ABSTRACT
Introduction
The use of gentamicin in the treatment of infectious diseases requires frequent monitoring to attain the best treatment outcomes.
Objective
This study aimed to evaluate the appropriateness of gentamicin therapeutic drug monitoring (TDM) at a tertiary care hospital in Qatar.
Methods
A one-year quantitative retrospective chart review of all gentamicin TDM records was conducted. Evidence-based criteria were applied to evaluate the appropriateness of gentamicin TDM in terms of indication, sampling times, and post-analytical actions.
Results
Out of 59 captured gentamicin TDM records, 58 gentamicin samples were eligible for evaluation. Overall, gentamicin TDM appropriateness was achieved in 50% (n = 29) of the evaluated records. However, 12% (n = 7) of gentamicin drug concentrations were below the assay quantification limits or were not sampled appropriately. Inappropriate post-analytical actions (22.4%, n = 13) and inappropriate sampling times (44.8%, n = 26) were recorded. Most of the gentamicin blood samples (n = 43; 74.2%) were taken appropriately at steady-state. Inappropriate sampling time relative to the last dose was captured in 31% (n = 18) of the cases. Although 27.6% (n = 16) of gentamicin concentrations were non-therapeutic, continuing gentamicin dosing without adjustment was the most frequent post-analytical action (69.8%, n = 37). Gentamicin dose regimen continuations, dose regimen decreases and dose regimen discontinuations were inappropriately applied in 27% (n = 10), 25% (n = 2) and 14% (n = 1) of the times, respectively.
Conclusion
Suboptimal gentamicin TDM practices exist in relation to sampling time and post-analytical actions. Studies exploring setting-specific reasons behind inappropriate TDM practices and methods of its optimisation are needed.
1. Introduction
Gentamicin is an aminoglycoside antibiotic used to treat complicated invasive bacterial infections. The broad-spectrum coverage of gentamicin, coupled with wide availability of low cost brands, made it one of the most commonly used antibiotics in critically-ill hospitalised patients worldwide (Crcek et al., Citation2019; Krzyżaniak et al., Citation2016; Mijović et al., Citation2020; Ohnishi & Mikamo, Citation2013). The pharmacokinetics of gentamicin is affected by many factors such as age, critical-illness state, renal function, body size and other pathophysiological factors (Crcek et al., Citation2019). The wide between-subject variability in gentamicin pharmacokinetic parameters, together with its dose-dependent toxicity (i.e. nephrotoxicity and ototoxicity) and narrow therapeutic index mandate the application of therapeutic drug monitoring (TDM) to guide individualised patient dosing (Crcek et al., Citation2019; Singu et al., Citation2018; Sweileh, Citation2009). Gentamicin TDM has been well established worldwide (Ben Romdhane et al., Citation2019; du Toit et al., Citation2019; Hodiamont et al., Citation2017; Ismail et al., Citation1990; Lim et al., Citation2020), and is associated with positive clinical outcomes and decreased costs (Crist et al., Citation1987; Ho et al., Citation1994; Hodiamont et al., Citation2017; Ismail et al., Citation1990). In the TDM context, ‘appropriateness’ includes the correctness of all activities included in the TDM continuum, in relation to indication, sampling time, interpretation, analytical technique and subsequent dosage adjustments (Al-Sulaiti et al., Citation2020).
The epidemiology of multi-drug resistant gram-negative bacterial strains in the Middle East shows increased emergence of gentamicin-resistant strains (Dandachi et al., Citation2019), possibly related to inappropriate clinical practices. Elsewhere, gentamicin was one of the top drugs associated with high resistance rates towards gram-negative infections (Mijović et al., Citation2020). The inappropriateness of antibiotic use may contribute toincreased rates of antibiotic resistance towards gram-negative infections, causing a threat to healthcare systems and public health (Dandachi et al., Citation2019; Mijović et al., Citation2020). Therefore, TDM of gentamicin needs to be emphasised to avoid antimicrobial resistance and to prevent therapeutic failure. In our institution, as the case elsewhere, gentamicin dosing and TDM practices are heterogeneous and follow variable dosing and monitoring schemes reported in the literature (Bauer, Citation2008; Gilbert et al., Citation2019; HMC, Citation2006-2020). Moreover, TDM is generally conducted by various health care providers who are mostly expatriates from Middle Eastern or Asian educational and practice backgrounds (Kheir et al., Citation2015). These are potential factors that could contribute to inconsistencies and inefficient TDM application due to different practice standards.
