service is of great importance to patients in critical care who are at greater risk of medication errors and antibiotic misuse. Implementing targeted education in specific clinical areas, such as infectious diseases should be promoted to enhance pharmacists’ clinical skills for achieving better patient care, especially during disasters.
6. Limitations
This study has several limitations. First, the study hospital was a single-center general hospital in a developing country during the COVID-19 pandemic in Behera, Egypt; therefore, the result may have limited generalizability due to its unique set of resources and geographic setting. Second, this study is a prospective interventional pre–post study. Therefore, there is a need for randomised controlled trials to confirm our findings. The main strength of this work is that we assessed two stages of medication use, prescribing, and dispensing, whereas previous research papers have focused mostly on one stage only.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Al-Hashar, A., Al-Zakwani, I., Eriksson, T., Sarakbi, A., Al-Zadjali, B., Al Mubaihsi, S., & Al Za’abi, M. (2018). Impact of medication reconciliation and review and counselling, on adverse drug events and healthcare resource use. International Journal of Clinical Pharmacy, 40(5), 1154–1164. https://doi.org/10.1007/s11096-018-0650-8
- Al Aqeel, S., Abanmy, N., AlShaya, H., & Almeshari, A. (2018). Interventions for improving pharmacist-led patient counselling in the community setting: A systematic review. Systematic Reviews, 7(1), 71. https://doi.org/10.1186/s13643-018-0727-4
- Alefan, Q., Alshareef, S., & Al-Shatnawi, S. (2019). Drug and therapeutics committees in Jordanian hospitals: A nation-wide survey of organization, activities, and drug selection procedures. Pharmacy Practice, 17(4), 1590. https://doi.org/10.18549/PharmPract.2019.4.1590
- Alenzi, K. A., Al-Malky, H. S., Altebainawi, A. F., Abushomi, H. Q., Alatawi, F. O., Atwadi, M. H., Khobrani, M. A., Almazrou, D. A., Alrubeh, N., Alsoliabi, Z. A., Kardam, A. M., Alghamdi, S. A., Alasiri, A., Albalwi, M. H., & Alshammari, T. M. (2022). Health economic burden of COVID-19 in Saudi Arabia. Frontiers in Public Health, 10, 927494. https://doi.org/10.3389/fpubh.2022.927494
- Almaleh, R., Helmy, Y., Farhat, E., Hasan, H., & Abdelhafez, A. (2017). Assessment of health literacy among outpatient clinics attendees at Ain Shams University Hospitals, Egypt: A cross-sectional study. Public Health, 151, 137–145. https://doi.org/10.1016/j.puhe.2017.06.024
- Al Meslamani, A. Z. (2023a). Medication errors during a pandemic: What have we learnt? Expert Opinion on Drug Safety, 22(2), 115–118. https://doi.org/10.1080/14740338.2023.2181954
- Al Meslamani, A. Z. (2023b). Underreporting of adverse drug events: A look into the extent, causes, and potential solutions. Expert Opinion on Drug Safety, 22(5), 351–354. https://doi.org/10.1080/14740338.2023.2224558
- Al Meslamani, A. Z., Kassem, A. B., El-Bassiouny, N. A., & Ibrahim, O. M. (2021). An emergency plan for management of COVID-19 patients in rural areas. International Journal of Clinical Practice, 75(10), https://doi.org/10.1111/ijcp.14563
- Anwar, W. A., Mostafa, N. S., Hakim, S. A., Sos, D. G., Abozaid, D. A., & Osborne, R. H. (2020). Health literacy strengths and limitations among rural fishing communities in Egypt using the Health Literacy Questionnaire (HLQ). PLoS One, 15(7), e0235550. https://doi.org/10.1371/journal.pone.0235550
- Arain, S., Thalapparambath, R., & Al Ghamdi, F. H. (2021). COVID-19 pandemic: Response plan by the Johns Hopkins Aramco Healthcare inpatient pharmacy department. Research in Social & Administrative Pharmacy: RSAP, 17(1), 2009–2011. https://doi.org/10.1016/j.sapharm.2020.05.016
