ABSTRACT
Background
Direct oral anticoagulants (DOACs) have demonstrated clinical benefits and better patient adherence over low-molecular-weight heparin (LMWH) in treating patients with cancer-associated venous thrombosis (CAT). We aimed to compare the cost-effectiveness of DOACs against LMWH in patients with CAT from the perspective of the Hong Kong healthcare system.
Methods
A Markov state-transition model was performed to estimate the incremental cost-effectiveness ratio (ICER) per quality-adjusted life years (QALYs) for DOACs and LMWH in a hypothetical cohort of 10,000 patients with CAT over a 5-year lifetime horizon. The model was primarily based on the health states of no event, recurrent venous thromboembolism, bleeding, and death. Transition probabilities, relative risks, and utilities were derived from the literature. Resource cost data were obtained from the Hong Kong Hospital Authority. Deterministic and probabilistic sensitivity analyses tested the robustness of the results.
Results
Relative to LMWH, DOACs were associated with increased QALYs (1.52 versus 1.50) at a lower medical cost of USD 2,232 versus 8,224 in five years. The cost of LMWH was the main contributor to the outcome. Out of 10,000 simulated cases, DOACs were dominant in 15.8% and cost-effective in 42.1%, at the willingness-to-pay threshold of USD 148,392 per additional QALY.
Conclusions
DOACs were associated with greater QALY improvements and lower overall costs compared to LMWH. Accounting for uncertainty, DOACs were between cost-effective and dominant in 57.9% of cases. DOACs are a cost-effective alternative to LMWH in the management of CAT in Hong Kong.
Introduction
Cancer-associated venous thrombosis (CAT) is the second leading cause of death in patients with cancer. It is associated with a high risk of mortality and morbidities, including recurrent venous thromboembolism (VTE) and bleeding (Khorana et al., Citation2007). A recent study highlighted that out of the 17,271 patients with CAT who participated in the worldwide RIETE registry between 2001 and 2020, 1,760 (10.2%) patients passed away within a 30-day follow-up period. Among these patients, 235 (13.4%) died due to fatal pulmonary embolism (PE), whereas 95 (5.4%) succumbed to fatal bleeding (Bertoletti et al., Citation2023). Compared to patients with active cancer and without VTE, patients with CAT seek medical care more frequently, leading to 2-fold higher healthcare costs in all settings and 4-fold higher drug costs, posing a significant financial burden to their families and the healthcare support system (Shah et al., Citation2018).
According to current guidelines, low-molecular-weight heparin (LMWH) and direct oral anticoagulants (DOACs) are the main anticoagulant medications used to manage CAT (Farge et al., Citation2022; Key et al., Citation2020; Lyman et al., Citation2021). As a newer class of oral anticoagulants since 2010, DOACs have been shown to have various advantages over parenteral LMWHs, including convenience, absence of injection-site reactions and infections, improved patient compliance, as well as comparable level of effectiveness and safety (McShane et al., Citation2020; Raskob et al., Citation2018). Apart from the safety and effectiveness profile, medical costs are also a critical consideration for healthcare institutions given limited budgets. Previous studies have compared DOACs and LMWH in managing CAT in various countries (de Jong et al., Citation2020; Li et al., Citation2019; Lopes et al., Citation2020; Wumaier et al., Citation2021). These studies revealed that DOACs were between dominant and cost-effective, with a wide range of incremental cost-effectiveness ratios (ICERs), ranging from 112,896 to 623,459 United States dollars (USD) per quality-adjusted life-year (QALY) gained by DOAC users over five years. This wide variability could be attributed to various locally-specific factors, including income levels, healthcare systems, patient ethnicities, and medication accessibility across different countries (Al Mukdad et al., Citation2019).
Hong Kong is globally recognised as one of the regions with top life expectancy (The World Bank, Citation2024). Its healthcare system receives approximately 19 percent of the government's recurrent expenditure annually (Hong Kong Special Administrative Region Government, Citation2024). All Hong Kong citizens are provided with healthcare services under this system, either free of charge or at a nominal fee. Thus, both clinical oncologists who prescribe anticoagulants and patients are not sensitive to the price of medications or the management of adverse drug reactions. In Hong Kong, LMWH remains the first-line treatment for most patients with CAT. However, there is currently a lack of economic evaluation studies in Hong Kong that compare the cost-effectiveness of DOACs versus LMWH in patients with CAT. This information is crucial to guide DOACs and LMWH usage from an institutional perspective, to help reduce government healthcare expenditure while ensuring that patients continue to receive optimal care. This approach enables the strategic allocation of limited healthcare resources to areas with greater clinical needs, without compromising patient outcomes.
Thus, our study aims to compare the cost-effectiveness of DOACs versus LMWHs in patients with CAT. Our results provide evidence for clinical decision-making and optimisation of medical resource allocation for patients with CAT in Hong Kong.