According to our institutional antimicrobial protocols, gentamicin can be administered as high-dose extended-interval dosing which is identified as once-daily dosing (ODD) or through conventional/traditional dosing (Bauer, Citation2008; HMC, Citation2006-2020). The clinician monitors the peak and trough steady-state gentamicin levels if conventional dosing is used. In ODD, clinicians can either measure steady-state peak and trough concentrations, measure a single concentration 6–14 hours post-dose with Hartford nomogram-based dose adjustment, or measure a single steady-state trough concentration (Bauer, Citation2008; Gilbert et al., Citation2019; HMC, Citation2006-2020). Setting-specific local audits assessing the compliance with gentamicin TDM institutional guidelines have not been conducted to date. Continuous TDM service quality optimisation based on setting-specific quality audits is important to design appropriate TDM services and achieve optimal treatment outcomes (Al-Sulaiti et al., Citation2020). To our knowledge, no previous studies have assessed the appropriateness of gentamicin TDM in Qatar. This study aimed to evaluate the appropriateness of TDM for gentamicin in relation to indication, sampling time, interpretation, and subsequent dosage adjustments at a tertiary hospital in Qatar.
2. Methods
2.1. Ethical approval
This retrospective study was approved by the Institutional Review Boards of Qatar University and the Medical Research Center of Hamad Medical Corporation.
2.2. Study setting and population
A quantitative retrospective chart review that included all gentamicin TDM records across a 12-month period was conducted at Al-Khor Hospital (AKH), a tertiary care hospital under Hamad Medical Corporation (HMC). HMC is the major public healthcare provider in Qatar (HMC, Citation2016). AKH is a 110-bed tertiary hospital that serves the northern region population of Qatar for both adult and paediatric patients (HMC, Citation2016). The clinical pharmacy practitioners at AKH are Asian or Middle Eastern-trained expatriates from various educational and clinical backgrounds.
In the study setting, TDM was not limited to clinical pharmacists with specialised pharmacokinetic training (Al-Sulaiti et al., Citation2020; Kheir et al., Citation2015). TDM clinical recommendations were provided by clinical pharmacists as part of their roles as members of the primary treating team. The acceptance or rejection of the TDM recommendations was dependent on the approval of the treating physician. The institutional guidelines summarised the published literature that reported various schemes for gentamicin TDM (Bauer, Citation2008).
Institutional peak and trough gentamicin targets for gram negative infections varied according to infection type as follows:
– Target peak concentrations: pneumonia (8–10 mg/L); bacteremia, skin/soft tissue infections (6–8 mg/L); urinary tract infections (5–6 mg/L)
– Target trough concentrations: pneumonia, bacteremia, skin/soft tissue (<2 mg/L); urinary tract infections (<1.5 mg/L)
The selection of dosing and monitoring scheme was the onus of the treating clinician.
2.3. Inclusion and exclusion criteria
The unit of analysis of this retrospective audit was a gentamicin TDM record/request. A gentamicin TDM record/request was defined as a gentamicin drug concentration recorded in the electronic medical record (EMR) used in our setting. A gentamicin TDM record/request was included in this analysis if it was associated with sufficient documentation to evaluate at least one TDM appropriateness criterion (). Exclusion criteria was defined as any gentamicin TDM record that was not associated with sufficient documentation in the EMR, hindering the assessment of at least one TDM appropriateness criterion as summarised in .
Table 1. Definitions of evaluation criteria and study endpoints.
2.4. Sample size and sampling technique
Given the expected low sample size of the eligible records, a universal sampling technique was applied. All eligible gentamicin TDM records were included in the study. No sample size calculations were conducted a priori.