- Aronson, J. K. (2009). Medication errors: Definitions and classification. British Journal of Clinical Pharmacology, 67(6), 599–604. https://doi.org/10.1111/j.1365-2125.2009.03415.x
- Arredondo, E., Udeani, G., Horseman, M., Hintze, T. D., & Surani, S. (2021). Role of clinical pharmacists in intensive care units. Cureus, 13(9), e17929. https://doi.org/10.7759/cureus.17929
- AWaRe classification. (n.d.). Retrieved December 10, 2023, from https://www.who.int/publications-detail-redirect/2021-aware-classification
- Baggs, J., Fridkin, S. K., Pollack, L. A., Srinivasan, A., & Jernigan, J. A. (2016). Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Internal Medicine, 176(11), 1639–1648. https://doi.org/10.1001/jamainternmed.2016.5651
- Balsom, C., Pittman, N., King, R., & Kelly, D. (2020). Impact of a pharmacist-administered deprescribing intervention on nursing home residents: A randomized controlled trial. International Journal of Clinical Pharmacy, 42(4), 1153–1167. https://doi.org/10.1007/s11096-020-01073-6
- Barlam, T. F., Cosgrove, S. E., Abbo, L. M., MacDougall, C., Schuetz, A. N., Septimus, E. J., Srinivasan, A., Dellit, T. H., Falck-Ytter, Y. T., Fishman, N. O., Hamilton, C. W., Jenkins, T. C., Lipsett, P. A., Malani, P. N., May, L. S., Moran, G. J., Neuhauser, M. M., Newland, J. G., Ohl, C. A., … Trivedi, K. K. (2016). Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the society for healthcare epidemiology of America. Clinical Infectious Diseases, 62(10), e51–e77. https://doi.org/10.1093/cid/ciw118
- Barlow, B. T., Roberts, R. J., Newman, K., Harrison, S. K., & Sin, J. H. (2022). Economic evaluation of a pharmacist-led 5-day therapeutic hold of IV levothyroxine at an academic medical center. Hospital Pharmacy, 57(1), 20–25. https://doi.org/10.1177/0018578720970457
- Beardsley, J. R., Williamson, J. C., Johnson, J. W., Luther, V. P., Wrenn, R. H., & Ohl, C. C. (2012). Show me the money: Long-term financial impact of an antimicrobial stewardship program. Infection Control & Hospital Epidemiology, 33(4), 398–400. https://doi.org/10.1086/664922
- Beshyah, S., Farooqi, M., Farghaly, M., Abusnana, S., Al Kaabi, J., Benbarka, M., & The MENA Endocrine Research Group. (2017). Management of diabetes during Ramadan fasting: A comprehensive survey of physicians’ knowledge, attitudes, and practices. Ibnosina Journal of Medicine and Biomedical Sciences, 09(02), 28–36. https://doi.org/10.4103/1947-489X.210107
- Bhimraj, A., & Falck-Yetter, Y. (2023). Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
- Bronkhorst, E., Gous, A. G. S., & Schellack, N. (2020). Practice guidelines for clinical pharmacists in middle to low income countries. Frontiers in Pharmacology, 11), https://www.frontiersin.org/articles/10.3389fphar.2020.00978.
- Cameron, G., Chandra, R. N., Ivey, M. F., Khatri, C. S., Nemire, R. E., Quinn, C. J., & Subramaniam, V. (2022). ASHP statement on the pharmacist’s role in public health. American Journal of Health-System Pharmacy, 79(5), 388–399. https://doi.org/10.1093/ajhp/zxab338
- Coralic, Z., Kanzaria, H. K., Bero, L., & Stein, J. (2014). Staff perceptions of an on-site clinical pharmacist program in an academic emergency department after one year. The Western Journal of Emergency Medicine, 15(2), 205–210. https://doi.org/10.5811/westjem.2013.11.18069
- Cunha, C. B. (2018). Antimicrobial stewardship programs. Medical Clinics of North America, 102(5), 797–803. https://doi.org/10.1016/j.mcna.2018.04.003
- Cunha, C. B., & Opal, S. M. (2018). Antibiotic stewardship. Medical Clinics of North America, 102(5), 831–843. https://doi.org/10.1016/j.mcna.2018.04.006