Materials and methods
Model overview
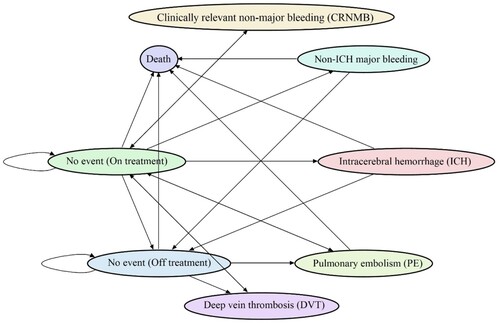
This study compared the cost-effectiveness of DOACs against LMWH in patients with CAT from the Hong Kong healthcare system perspective. A Markov state-transition model was constructed to evaluate the cost-effectiveness of DOACs versus LMWH by simulating the accumulative medical costs and quality-adjusted life years for patients with CAT (). We used a hypothetical cohort of 10,000 patients with new CAT diagnoses who were eligible for either drug. The model was based on eight transition states: ‘no event (on treatment)’, ‘no event (off treatment)’, ‘recurrent VTE’ (including ‘recurrent PE’ and ‘recurrent deep vein thrombosis [DVT] ‘), ‘major bleeding’ (MB, including ‘intracranial hemorrhage [ICH]‘ and ‘non-ICH MB’), ‘clinically relevant non-major bleeding (CRNMB)‘, and ‘death’ (including death related to PE, ICH, non-ICH MB, and other reasons such as cancer progression). All patients entered the state of ‘no event (On treatment)’ at the first cycle. They were allowed to either remain on the initial health state or transfer to other health states, as depicted in , at a monthly interval. A duration of five years was selected as an approximation for the survival period based on the nearly 40% death rate in a twelve-month randomised controlled trial (RCT) by Raskob et al. (Citation2018). A 3.5% yearly discount rate was applied for both costs and utilities (National Institute for Health and Care Excellence, Citation2022).
Figure 1. Markov transition-state model diagram. This chart displays the various health conditions that a patient can experience within the Markov transition-state model. The arrows indicate the possible changes in the patient's health status. The patients are susceptible to several risks, including recurrent deep vein thrombosis (DVT), recurrent pulmonary embolism (PE), intracranial haemorrhage (ICH), non-ICH major bleeding (MB), clinically relevant non-major bleeding (CRNMB), and death. All death states are self-absorbing.
Model assumptions
Our Markov model had several assumptions: (1) Patients could not have more than one adverse event simultaneously. (2) Patients could only continue, quit, or restart the same class of anticoagulation treatment, which means switching between LMWH and DOACs was not possible. (3) Enoxaparin, nadroparin, and tinzaparin were equally considered for the treatment of CAT in the LMWH treatment group. Apixaban, dabigatran, edoxaban, and rivaroxaban were equally considered for the treatment of CAT in the DOAC treatment group. (4) Death could occur at any time.
Model inputs
Population
The study’s hypothetical cohort of 10,000 patients with new CAT diagnoses is defined as patients with active cancer followed by an incident diagnosis of VTE. Based on the baseline characteristics of patients with CAT in Hong Kong, the mean age of this cohort was 67 years, and the mean weight was 70 kg. A new cancer diagnosis, recurrent diagnosis of cancer, metastasis, any cancer-related treatment, or palliative care in the last six months before the first VTE diagnosis were considered active cancer (Khorana et al., Citation2018). Patients in the cohort shared similar baseline characteristics and were eligible for either LMWH or DOACs as treatment options.
Transition probabilities and relative risks
For the purpose of the current study, a meta-analysis of adverse event rates of interest was performed using published data from the all existing RCTs completed prior to 2022, which compared DOACs to LMWH in patients with CAT, including Hokusai-VTE, SELECT-D, Caravaggio, and ADAM VTE trials (Agnelli et al., Citation2020; McBane et al., Citation2020; Raskob et al., Citation2018; Young et al., Citation2018). Relative risks and transition probabilities of recurrent DVT, recurrent PE, ICH, non-ICH MB, CRNMB, and death due to other reasons within the first six months, as well as relative risks and transition probabilities of fatal PE, fatal ICH, fatal non-ICH MB, and treatment discontinuation during the entire study period were derived from the meta-analysis results. Due to the higher occurrence of recurrent VTE and bleeding events within the first six months, only the Hokusai-VTE study investigated the rates for adverse events beyond this period. Therefore, the relative risks and transition probabilities of recurrent DVT, recurrent PE, ICH, non-ICH MB, CRNMB, and death due to other reasons after the first six months were directly derived from the 7–12 months results of the Hokusai-VTE study (Di Nisio et al., Citation2019; Raskob et al., Citation2018). Transition probability for each cycle was calculated using the formula TP1 = 1 − (1 − TPt)(1/t), where TP1 is the probability of event each cycle, TPt is the event probability as derived from our meta-analysis or reported in the Hokusai-VTE trial, and t is six months in this study (Gidwani & Russell, Citation2020).
Utilities
Utility values were to the best available extracted from different sources. Utilities of base case without treatment, recurrent PE, recurrent DVT, non-ICH MB, and CRNMB were derived from the CATCH trial conducted using the EQ-5D-3L questionnaire for patients with CAT (Lloyd et al., Citation2018). Utilities of base case with DOAC or LMWH treatment for patients with DVT and/or PE were obtained from technology appraisal guidance published by the National Institute for Health and Care Excellence (Citation2015). Utility of ICH was derived from a standard gamble interview for patients with acute venous thrombosis conducted in a single clinic (Hogg et al., Citation2013).
Costs
Given the perspective of the study, only the cost of direct medical resources was included. Drug and adverse event management costs were obtained from the Hospital Authority Gazette (Hospital Authority, Citation2023). Consistent with the pooled RCTs and local clinical practice, the assumed dosages for different anticoagulants were as follows: enoxaparin at 1 mg/kg administrated twice daily; nadroparin at 171 units/kg once daily; tinzaparin at 175 units/kg once daily. Apixaban was taken at 10 mg twice daily for seven days, followed by 5 mg twice daily; dabigatran was taken at 150 mg twice daily after 7.5 days of LMWH; edoxaban was taken at 60 mg once daily after initial 7.5 days of LMWH; rivaroxaban was assumed at 15 mg twice daily for 21 days, followed by 20 mg once daily (Hull & Lip, Citation2023; Leung, Citation2023; Lip & Hull, Citation2023). Costs of drugs and adverse event management were reported in USD with an average exchange rate in 2022 of 1 USD = 7.8307 Hong Kong Dollars (HKD) (Hong Kong Monetary Authority, Citation2023).