2.5. Data collection
The previously published multi-domain pretested and pilot-tested TDM appropriateness evaluation data collection tool was used for data collection (Al-Sulaiti et al., Citation2020). Multiple institutional databases were screened to capture all potential gentamicin TDM records. The Central Laboratory electronic database, pharmacy gentamicin prescriptions and EMR were screened to identify any ordered gentamicin blood concentrations. The data collection window was limited to the duration of gentamicin treatment period. Pharmacists with training in clinical pharmacokinetics captured the data into the data collection sheet.
2.6. TDM quality assessment and relevant clinical outcomes
Appropriateness of gentamicin TDM practices was determined based on a priori criteria specific per each gentamicin TDM record () (Bauer, Citation2008; HMC, Citation2006-2020). A gentamicin TDM record corresponded to one gentamicin drug concentration and all accompanying pre-analytical (i.e. indication and sampling time) and post-analytical actions (interpretation, dose adjustment and clinical recommendation). Evidence-based clinical practice guidelines and clinical pharmacokinetic principles were used for the development and definition of gentamicin TDM Quality Assessment Criteria () (Bauer, Citation2008; HMC, Citation2006-2020). Evaluation criteria included the appropriateness of: (1) indication; (2) sampling time in relation to the last dose (AST-LD); (3) sampling time in relation to steady-state attainment (AST-SS); (4) composite sampling time (AST-C) (i.e: the appropriateness of both AST-SS and AST-LD); (5) post-analytical action (PAA). Composite TDM appropriateness was achieved if all quality assessment criteria were appropriate. The pragmatic nature of the data collection tool was assured by pretesting and pilot testing by a panel of three expert clinical pharmacists. For each evaluation criterion, the evaluator selected either appropriate, inappropriate, or undetermined (i.e. insufficient documentation). Clinical outcomes were determined using evidence-based definitions as per . Clinical effectiveness, gentamicin treatment duration, length of hospitalisation (LOS) and nephrotoxicity were captured per gentamicin TDM record.
Table 2. Definitions of clinical outcome measures.
2.7. Statistical analysis
Descriptive statistics were performed using the IBM Statistical Package for Social Sciences (SPSS) Version 23. The unit of analysis was gentamicin TDM record/request. Shapiro–Wilk test was used to assess normality. Given that the continuous variables were not normally distributed, medians and interquartile range (IQR) were calculated to summarise non-normally distributed continuous variables. For categorical variables, frequencies and percentages were generated as appropriate.
3. Results
3.1. Characteristics of gentamicin TDM records
A total of 59 gentamicin samples were captured from the electronic records. One gentamicin sample was not accompanied with any dosing and clinical documentation in the EMR databases and thus was excluded from the analysis. The final study sample size included 58 gentamicin samples (i.e. gentamicin TDM records) that were collected from 25 patients (22 neonates and 3 adults). Of those, 14 gentamicin samples were for one elderly 70-year-old patient who was on renal dialysis. Most of the gentamicin TDM records were from neonates hospitalised in the neonatal intensive care unit (NICU) (n = 34, 58.6%). Sepsis or septic shock (n = 18, 31.6%), respiratory tract infections (n = 18, 31.6%), and device-related infections (n = 14, 24.6%) were the most frequently documented primary diagnoses. Sixteen patients received ODD schemes, corresponding to 69% (n = 40) gentamicin TDM records. Other dosing schemes such as q 8, 34, 36, 48 and 72 hourly dosing were also used (). Peak, trough and random gentamicin drug concentrations were routinely ordered and were within the therapeutic window in 72.4% (n = 42) of the evaluated records. The characteristics of the gentamicin TDM records are summarised in .
Table 3. Characteristics of gentamicin therapeutic drug monitoring (TDM) records.