- Cypes, I. N., Prohaska, E. S., & Melton, B. L. (2021). Pharmacist impact on medication dosing and billable coding accuracy in outpatients with chronic kidney disease. Journal of the American Pharmacists Association: JAPhA, 61(2), e153–e158. https://doi.org/10.1016/j.japh.2020.10.009
- Deawjaroen, K., Sillabutra, J., Poolsup, N., Stewart, D., & Suksomboon, N. (2022). Characteristics of drug-related problems and pharmacist’s interventions in hospitalized patients in Thailand: A prospective observational study. Scientific Reports, 12(1), Article 1. https://doi.org/10.1038/s41598-022-21515-7
- Di Simone, E., Tartaglini, D., Fiorini, S., Petriglieri, S., Plocco, C., & Di Muzio, M. (2016). Medication errors in intensive care units: Nurses’ training needs. Emergency Nurse: The Journal of the RCN Accident and Emergency Nursing Association, 24(4), 24–29. https://doi.org/10.7748/en.2016.11577
- Eldin, M. K., Mohyeldin, M., Zaytoun, G. A., Elmaaty, M. A., Hamza, M., Fikry, S., Kamal, S., Khalifa, A. E., & Abouelnaga, S. (2022). Factors hindering the implementation of clinical pharmacy practice in Egyptian hospitals. Pharmacy Practice, 20(1), 2607. https://doi.org/10.18549/PharmPract.2022.1.2607
- Elhadi, M., Msherghi, A., Alkeelani, M., Alsuyihili, A., Khaled, A., Buzreg, A., Boughididah, T., Abukhashem, M., Alhashimi, A., Khel, S., Gaffaz, R., Ben Saleim, N., Bahroun, S., Elharb, A., Eisay, M., Alnafati, N., Almiqlash, B., Biala, M., & Alghanai, E. (2020). Concerns for low-resource countries, with under-prepared intensive care units, facing the COVID-19 pandemic. Infection, Disease & Health, 25(4), 227–232. https://doi.org/10.1016/j.idh.2020.05.008
- Elsayed, A. A., Darwish, S. F., Zewail, M. B., Mohammed, M., Saeed, H., & Rabea, H. (2021). Antibiotic misuse and compliance with infection control measures during COVID-19 pandemic in community pharmacies in Egypt. International Journal of Clinical Practice, 75(6), e14081. https://doi.org/10.1111/ijcp.14081
- Elsorady, K. E., Hassan, R. A., Abdelhamid, D. H., & Abd El-Mohsen, M. (2022). Antibiotics use and its association with multi-drug resistance in a tertiary care geriatrics hospital in Egypt. Journal of Infection in Developing Countries, 16(12), 1860–1869. https://doi.org/10.3855/jidc.17257
- Erstad, B. L. (2021). Caring for the COVID patient: A clinical pharmacist’s perspective. The Annals of Pharmacotherapy, 55(3), 413–414. https://doi.org/10.1177/1060028020954224
- Essam, N., Khafagy, M. A., & Alemam, D. S. (2022). Health literacy of pregnant women attending antenatal care clinics in Mansoura district, Egypt. The Journal of the Egyptian Public Health Association, 97(1), 24. https://doi.org/10.1186/s42506-022-00119-z
- Fair, L., Burns, C., & Lindsley, J. (2023). Improving medication safety in an ICU. The American Journal of Nursing, 123(7), 39–45. https://doi.org/10.1097/01.NAJ.0000944924.15137.c8
- Fick, D. M., & Semla, T. P. (2012). 2012 American Geriatrics Society beers criteria: New year, new criteria, new perspective. Journal of the American Geriatrics Society, 60(4), 614–615. https://doi.org/10.1111/j.1532-5415.2012.03922.x
- Founou, R. C., Blocker, A. J., Noubom, M., Tsayem, C., Choukem, S. P., Dongen, M. V., & Founou, L. L. (2021). The COVID-19 pandemic: A threat to antimicrobial resistance containment. Future Science OA, 7(8), FSO736. https://doi.org/10.2144/fsoa-2021-0012
- Garg, S. K. (2021). Antibiotic misuse during COVID-19 pandemic: A recipe for disaster. Indian Journal of Critical Care Medicine: Peer-Reviewed, Official Publication of Indian Society of Critical Care Medicine, 25(6), 617–619. https://doi.org/10.5005/jp-journals-10071-23862
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021. (2021). https://www.who.int/publications-detail-redirect/9789240027336.