Model outputs
Base case analysis
In the base-case analysis, the model estimated the cumulative cost and QALYs for each treatment over five years. This time horizon was considered based on the life expectancy of patients with cancer and has been widely used in previous studies (Chiang et al., Citation2021; Li et al., Citation2019). The QALY was calculated by summing the utilities for follow-up cycles throughout the 5-year time horizon. The ICER was computed as the difference in cost over the difference in QALYs with DOAC relative to the LMWH treatment. A willingness-to-pay (WTP) threshold of 3-fold Gross Domestic Product (GDP, USD 49,464 per capita in 2022) was adapted to determine the cost-effectiveness of competing therapies in this study (The Hong Kong Trade Development Council (HKTDC), Citation2023).
Sensitivity analyses
A one-way deterministic sensitivity analysis with a time horizon of five years was performed for DOACs versus LMWH, where each input parameter varied within its standard deviations (SD). In cases where SD was not applicable, upper and lower bounds were assumed to vary by ±20%.
A probabilistic sensitivity analysis with a time horizon of 5 years was also conducted to simulate 10,000 iterations to generate a cost-effectiveness scatter plot and acceptance curve. Log-normal type of random distributions was used for relative risk inputs, beta distributions were used for transition probabilities and utility inputs, and gamma distributions were used for cost inputs.
To assess the short-term cost-effectiveness of DOACs compared to LMWH for CAT, a base-case analysis was re-conducted based on a 6-month time horizon as a scenario analysis. This time horizon was chosen considering that patients with CAT typically suffer from the highest risk of adverse events and death within the first six months (Raskob et al., Citation2018).
Statistical analysis
The Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS) was used to guide the methodological quality of our study (Husereau et al., Citation2022). TreeAge Pro (Healthcare Version) 2020 was used for all health economic analyses. R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for the meta-analysis. All analyses were conducted by WK and cross-checked independently by KP for quality assurance.
Results
Base case analysis
The results from the meta-analysis of four RCTs are displayed in Supplemental Table S1, and all parameters used in the model are listed in Supplemental Table S2. The results from the base-case analysis are shown in . In the 5-year time horizon, patients on DOACs were observed to experience 190 fewer recurrent PEs, 395 fewer recurrent DVTs, 25 fewer ICHs, 304 more non-ICH MBs, 176 more CRNMBs, and 14 fewer deaths (including equal number of PE-related, no ICH-related deaths, five more non-ICH MB related deaths, and 19 fewer deaths due to other reasons) per 10,000 patients, compared to LMWH. The total cost of treating patients with DOACs over five years was USD 2,232 per patient, while it was USD 8,224 per patient for LMWH. There was a higher QALY among patients on DOACs (1.52) compared to LMWH (1.50), resulting in a cost-saving of USD 5,992 and an incremental QALY difference of 0.02, in favour of the former.
Table 1. Base-case analysis results for a 5-year time horizon.
One-way deterministic sensitivity analysis
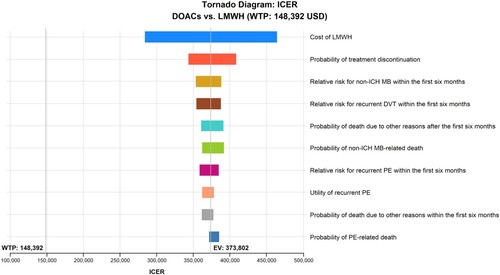
presents the one-way deterministic analysis of the top ten most influential parameters that contributed to the ICER outcome. The top three most influential parameters over a 5-year time horizon were the cost of LMWH, probabilities of LMWH treatment discontinuation, and relative risk for non-ICH MB within the first six months. The cost of LMWH had the highest impact on ICER, ranging from USD 283,654 to 463,950 per QALY. Variations in the other relevant parameters had a minimal observed impact on the ICER results.
Figure 2. One-way deterministic sensitivity analysis over a 5-year time horizon. LMWH: low-molecular-weight heparins; PE: pulmonary embolism; DOACs: direct oral anticoagulants; ICH: intracranial haemorrhage; MB: major bleeding; DVT: deep vein thrombosis; EV: expected value; ICER: incremental cost-effectiveness ratio; USD: United States dollars; WTP: Willing-To-Pay.
Probabilistic sensitivity analysis
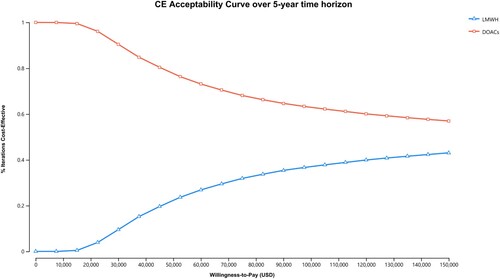
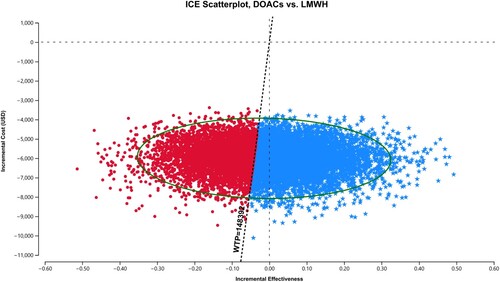
For the probabilistic sensitivity analysis, DOACs maintained dominance over LMWH in 1,578 (15.8%) out of 10,000 simulated cases, and were cost-effective in 4,211 (42.1%) out of the 10,000 cases simulated cases (). As cost-effectiveness acceptability curves showed in , throughout a range of lower WTP thresholds, relative to the base case, DOACs maintained a higher probability of being cost-effective in 57.9%−100% of cases over a 5-year study period.