3.2. Pre-analytical appropriateness of gentamicin TDM records
The indications for ordering TDM requests were appropriate in all the gentamicin TDM records (). Indications for ordering gentamicin drug concentrations were to prevent toxicity (n = 32; 55.2%) or assure therapeutic concentration achievement (n = 21, 36.2%). Despite that 12% (n = 7) of gentamicin drug concentrations were below the assay quantification limit (i.e. undetectable) or were not sampled appropriately to allow classification (unevaluable) (), none of the indications included correction of inappropriate sampling or probable laboratory errors.
Table 4. Appropriateness of gentamicin TDM service.
Composite sampling time appropriateness (AST-C) was only achieved in 50% (n = 29) of gentamicin TDM records (). Most of the blood samples (n = 43; 74.2%) were appropriately taken at steady-state. Contrastingly, gentamicin samples were not appropriately timed in relation to the dosing schedule of the patient in 31% (n = 18) of specimens (). summarises the pre-analytical appropriateness indices of gentamicin TDM services.
3.3. Post-analytical appropriateness indices
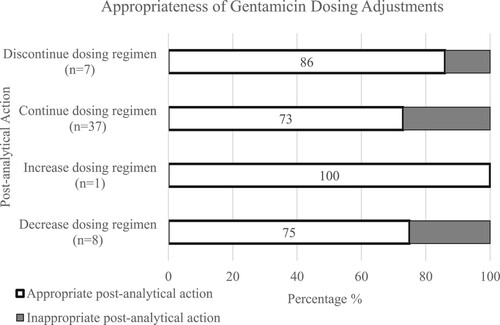
Fifty-three TDM records possessed sufficient documentation to assess the post-analytical actions (). Five records were not evaluated in terms of post-analytical appropriateness due to missing documentation. Gentamicin dosing regimens were frequently continued without change (n = 37, 69.8%), decreased (n = 8, 15%) or discontinued (n = 7, 13.2%) (). Despite that 27.5% (n = 16) of gentamicin concentrations were out of the therapeutic window (), the top-rated post-analytical action taken was to continue the dosing regimen without change. No concentrations were reordered in patient charts in cases of incorrect sampling times or suspected laboratory or technical errors and the turn-around time for the gentamicin assay was about 8 hours. The post-analytical actions were appropriate in 69% (n = 40) of gentamicin TDM records (). Some of the applied dosing adjustments were inappropriate. Gentamicin dose regimen continuations, decreases and discontinuations were inappropriate in 27% (n = 10), 25% (n = 2) and 14% (n = 1) of the times, respectively ().
3.4. Composite gentamicin TDM appropriateness and relevant clinical outcomes
Median LOS [IQR] was 7 [4-26] days for the studied cases. Overall gentamicin TDM appropriateness was achieved in 50% (n = 29) of the evaluated records. Inappropriate post-analytical actions and inappropriate sampling times were the main reasons for inappropriate composite TDM services (). Therapeutic cure was seen in 81.2% (n = 39) of the cases. Gentamicin TDM practices were accompanied with therapeutic failure, including nephrotoxicity in 18.8% (n = 9) of the records.
4. Discussion
To the best of our knowledge, this is the first retrospective audit evaluating the quality of gentamicin TDM service in Qatar. Our study shows the presence of inappropriate gentamicin TDM practices across various pre-analytical and post-analytical TDM aspects. The main areas of inappropriate practices were inappropriate sampling times and inappropriate post-analytical actions including inappropriate dose adjustments. Globally, challenges related to gentamicin optimal dosing, monitoring and TDM application in clinical settings continue to be published in the recent literature (Al-Lanqawi et al., Citation2007; Crcek et al., Citation2019; Hartman et al., Citation2020; Kadambari et al., Citation2011; Lee et al., Citation2014; Valitalo et al., Citation2015). Compelling evidence has proven that gentamicin dosing nomograms performed poorly in achieving therapeutic gentamicin pharmacokinetic targets and emphasise the crucial need for appropriate TDM to guide gentamicin dosing in various populations (Al-Lanqawi et al., Citation2007; Lee et al., Citation2014). For example, a recent systematic review reported decreased target attainment with gentamicin in critically ill children (Hartman et al., Citation2020). Interestingly, a review of population pharmacokinetic models of gentamicin in different patient groups highlighted that administered gentamicin doses sometimes differerd from the proposed evidence-based doses (Crcek et al., Citation2019). These findings highlight possible poor adherence to nomogram-guided dosing practices and the indispensable need for appropriate TDM application in all patients receiving gentamicin.