- Guan, W., Ni, Z., Hu, Y., Liang, W., Ou, C., He, J., Liu, L., Shan, H., Lei, C., Hui, D. S. C., Du, B., Li, L., Zeng, G., Yuen, K.-Y., Chen, R., Tang, C., Wang, T., Chen, P., Xiang, J., … Zhong, N. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. https://doi.org/10.1056/NEJMoa2002032
- Guignard, B., Bonnabry, P., Perrier, A., Dayer, P., Desmeules, J., & Samer, C. F. (2015). Drug-related problems identification in general internal medicine: The impact and role of the clinical pharmacist and pharmacologist. European Journal of Internal Medicine, 26(6), 399–406. https://doi.org/10.1016/j.ejim.2015.05.012
- Gwatkin, D. R. (2017). Trends in health inequalities in developing countries. The Lancet Global Health, 5(4), e371–e372. https://doi.org/10.1016/S2214-109X(17)30080-3
- Heil, E. L., Kuti, J. L., Bearden, D. T., & Gallagher, J. C. (2016). The essential role of pharmacists in antimicrobial stewardship. Infection Control and Hospital Epidemiology, 37(7), 753–754. https://doi.org/10.1017/ice.2016.82
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z., Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, People’s Republic of China. Lancet (London, England), 395(10223), 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
- Hyland, S. J., Kramer, B. J., Fada, R. A., & Lucki, M. M. (2020). Clinical pharmacist service associated with improved outcomes and cost savings in total joint arthroplasty. The Journal of Arthroplasty, 35(9), 2307–2317.e1. https://doi.org/10.1016/j.arth.2020.04.022
- Jamshed, S., Padzil, F., Shamsudin, S. H., Bux, S. H., Jamaluddin, A. A., Bhagavathula, A. S., Azhar, S., & Hassali, M. A. (2018). Antibiotic stewardship in community pharmacies: A scoping review. Pharmacy (Basel, Switzerland), 6(3), E92. https://doi.org/10.3390/pharmacy6030092
- Johansen, E. T., Haustreis, S. M., Mowinckel, A. S., & Ytrebø, L. M. (2016). Effects of implementing a clinical pharmacist service in a mixed Norwegian ICU. European Journal of Hospital Pharmacy: Science and Practice, 23(4), 197–202. https://doi.org/10.1136/ejhpharm-2015-000751
- Kabba, J. A., Koroma, M. M., James, P. B., Kitchen, C., Xu, S., Chang, J., Saeed, A., Malik, U. R., Jiang, M., & Fang, Y. (2023). Pilot drug and therapeutic committee programme in Sierra Leone: A descriptive mixed method study. British Journal of Clinical Pharmacology, 89(1), 20–33. https://doi.org/10.1111/bcp.14716
- Kajal, K., Naik, B. N., Singh, A., Soni, S. L., Hazarika, A., Saini, K., Jaswal, S., Meena, S. C., Pandey, N., & Puri, G. D. (2020). Preparing intensive care unit in resource-constraint setting amid COVID-19 pandemic: Our experience and review. Anesthesia, Essays and Researches, 14(3), 366–369. https://doi.org/10.4103/aer.AER_86_20
- Kale, A., Shelke, V., Dagar, N., Anders, H.-J., & Gaikwad, A. B. (2023). How to use COVID-19 antiviral drugs in patients with chronic kidney disease. Frontiers in Pharmacology, 14, 1053814. https://doi.org/10.3389/fphar.2023.1053814
- Kassem, A. B., Ghoneim, A. I., Nounou, M. I., & El-Bassiouny, N. A. (2021). Community pharmacists’ needs, education, and readiness in facing COVID-19. Actions & Recommendations in Egypt. International Journal of Clinical Practice, 75(11)), https://doi.org/10.1111/ijcp.14762
- Khalili, H., Karimzadeh, I., Mirzabeigi, P., & Dashti-Khavidaki, S. (2013). Evaluation of clinical pharmacist’s interventions in an infectious diseases ward and impact on patient’s direct medication cost. European Journal of Internal Medicine, 24(3), 227–233. https://doi.org/10.1016/j.ejim.2012.11.014
- Kharaba, Z., Moutraji, S. A., Al Khawaldeh, R. A., Alfoteih, Y., & Al Meslamani, A. Z. (2022). What has changed in the pharmaceutical care after COVID-19: Pharmacists’ perspective. Pharmacy Practice, 20(2), 01–08. https://doi.org/10.18549/PharmPract.2022.2.2656