Figure 3. Probabilistic sensitivity analysis over a 5-year time horizon. DOACs: direct oral anticoagulants; LMWH: low-molecular-weight heparins; USD, United States dollars; WTP: Willingness-To-Pay; ICE: Incremental cost-effectiveness. Spots represent 10,000 draws of the probabilistic analysis, and the vertical dotted line represents the WTP limit. Values of blue pentagonal spots on the right side of the WTP line are considered dominant or cost-effective.
Scenario analysis
As seen in Supplemental Table S3, based on the first 6-month short-term scenario analysis time horizons, it was observed that the DOACs group had 104 fewer recurrent PEs, 110 fewer recurrent DVTs, 22 fewer ICHs, 121 more non-ICH MBs, 22 more CRNMBs, and nine more deaths (including an equal number of PE-related deaths, no ICH-related deaths, three more non-ICH MB-related deaths, and six more deaths due to other reasons) per 10,000 patients, compared to LMWH. The total cost of treating patients with DOACs over six months was USD 668 per patient, while it was USD 2,670 per patient for LMWH. QALYs for both treatment groups were equivalent, at 0.28.
Discussion
In this cost-effectiveness analysis of DOACs and LMWH for the treatment of CAT, we found that DOACs were associated with lower costs and higher QALYs over a 5-year time horizon compared to LMWH. Even though there was no difference in QALYs in the short-term (6-month) cost-effectiveness analysis, DOAC treatment reduced total medical costs by USD 2,002 per patient. Possible explanations for the cost reduction include: Firstly, DOACs have lower costs in comparison to LMWH due to factors such as generic availability in Hong Kong (The Pharmacy and Poisons Board of Hong Kong, Citation2024). Secondly, DOAC treatment may result in lower costs associated with managing adverse events or complications, particularly recurrent VTE (Chen et al., Citation2021).
The latest Hong Kong Cancer Statistics published in 2023, reported 38,462 new cancer cases diagnosed in 2021 (Hong Kong Hospital Authority, Citation2023). Assuming a 5% cumulative incidence of VTE during cancer survival (Mulder et al., Citation2021). DOAC could potentially save approximately USD 6.6 million (HKD 51 million) annually while ensuring the quality of life of these vulnerable patients. The results of this study underscore the significant importance of DOACs for the treatment of VTE. Importantly, our findings are consistent with previously published data confirming the cost-effectiveness of DOACs over LMWH in various territories, including the US, UK, Brazil, Mainland China, and Spain (de Jong et al., Citation2020; Gulati & Eckman, Citation2023; Li et al., Citation2019; Lopes et al., Citation2020; Munoz et al., Citation2022; Wumaier et al., Citation2021). Moreover, a recent Canadian cost-utility study has also highlighted the value of apixaban as a cost-effective option for primary thromboprophylaxis in ambulatory cancer patients initiating chemotherapy, particularly those at intermediate-to-high risk of developing VTE (Kimpton et al., Citation2021). These collective findings emphasise the critical role of DOACs in effectively managing CAT and optimising the utilisation of healthcare resources. The implications of these findings extend beyond clinical practice and should inform the development of evidence-based guidelines for the treatment of CAT in Hong Kong, and potentially worldwide.
To address the uncertainty of input parameters in the model, we performed a series of sensitivity analyses. First, in the one-way deterministic sensitivity analysis, we varied all parameter inputs listed in Supplemental Table S2 independently. Notably, including the cost of LMWH, which was identified as the most influential parameter, no individual parameter impacted the cost-effectiveness of DOACs compared to LMWH. Second, the probabilistic sensitivity analysis results demonstrated that the probability of DOACs being dominant or cost-effective exceeded 57%. Additionally, in the scenario analysis conducted within the first six-month time horizon, DOACs and LMWH consistently resulted in similar QALYs, with a cost-saving of USD 2,002 per patient. These findings provide robust evidence that further supports the cost-effectiveness and clinical advantages of DOACs for both short-term and long-term use in this patient population.
Previous RCTs have reported that the DOACs were non-inferior to parenteral LMWH for preventing recurrent VTE over a 6-month follow-up period among patients with CAT (Planquette et al., Citation2022; Schrag et al., Citation2023). The American Society of Hematology, American Society of Clinical Oncology, and International Initiative on Thrombosis and Cancer guidelines suggest that DOACs and LMWH are the first-line treatment options for CAT during the first three to six months (Farge et al., Citation2022; Key et al., Citation2020; Lyman et al., Citation2021). However, long-term use of parenteral medications is generally not preferred by patients due to the inconvenience of daily subcutaneous injections and injection-site adverse reactions, leading to low treatment adherence and a high discontinuation rate, with the price of LMWH and resources required for drug administration further contributing to its burden (van der Wall et al., Citation2017). In actual practice, DOACs might have better compliance than LMWH (Chen et al., Citation2021; Schaefer et al., Citation2021; Streiff et al., Citation2018). Thus, it is associated with better clinical benefits ignored in RCTs. Therefore, our model might underestimate the benefits of DOACs as the clinical efficacy is solely extracted from RCTs. Nevertheless, our findings still support the use of DOACs as cost-effective. This is important as the medication expenses for CAT can be up to four times higher than in patients with non-VTE cancer (Shah et al., Citation2018).
To the best of our knowledge, this is the first study to compare the cost-effectiveness of anticoagulation treatments for patients with CAT in Hong Kong. While previous studies from other countries have explored the cost-effectiveness of DOACs versus LMWH for CAT, the anticoagulant availability and healthcare systems may vary. In this study, we assessed the cost-effectiveness of dabigatran, apixaban, rivaroxaban, and edoxaban (i.e. DOACs), in comparison to LMWH, which includes enoxaparin, nadroparin, and tinzaparin. By examining these treatment options collectively, we aimed to provide a comprehensive analysis of their cost-effectiveness for CAT management. Also noteworthy is that this is the first cost-effectiveness study to compare DOACs to LMWH based on meta-analysis results of all four RCTs published before 2022. These trials provided valuable clinical efficacy and safety data on edoxaban, apixaban, and rivaroxaban. Transition probabilities, to the best of the available resources, were separately calculated for 5-year and 6-month Markovian follow-up durations (Agnelli et al., Citation2020; Di Nisio et al., Citation2019; McBane et al., Citation2020; Raskob et al., Citation2018; Young et al., Citation2018). This approach is credible as it avoids using consistent transition probabilities throughout the entire 5-year lifetime horizon, which would overlook the sharp decrease in the incidence of adverse events after the initial six months (Di Nisio et al., Citation2019; Li et al., Citation2019; Lopes et al., Citation2020; Munoz et al., Citation2022; Wumaier et al., Citation2021). In addition, unlike the majority of relevant cost-effectiveness studies, we obtained the utilities of base case, recurrent VTE, MB, and CRNMB from the CATCH trial, which is the only one that has explicit data on the utilities of patients with CAT instead of general patients with VTE, noting the fact that there is limited literature available in this area, where further studies are needed.
Nevertheless, this study has limitations. Firstly, participants from the meta-analyzed RCTs were from western countries, where the relative risks and transition probabilities may not be generalisable to the Chinese population. There is a possibility of a higher risk of bleeding with DOACs in the Chinese populations, which may result in a lower QALY gain for DOAC users in the real world in Hong Kong (Chiang et al., Citation2014; Kim et al., Citation2021). While our sensitivity analyses confirmed the robustness of the study conclusion against variability in study inputs, further studies using data from RCTs conducted in Hong Kong or other Asian populations may strengthen the findings of our study. Second, our analysis assumed that each medication in the classes of DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) and LMWH (enoxaparin, nadroparin, and tinzaparin) was equally likely to be used for the treatment of CAT. In reality, dabigatran had the highest monthly cost, which was three times that of edoxaban in Hong Kong (Hospital Authority, Citation2023). Therefore, the monthly cost of anticoagulants will fluctuate depending on the actual proportion of usage for each medication. In any case, the sensitivity analyses performed in our current study have confirmed the study conclusion against input uncertainty, including the cost of medications. Third, generalizability of parameters from RCTs may differ from real-world practice because of strict inclusion and exclusion criteria, which may exclude vulnerable patients with complex comorbidity or severe cancer progression. Patients in the real world may be followed up less frequently and not receiving the same level of close monitoring, which can lead to underestimated transition probabilities for mild-to-moderate adverse events and overestimated transition probabilities for severe events like death. The use of literature data is not uncommon in health economics when it is the best available source of data and local data is not available. Finally, although our results indicate that DOACs could be advantageous for patients with CAT compared to LMWH, the decision for anticoagulant choice must be evaluated based on the individual risks and benefits, especially for patients with high bleeding risk and contraindications to the study medications.
Conclusions
DOACs are a viable cost-effective anticoagulant alternative to LMWH for the treatment of CAT in the Hong Kong healthcare system. In almost two-thirds of simulated cases, DOACs were between cost-effective and dominant. Our study presents crucial evidence for the prioritisation of DOACs in clinical practice for their potential to deliver substantial economic and clinical benefits. The implementation of effective education programmes and policies to facilitate the broader utilisation of DOACs in Hong Kong and beyond is highly recommended. We believe that our study will serve as a valuable reference for policymakers and healthcare professionals in making informed decisions regarding the use of DOACs.
Authors’ contributions
Conception and design: WK, KP, VKCY and EWC; Acquisition, analysis, or interpretation of data: WK, KP, and EWC; Drafting of the manuscript: WK; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: WK and KP; Administrative, technical, or material support: EWC; Supervision: EWC and ICKW. All authors reviewed the manuscript.
Suppl_CEA_CAT_v18_tracked cleaned.docx
Download MS Word (301.2 KB)Acknowledgments
We thank Lisa Y Lam, MJ, Department of Pharmacology and Pharmacy, The University of Hong Kong, for proofreading the manuscript. Ms. Lam was compensated for her contribution. We thank the Information Technology Services, the University of Hong Kong, for offering research computing facilities for the computations in this study.
Disclosure statement
ICK Wong received research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, Takeda, the Hong Kong Research Grants Council, the Hong Kong Health and Medical Research Fund, the National Institute for Health Research in England, the European Commission, the National Health and Medical Research Council in Australia and the European Union's Seventh Framework Programme for research and technological development. He has also received consulting fees from IQVIA, the WHO and expert testimony for the Appeal Court in Hong Kong over the past three years. He is an advisory member of the Pharmacy and Poisons Board, the Expert Committee on Clinical Events Assessment Following COVID-19 Immunization and the Advisory Panel on COVID-19 Vaccines of the Hong Kong Government. He is also a non-executive director of Jacobson Medical Hong Kong Advanced Data Analytics for Medical Science (ADAMS) Limited and OCUS Innovation Limited (Hong Kong, Ireland and United Kingdom) and the founder and director of Therakind Limited (United Kingdom). EWC reported receiving grants from the Research Grants Council of Hong Kong, the Research Fund Secretariat of the Food and Health Bureau of Hong Kong, the National Natural Science Fund of China, the National Health and Medical Research Council (Australia), Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, Novartis, Wellcome Trust and Narcotics Division of the Security Bureau of Hong Kong; honorarium from Hospital Authority; outside the submitted work. Other authors report there are no competing interests to declare.