- King, L. M., Talley, P., Kainer, M. A., Evans, C. D., Adre, C., Hicks, L. A., & Fleming-Dutra, K. E. (2019). Inappropriate ceftriaxone use in outpatient acute respiratory infection management. Infection Control & Hospital Epidemiology, 40(4), 487–490. https://doi.org/10.1017/ice.2019.21
- Klein, E. Y., Van Boeckel, T. P., Martinez, E. M., Pant, S., Gandra, S., Levin, S. A., Goossens, H., & Laxminarayan, R. (2018). Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proceedings of the National Academy of Sciences, 115(15), https://doi.org/10.1073/pnas.1717295115
- Klopotowska, J. E., Kuiper, R., van Kan, H. J., de Pont, A.-C., Dijkgraaf, M. G., Lie-A-Huen, L., Vroom, M. B., & Smorenburg, S. M. (2010). On-ward participation of a hospital pharmacist in a Dutch intensive care unit reduces prescribing errors and related patient harm: An intervention study. Critical Care (London, England), 14(5), R174. https://doi.org/10.1186/cc9278
- Lambert, M., Smit, C. C. H., De Vos, S., Benko, R., Llor, C., Paget, W. J., Briant, K., Pont, L., Van Dijk, L., & Taxis, K. (2022). A systematic literature review and meta-analysis of community pharmacist-led interventions to optimise the use of antibiotics. British Journal of Clinical Pharmacology, 88(6), 2617–2641. https://doi.org/10.1111/bcp.15254
- Lee, M., Badowski, M. E., Acquisto, N. M., Covey, D. F., Fox, B. D., Gaffney, S. M., Haines, S. T., Hilaire, M. L., Raymond, A., Salvo, M. C., & Turner, K. (2017). ACCP template for evaluating a clinical pharmacist. Pharmacotherapy, 37(5), e21–e29. https://doi.org/10.1002/phar.1927
- Leguelinel-Blache, G., Nguyen, T.-L., Louart, B., Poujol, H., Lavigne, J.-P., Roberts, J. A., Muller, L., Kinowski, J.-M., Roger, C., & Lefrant, J.-Y. (2018). Impact of quality bundle enforcement by a critical care pharmacist on patient outcome and costs. Critical Care Medicine, 46(2), 199–207. https://doi.org/10.1097/CCM.0000000000002827
- Li, H., Zheng, S., Liu, F., Liu, W., & Zhao, R. (2021). Fighting against COVID-19: Innovative strategies for clinical pharmacists. Research in Social and Administrative Pharmacy, 17(1), 1813–1818. https://doi.org/10.1016/j.sapharm.2020.04.003
- Lim, C. J., Kong, D. C., & Stuart, R. L. (2014). Reducing inappropriate antibiotic prescribing in the residential care setting: Current perspectives. Clinical Interventions in Aging, 9, 165–177. https://doi.org/10.2147/CIA.S46058
- Liou, W.-S., Huang, S.-M., Lee, W.-H., Chang, Y.-L., & Wu, M.-F. (2021). The effects of a pharmacist-led medication review in a nursing home: A randomized controlled trial. Medicine, 100(48), e28023. https://doi.org/10.1097/MD.0000000000028023
- Lui, B., Zheng, M., White, R. S., & Hoyler, M. (2021). Economic burden of lives lost due to COVID-19 in New York state. Journal of Comparative Effectiveness Research, 10(11), 893–897. https://doi.org/10.2217/cer-2021-0086
- MacLaren, R., & Bond, C. A. (2009). Effects of pharmacist participation in intensive care units on clinical and economic outcomes of critically ill patients with thromboembolic or infarction-related events. Pharmacotherapy, 29(7), 761–768. https://doi.org/10.1592/phco.29.7.761
- Maharaj, S., Brahim, A., Brown, H., Budraj, D., Caesar, V., Calder, A., Carr, D., Castillo, D., Cedeno, K., & Janodia, M. D. (2020). Identifying dispensing errors in pharmacies in a medical science school in Trinidad and Tobago. Journal of Pharmaceutical Policy and Practice, 13(1), 67. https://doi.org/10.1186/s40545-020-00263-x