Data availability statement
All data generated or analysed during this study are included in this published article and its supplementary information files.
Additional information
Funding
References
- Agnelli, G., Becattini, C., Meyer, G., Munoz, A., Huisman, M. V., Connors, J. M., Cohen, A., Bauersachs, R., Brenner, B., Torbicki, A., Sueiro, M. R., Lambert, C., Gussoni, G., Campanini, M., Fontanella, A., Vescovo, G., Verso, M., & Caravaggio, I. (2020). Apixaban for the treatment of venous thromboembolism associated with cancer. New England Journal of Medicine, 382(17), 1599–1607. https://doi.org/10.1056/NEJMoa1915103
- Al Mukdad, M., Al-Badriyeh, D., & Elewa, H. F. (2019). Cost-effectiveness evaluations among the direct oral anticoagulants for the prevention and treatment of venous thromboembolism: Systematic review. Clinical and Applied Thrombosis/Hemostasis, 25, 107602961984910. https://doi.org/10.1177/1076029619849103
- Bertoletti, L., Madridano, O., Jimenez, D., Muriel, A., Bikdeli, B., Ay, C., Trujillo-Santos, J., Bosevski, M., Siguenza, P., & Monreal, M. (2023). Cancer-associated thrombosis: Trends in clinical features, treatment, and outcomes from 2001 to 2020. JACC: CardioOncology, 5(6), 758–772. https://doi.org/10.1016/j.jaccao.2023.09.003
- Chen, D. Y., Tseng, C. N., Hsieh, M. J., Lan, W. C., Chuang, C. K., Pang, S. T., Chen, S. W., Chen, T. H., Chang, S. H., Hsieh, I. C., Chu, P. H., Wen, M. S., Chen, J. S., Chang, J. W., See, L. C., & Huang, W. K. (2021). Comparison between non-vitamin K antagonist oral anticoagulants and low-molecular-weight heparin in Asian individuals with cancer-associated venous thromboembolism. JAMA Network Open, 4(2), e2036304. https://doi.org/10.1001/jamanetworkopen.2020.36304
- Chiang, C. L., Chan, S. K., Lee, S. F., & Choi, H. C. (2021). First-line atezolizumab plus bevacizumab versus sorafenib in hepatocellular carcinoma: A cost-effectiveness analysis. Cancers, 13(5), 931. https://doi.org/10.3390/cancers13050931
- Chiang, C. E., Wang, K. L., & Lip, G. Y. (2014). Stroke prevention in atrial fibrillation: An Asian perspective. Thrombosis and Haemostasis, 112(5), 789–797. https://doi.org/10.1160/TH13-11-0948
- de Jong, L. A., van der Velden, A. W. G., Hulst, M. V., & Postma, M. J. (2020). Cost-effectiveness analysis and budget impact of rivaroxaban compared with dalteparin in patients with cancer at risk of recurrent venous thromboembolism. BMJ Open, 10(11), e039057. https://doi.org/10.1136/bmjopen-2020-039057
- Di Nisio, M., van Es, N., Carrier, M., Wang, T. F., Garcia, D., Segers, A., Weitz, J., Buller, H., & Raskob, G. (2019). Extended treatment with edoxaban in cancer patients with venous thromboembolism: A post-hoc analysis of the Hokusai-VTE Cancer study. Journal of Thrombosis and Haemostasis, 17(11), 1866–1874. https://doi.org/10.1111/jth.14561
- Farge, D., Frere, C., Connors, J. M., Khorana, A. A., Kakkar, A., Ay, C., Muñoz, A., Brenner, B., Prata, P. H., Brilhante, D., Antic, D., Casais, P., Guillermo Esposito, M. C., Ikezoe, T., Abutalib, S. A., Meillon-García, L. A., Bounameaux, H., Pabinger, I., Douketis, J., … Yasuda, C. (2022). 2022 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. The Lancet Oncology, 23(7), e334–e347. https://doi.org/10.1016/S1470-2045(22)00160-7
- Gidwani, R., & Russell, L. B. (2020). Estimating transition probabilities from published evidence: A tutorial for decision modelers. Pharmacoeconomics, 38(11), 1153–1164. https://doi.org/10.1007/s40273-020-00937-z
- Gulati, S., & Eckman, M. H. (2023). Anticoagulant therapy for cancer-associated thrombosis: A cost-effectiveness analysis. Annals of Internal Medicine, 176(1), 1–9. https://doi.org/10.7326/M22-1258
- Hogg, K., Kimpton, M., Carrier, M., Coyle, D., Forgie, M., & Wells, P. (2013). Estimating quality of life in acute venous thrombosis. JAMA Internal Medicine, 173(12), 1067–1072. https://doi.org/10.1001/jamainternmed.2013.563
- Hong Kong Hospital Authority. (2023, October 31). Overview of Hong Kong cancer statistics. Retrieved October 31, 2024, from https://www3.ha.org.hk/cancereg/pub.html
- Hong Kong Monetary Authority. (2023, December 8). Monthly statistical bulletin. Retrieved December 8, 2024, from https://www.hkma.gov.hk/eng/data-publications-and-research/data-and-statistics/monthly-statistical-bulletin/table/
- Hong Kong Special Administrative Region Government. (2024, February 28). The 2024–25 budget. Retrieved February 28, 2024, from https://www.budget.gov.hk/2024/eng/speech.html
- The Hong Kong Trade Development Council (HKTDC). (2023, November 30). Economic and trade information on Hong Kong. Retrieved November 30, 2024, from https://research.hktdc.com/en/article/MzIwNjkzNTY5
- Hospital Authority. (2023, January 10). Fees and charges. Retrieved June 18, 2024, from https://www.ha.org.hk/visitor/ha_visitor_index.asp?Content_ID=10045&Lang=ENG