- Malani, A. N., Richards, P. G., Kapila, S., Otto, M. H., Czerwinski, J., & Singal, B. (2013). Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. American Journal of Infection Control, 41(2), 145–148. https://doi.org/10.1016/j.ajic.2012.02.021
- Martin, P., Tamblyn, R., Benedetti, A., Ahmed, S., & Tannenbaum, C. (2018). Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: The D-PRESCRIBE randomized clinical trial. JAMA, 320(18), 1889–1898. https://doi.org/10.1001/jama.2018.16131
- Matrat, L., Plaisant, F., Barreto, C., Claris, O., & Butin, M. (2020). Increasing use of linezolid in a tertiary NICU during a 10-year period: Reasons and concerns for the future. Antimicrobial Resistance & Infection Control, 9(1), 156. https://doi.org/10.1186/s13756-020-00818-2
- McLellan, L., Dornan, T., Newton, P., Williams, S. D., Lewis, P., Steinke, D., & Tully, M. P. (2016). Pharmacist-led feedback workshops increase appropriate prescribing of antimicrobials. The Journal of Antimicrobial Chemotherapy, 71(5), 1415–1425. https://doi.org/10.1093/jac/dkv482
- Medic, D., Bozic Cvijan, B., & Bajcetic, M. (2023). Impact of antibiotic consumption on antimicrobial resistance to invasive hospital pathogens. Antibiotics (Basel, Switzerland), 12(2), 259. https://doi.org/10.3390/antibiotics12020259
- Merino, P., Martín, M. C., Alonso, A., Gutiérrez, I., Álvarez, J., & Becerril, F. (2013). Medication errors in Spanish intensive care units. Medicina Intensiva (English Edition), 37(6), 391–399. https://doi.org/10.1016/j.medine.2012.11.004
- Moody, J., Cosgrove, S. E., Olmsted, R., Septimus, E., Aureden, K., Oriola, S., Patel, G. W., & Trivedi, K. K. (2012). Antimicrobial stewardship: A collaborative partnership between infection preventionists and health care epidemiologists. American Journal of Infection Control, 40(2), 94–95. https://doi.org/10.1016/j.ajic.2012.01.001
- Morgan, S. R., Acquisto, N. M., Coralic, Z., Basalyga, V., Campbell, M., Kelly, J. J., Langkiet, K., Pearson, C., Sokn, E., & Phelan, M. (2018). Clinical pharmacy services in the emergency department. The American Journal of Emergency Medicine, 36(10), 1727–1732. https://doi.org/10.1016/j.ajem.2018.01.056
- Moustafa, H. A. M., & Kassem, A. B. (2023). COVID-19-related health literacy and preparedness to what may come: A cross-sectional study. Beni-Suef University Journal of Basic and Applied Sciences, 12(1), 114. https://doi.org/10.1186/s43088-023-00452-y
- Nguyen, H.-T., Pham, H.-T., Vo, D.-K., Nguyen, T.-D., van den Heuvel, E. R., Haaijer-Ruskamp, F. M., & Taxis, K. (2014). The effect of a clinical pharmacist-led training programme on intravenous medication errors: A controlled before and after study. BMJ Quality & Safety, 23(4), 319–324. https://doi.org/10.1136/bmjqs-2013-002357
- Niznik, J. D., He, H., & Kane-Gill, S. L. (2018). Impact of clinical pharmacist services delivered via telemedicine in the outpatient or ambulatory care setting: A systematic review. Research in Social & Administrative Pharmacy: RSAP, 14(8), 707–717. https://doi.org/10.1016/j.sapharm.2017.10.011
- Nowak, M. A., Nelson, R. E., Breidenbach, J. L., Thompson, P. A., & Carson, P. J. (2012). Clinical and economic outcomes of a prospective antimicrobial stewardship program. American Journal of Health-System Pharmacy: AJHP: Official Journal of the American Society of Health-System Pharmacists, 69(17), 1500–1508. https://doi.org/10.2146/ajhp110603
- Patil, S., Mugalikar, A., Patil, D., & Gondhali, G. (2022). Remdesivir use and controversies in COVID-19 pneumonia: Myths and reality!. Journal of Translational Critical Care Medicine, 4(1), 15. https://doi.org/10.4103/JTCCM-D-22-00010
- Peramo-Álvarez, F. P., López-Zúñiga, MÁ, & López-Ruz, MÁ. (2021). Medical sequels of COVID-19. Medicina Clinica, 157(8), 388–394. https://doi.org/10.1016/j.medcli.2021.04.023
- Perez, M., Masse, M., Deldicque, A., Beuscart, J. B., De Groote, P., Desbordes, J., Fry, S., Musy, E., Odou, P., Puisieux, F., Lambert, M., Scherpereel, A., & Décaudin, B. (2022). Analysis of clinical pharmacist interventions in the COVID-19 units of a French university hospital. European Journal of Hospital Pharmacy: Science and Practice, 29(e1), e30–e35. https://doi.org/10.1136/ejhpharm-2020-002542
- Presley, B., Groot, W., & Pavlova, M. (2019). Pharmacy-led interventions to improve medication adherence among adults with diabetes: A systematic review and meta-analysis. Research in Social and Administrative Pharmacy, 15(9), 1057–1067. https://doi.org/10.1016/j.sapharm.2018.09.021
- Riaz, M. K., Hashmi, F. K., Bukhari, N. I., Riaz, M., & Hussain, K. (2014). Occurrence of medication errors and comparison of manual and computerized prescription systems in public sector hospitals in Lahore, Pakistan. PLoS One, 9(8), e106080. https://doi.org/10.1371/journal.pone.0106080
- Richardson, S. R., Neuner, E. A., Athans, V., Srinivas, P., Wesolowski, J., Gordon, S. M., & Fraser, T. G. (2019). Evaluation of an electronic antimicrobial time-out on antimicrobial utilization at a large health system. Infection Control & Hospital Epidemiology, 40(7), 807–809. https://doi.org/10.1017/ice.2019.105
- [email protected]. (2014, July 18). About Medication Errors [Text]. NCC MERP. https://www.nccmerp.org/about-medication-errors
- Said, A., Hussain, N., & Abdelaty, L. N. (2020). Physicians’ and pharmacists’ perception and practice of hospital pharmacist professional role in Egypt. The International Journal of Pharmacy Practice, 28(5), 491–497. https://doi.org/10.1111/ijpp.12638
- Santhosh, L., Lyons, P. G., Rojas, J. C., Ciesielski, T. M., Beach, S., Farnan, J. M., & Arora, V. (2019). Characterising ICU-ward handoffs at three academic medical centres: Process and perceptions. BMJ Quality & Safety, 28(8), 627–634. https://doi.org/10.1136/bmjqs-2018-008328
- Shaker, H. O., Sabry, A. A. F., Salah, A., Ragab, G. M., Sedik, N. A., Ali, Z., Magdy, D., & Alkafafy, A. M. (2023). The impact of clinical pharmacists’ medication reconciliation upon patients’ admission to reduce medication discrepancies in the emergency department: A prospective quasi-interventional study. International Journal of Emergency Medicine, 16(1), 89. https://doi.org/10.1186/s12245-023-00568-z
- Shawki, M. A., Sabri, N. A., Ibrahim, D. M., Samady, M. M., & Hamza, M. S. (2022). The impact of clinical pharmacist implemented education on the incidence of prescribing errors in COVID-19 patients. Saudi Pharmaceutical Journal: SPJ: The Official Publication of the Saudi Pharmaceutical Society, 30(8), 1101–1106. https://doi.org/10.1016/j.jsps.2022.06.007
- Shendy, E. M., Elberry, A. A., Hamed Mohamed, L., & Boshra, M. S. (2022). Perceptions and attitudes of ICU physicians toward antibiotics prescribing and resistance: A cross-sectional study. PLoS One, 17(9), e0273673. https://doi.org/10.1371/journal.pone.0273673
- Shrestha, S., Danekhu, K., Thapa, S., Khanal, S., & Shankar, P. R. (2019). The unmet need for pharmacists as important members of the emergency department team in resource-limited settings: A case of Nepal. The Journal of Pharmacy Technology: Jpt: Official Publication of the Association of Pharmacy Technicians, 35(5), 219–224. https://doi.org/10.1177/8755122519850118
- Smith Jervelund, S., & Eikemo, T. A. (2021). The double burden of COVID-19. Scandinavian Journal of Public Health, 49(1), 1–4. https://doi.org/10.1177/1403494820984702