- Hull, R. D., & Lip, G. Y. (2023, June 7). Venous thromboembolism: Anticoagulation after initial management. Retrieved June 7, 2024, from https://www-uptodate-com.eproxy.lib.hku.hk/contents/venous-thromboembolism-anticoagulation-after-initial-management?search=apixaban&source=search_result&selectedTitle=4~150&usage_type=default&display_rank=2
- Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., Caulley, L., Chaiyakunapruk, N., Greenberg, D., Loder, E., Mauskopf, J., Mullins, C. D., Petrou, S., Pwu, R. F., Staniszewska, S., & Force, C. I. G. R. P. T. (2022). Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Value in Health, 25(1), 3–9. https://doi.org/10.1016/j.jval.2021.11.1351
- Key, N. S., Khorana, A. A., Kuderer, N. M., Bohlke, K., Lee, A. Y. Y., Arcelus, J. I., Wong, S. L., Balaban, E. P., Flowers, C. R., Francis, C. W., Gates, L. E., Kakkar, A. K., Levine, M. N., Liebman, H. A., Tempero, M. A., Lyman, G. H., & Falanga, A. (2020). Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. Journal of Clinical Oncology, 38(5), 496–520. https://doi.org/10.1200/JCO.19.01461
- Khorana, A. A., Francis, C. W., Culakova, E., Kuderer, N. M., & Lyman, G. H. (2007). Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of Thrombosis and Haemostasis, 5(3), 632–634. https://doi.org/10.1111/j.1538-7836.2007.02374.x
- Khorana, A. A., Noble, S., Lee, A. Y. Y., Soff, G., Meyer, G., O'Connell, C., & Carrier, M. (2018). Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. Journal of Thrombosis and Haemostasis, 16(9), 1891–1894. https://doi.org/10.1111/jth.14219
- Kim, S. M., Jeon, E. T., Jung, J. M., & Lee, J. S. (2021). Real-world oral anticoagulants for Asian patients with non-valvular atrial fibrillation: A PRISMA-compliant article. Medicine, 100(32), e26883. https://doi.org/10.1097/MD.0000000000026883
- Kimpton, M., Kumar, S., Wells, P. S., Coyle, D., Carrier, M., & Thavorn, K. (2021). Cost-utility analysis of apixaban compared with usual care for primary thromboprophylaxis in ambulatory patients with cancer. Canadian Medical Association Journal, 193(40), E1551–E1560. https://doi.org/10.1503/cmaj.210523
- Leung, L. L. (2023 July 27). Direct oral anticoagulants (DOACs) and parenteral direct-acting anticoagulants: Dosing and adverse effects. Retrieved July 27, 2024, from https://www-uptodate-com.eproxy.lib.hku.hk/contents/direct-oral-anticoagulants-doacs-and-parenteral-direct-acting-anticoagulants-dosing-and-adverse-effects?search=apixaban&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=1#H10489655
- Li, A., Manohar, P. M., Garcia, D. A., Lyman, G. H., & Steuten, L. M. (2019). Cost effectiveness analysis of direct oral anticoagulant (DOAC) versus dalteparin for the treatment of cancer associated thrombosis (CAT) in the United States. Thrombosis Research, 180, 37–42. https://doi.org/10.1016/j.thromres.2019.05.012
- Lip, G. Y., & Hull, R. D. (2023 March 15). Venous thromboembolism: Initiation of anticoagulation. Retrieved March 15, 2024, from https://www-uptodate-com.eproxy.lib.hku.hk/contents/venous-thromboembolism-initiation-of-anticoagulation?sectionName=Low%20molecular%20weight%20heparin&search=apixaban&topicRef=95395&anchor=H796357734&source=see_link#H105734355
- Lloyd, A. J., Dewilde, S., Noble, S., Reimer, E., & Lee, A. Y. Y. (2018). What Impact does venous thromboembolism and bleeding have on cancer patients’ quality of life? Value in Health, 21(4), 449–455. https://doi.org/10.1016/j.jval.2017.09.015
- Lopes, D. G., Tamayo, A., Schipp, B., & Siepmann, T. (2020). Cost-effectiveness of edoxaban vs low-molecular-weight heparin and warfarin for cancer-associated thrombosis in Brazil. Thrombosis Research, 196, 4–10. https://doi.org/10.1016/j.thromres.2020.08.014
- Lyman, G. H., Carrier, M., Ay, C., Di Nisio, M., Hicks, L. K., Khorana, A. A., Leavitt, A. D., Lee, A. Y. Y., Macbeth, F., Morgan, R. L., Noble, S., Sexton, E. A., Stenehjem, D., Wiercioch, W., Kahale, L. A., & Alonso-Coello, P. (2021). American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Advances, 5(4), 927–974. https://doi.org/10.1182/bloodadvances.2020003442
- McBane, R. D., II, Wysokinski, W. E., Le-Rademacher, J. G., Zemla, T., Ashrani, A., Tafur, A., Perepu, U., Anderson, D., Gundabolu, K., Kuzma, C., Perez Botero, J., Leon Ferre, R. A., Henkin, S., Lenz, C. J., Houghton, D. E., Vishnu, P., & Loprinzi, C. L. (2020). Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. Journal of Thrombosis and Haemostasis, 18(2), 411–421. https://doi.org/10.1111/jth.14662
- McShane, M., Senchak, J., Stack, A., Frimpong, J., Hellerslia, V., & Zhao, H. Q. (2020). A retrospective single-center evaluation of the safety and efficacy of direct oral anticoagulants versus low molecular weight heparin in patients with cancer-associated thrombosis. Journal of Clinical Oncology, 38(15), e24102. https://doi.org/10.1200/JCO.2020.38.15_suppl.e24102