- Solà, I., Carrasco, J. M., Díaz Del Campo, P., Gracia, J., Orrego, C., Martínez, F., Kotzeva, A., Guillamón, I., Calderón, E., De Gaminde, I., Louro, A., Rotaeche, R., Salcedo, F., Velázquez, P., & Alonso-Coello, P. (2014). Attitudes and perceptions about clinical guidelines: A qualitative study with Spanish physicians. PLoS One, 9(2), e86065. https://doi.org/10.1371/journal.pone.0086065
- Stasiak, P., Afilalo, M., Castelino, T., Xue, X., Colacone, A., Soucy, N., & Dankoff, J. (2014). Detection and correction of prescription errors by an emergency department pharmacy service. Canadian Journal of Emergency Medical Care, 16(3), 193–206. https://doi.org/10.2310/8000.2013.130975
- Suclupe, S., Martinez-Zapata, M. J., Mancebo, J., Font-Vaquer, A., Castillo-Masa, A. M., Viñolas, I., Morán, I., & Robleda, G. (2020). Medication errors in prescription and administration in critically ill patients. Journal of Advanced Nursing, 76(5), 1192–1200. https://doi.org/10.1111/jan.14322
- Tang, L. Y., & Wang, J. (2020). Anesthesia and COVID-19: What we should know and what we should do. Seminars in Cardiothoracic and Vascular Anesthesia, 24(2), 127–137. https://doi.org/10.1177/1089253220921590
- Tannenbaum, C., Martin, P., Tamblyn, R., Benedetti, A., & Ahmed, S. (2014). Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: The EMPOWER cluster randomized trial. JAMA Internal Medicine, 174(6), 890–898. https://doi.org/10.1001/jamainternmed.2014.949
- Thom, K. A., Tamma, P. D., Harris, A. D., Dzintars, K., Morgan, D. J., Li, S., Pineles, L., Srinivasan, A., Avdic, E., & Cosgrove, S. E. (2019). Impact of a prescriber-driven antibiotic time-out on antibiotic use in hospitalized patients. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 68(9), 1581–1584. https://doi.org/10.1093/cid/ciy852
- USP Drug Safety Review: Top 10 drugs involved in medication errors. (2023). https://www.drugtopics.com/view/usp-drug-safety-review-top-10-drugs-involved-medication-errors.
- Van der Linden, L., Hias, J., Walgraeve, K., Flamaing, J., Tournoy, J., & Spriet, I. (2020). Clinical pharmacy services in older inpatients: An evidence-based review. Drugs & Aging, 37(3), 161–174. https://doi.org/10.1007/s40266-019-00733-1
- Van Schooneveld, T. C., Rupp, M. E., Cavaleiri, R. J., Lyden, E., & Rolek, K. (2020). Cluster randomized trial of an antibiotic time-out led by a team-based pharmacist. Infection Control & Hospital Epidemiology, 41(11), 1266–1271. https://doi.org/10.1017/ice.2020.347
- Vessal, G. (2010). Detection of prescription errors by a unit-based clinical pharmacist in a nephrology ward. Pharmacy World & Science: PWS, 32(1), 59–65. https://doi.org/10.1007/s11096-009-9341-9
- Visacri, M. B., Figueiredo, I. V., Lima, T., & de, M. (2021). Role of pharmacist during the COVID-19 pandemic: A scoping review. Research in Social and Administrative Pharmacy, 17(1), 1799–1806. https://doi.org/10.1016/j.sapharm.2020.07.003
- Volpe, C. R. G., Melo, E. M. M. d., Aguiar, L. B. d., Pinho, D. L. M., & Stival, M. M. (2016). Risk factors for medication errors in the electronic and manual prescription. Revista Latino-Americana De Enfermagem, 24, e2742. https://doi.org/10.1590/1518-8345.0642.2742
- Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., Wang, B., Xiang, H., Cheng, Z., Xiong, Y., Zhao, Y., Li, Y., Wang, X., & Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA, 323(11), 1061. https://doi.org/10.1001/jama.2020.1585
- Weinberger, J., Cocoros, N., & Klompas, M. (2021). Ventilator-Associated events: Epidemiology, risk factors, and prevention. Infectious Disease Clinics of North America, 35(4), 871–899. https://doi.org/10.1016/j.idc.2021.07.005
- Wittich, C. M., Burkle, C. M., & Lanier, W. L. (2014). Medication errors: An overview for clinicians. Mayo Clinic Proceedings, 89(8), 1116–1125. https://doi.org/10.1016/j.mayocp.2014.05.007