- Mulder, F. I., Horvath-Puho, E., van Es, N., van Laarhoven, H. W. M., Pedersen, L., Moik, F., Ay, C., Buller, H. R., & Sorensen, H. T. (2021). Venous thromboembolism in cancer patients: A population-based cohort study. Blood, 137(14), 1959–1969. https://doi.org/10.1182/blood.2020007338
- Munoz, A., Gallardo, E., Agnelli, G., Crespo, C., Forghani, M., Arumi, D., Fernandez de Cabo, S., & Soto, J. (2022). Cost-effectiveness of direct oral anticoagulants compared to low-molecular-weight-heparins for treatment of cancer associated venous thromboembolism in Spain. Journal of Medical Economics, 25(1), 840–847. https://doi.org/10.1080/13696998.2022.2087998
- National Institute for Health and Care Excellence. (2015, June 4). Apixaban for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism. Retrieved from https://www.nice.org.uk/guidance/ta341/resources/apixaban-for-the-treatment-and-secondary-prevention-of-deep-veinthrombosis-andor-pulmonary-embolism-pdf-82602602803141
- National Institute for Health and Care Excellence. (2022, January 31). NICE health technology evaluations: The manual. Retrieved January 31, 2024, from https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741
- The Pharmacy and Poisons Board of Hong Kong. (2024, April 19). Registered pharmaceutical products. Retrieved April 19, 2024, from https://www.drugoffice.gov.hk/eps/do/en/pharmaceutical_trade/reg_pharm_products/index.html
- Planquette, B., Bertoletti, L., Charles-Nelson, Chest, Laporte, S., Grange, C., Mahe, I., Pernod, G., Elias, A., Couturaud, F., Falvo, N., Sevestre, M. A., Ray, V., Burnod, A., Brebion, N., Roy, P. M., Timar-David, M., Aquilanti, S., Constans, J., Bura-Riviere, A., … Investigators, C. D. T. (2022). Rivaroxaban vs dalteparin in cancer-associated thromboembolism. Chest, 161(3), 781–790. https://doi.org/10.1016/j.chest.2021.09.037
- Raskob, G. E., van Es, N., Verhamme, P., Carrier, M., Di Nisio, M., Garcia, D., Grosso, M. A., Kakkar, A. K., Kovacs, M. J., Mercuri, M. F., Meyer, G., Segers, A., Shi, M., Wang, T. F., Yeo, E., Zhang, G., Zwicker, J. I., Weitz, J. I., Buller, H. R., & Hokusai, V. T. E. C. I. (2018). Edoxaban for the treatment of cancer-associated venous thromboembolism. New England Journal of Medicine, 378(7), 615–624. https://doi.org/10.1056/NEJMoa1711948
- Schaefer, J. K., Li, M., Wu, Z., Basu, T., Dorsch, M. P., Barnes, G. D., Carrier, M., Griggs, J. J., & Sood, S. L. (2021). Anticoagulant medication adherence for cancer-associated thrombosis: A comparison of LMWH to DOACs. Journal of Thrombosis and Haemostasis, 19(1), 212–220. https://doi.org/10.1111/jth.15153
- Schrag, D., Uno, H., Rosovsky, R., Rutherford, C., Sanfilippo, K., Villano, J. L., Drescher, M., Jayaram, N., Holmes, C., Feldman, L., Zattra, O., Farrar-Muir, H., Cronin, C., Basch, E., Weiss, A., Connors, J. M., & Investigators, C. (2023). Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer. JAMA, 329(22), 1924–1933. https://doi.org/10.1001/jama.2023.7843
- Shah, S., Rubin, N., & Khorana, A. A. (2018). Economic burden of venous thromboembolism in cancer patients—A comparative analysis between matched patients with cancer with and without a diagnosis of venous thromboembolism. Blood, 132(Supplement 1), 366. https://doi.org/10.1182/blood-2018-99-113581
- Streiff, M. B., Milentijevic, D., McCrae, K., Yannicelli, D., Fortier, J., Nelson, W. W., Laliberte, F., Crivera, C., Lefebvre, P., Schein, J., & Khorana, A. A. (2018). Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. American Journal of Hematology, 93(5), 664–671. https://doi.org/10.1002/ajh.25059
- van der Wall, S. J., Klok, F. A., den Exter, P. L., Barrios, D., Morillo, R., Cannegieter, S. C., Jimenez, D., & Huisman, M. V. (2017). Continuation of low-molecular-weight heparin treatment for cancer-related venous thromboembolism: A prospective cohort study in daily clinical practice. Journal of Thrombosis and Haemostasis, 15(1), 74–79. https://doi.org/10.1111/jth.13563
- The World Bank. (2024, March 28). Life expectancy at birth, total (years) – World. Retrieved March 28, 2024, from https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=1W
- Wumaier, K., Li, W., Chen, N., & Cui, J. (2021). Direct oral anticoagulants versus low molecular weight heparins for the treatment of cancer-associated thrombosis: A cost-effectiveness analysis. Thrombosis Journal, 19(1), 68. https://doi.org/10.1186/s12959-021-00319-1
- Young, A. M., Marshall, A., Thirlwall, J., Chapman, O., Lokare, A., Hill, C., Hale, D., Dunn, J. A., Lyman, G. H., Hutchinson, C., MacCallum, P., Kakkar, A., Hobbs, F. D. R., Petrou, S., Dale, J., Poole, C. J., Maraveyas, A., & Levine, M. (2018). Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). Journal of Clinical Oncology, 36(20), 2017–2023. https://doi.org/10.1200/JCO.2018.78.8034