Suboptimal gentamicin TDM practices highlighted in this study can be due to three main reasons. First, gentamicin TDM practices are not standardised in our institution; heterogenous gentamicin dosing and monitoring methods are applied by heterogeneous types of clinicians in our setting. The absence of a standardised institutional protocol or guideline contributes to variation in gentamicin prescribing and monitoring and adds to the potential errors related to its administration, sampling time and dose adjustment (Saddi et al., Citation2017). Thus, gentamicin TDM application in our setting presents a significant challenge to clinicians and is subject to variations and inconsistencies. This variation becomes more profound in our setting since clinicians are mostly expatriates coming from different educational and training backgrounds. Institutional inconsistencies in TDM protocols have been reported in the literature. To exemplify, variations in dosage and monitoring were recorded in TDM protocols between multiple UK hospital units (Kadambari et al., Citation2011). Flannigan et al. reported a decrease in mean gentamicin-related medication errors since the implementation of a standardised gentamicin dosing and monitoring chart (Flannigan et al., Citation2010). Second, our institution does not have standardised gentamicin-specific TDM documentation forms, which is the main reason for poor TDM documentation practices. Incomplete, inaccurate, and ambiguous documentation practices across TDM activities is another factor that impacts the TDM workflow negatively, presenting a potential reason for miscommunication-related errors between the treating clinicians. Third, gentamicin TDM computerisation is not applied in our setting. The use of computerised TDM methods to guide individualised antibiotic dosing may result in better achievement of target pharmacokinetic-pharmacodynamic measures (Bauer, Citation2008; Roberts et al., Citation2012). However, our institution does not have access to these computer programmes and continues to use nomograms. Thus, it appears that a collaborative effort between pharmacists and clinicians is needed to develop gentamicin-specific standardised institutional TDM protocol and documentation forms while applying computerisation of gentamicin dosing and monitoring.
Inappropriate TDM practices present potential sources of economic burden on the healthcare system and potential unexplored sources of treatment failure, prolonged hospitalisations and the emergence of antimicrobial-resistant strains (Al-Sulaiti et al., Citation2020; Mijović et al., Citation2020). The inappropriate pre-analytical and post-analytical TDM aspects reported in this study are confirmed by similar findings of a vancomycin TDM quality audit in our setting (Al-Sulaiti et al., Citation2020). Similar observations were reported in a teaching hospital in Oman; the reported sampling times were found to be inappropriate in 71.5% of the cases due to wrong sampling time or sampling before reaching steady-state (Al Za'abi et al., Citation2015). TDM principles mandate correct sampling times to assure correct dosing adjustments. Inappropriate sampling times may result in inappropriately high or low calculated new doses presenting a source of antibiotic misuse. Moreover, incorrect sampling necessitates resampling of the gentamicin TDM. Obtaining unnecessary and avoidable blood samples in critically ill patients contributes to blood loss, pain and increases the financial burden on health care systems (Reynolds et al., Citation2012). Repeated exposure to painful procedures in neonates may have negative long-term effects on the central nervous system (Batton et al., Citation2006). Thus, inappropriately timed samples are potential reasons for erroneous clinical decisions, subjecting patients to avoidable painful procedures, and subjecting healthcare systems to avoidable costs. On the other hand, appropriate gentamicin TDM application was associated with achievements of target drug therapeutic concentrations and less subtherapeutic concentrations (Hodiamont et al., Citation2017; Ismail et al., Citation1990). More importantly, optimum pharmacy-based gentamicin TDM translated into better clinical outcomes such as significantly shorter fever days, less need for dose adjustments and less need of drug concentration monitoring compared to the comparator group (Ho et al., Citation1994). Appropriate aminoglycoside TDM programmes markedly decreased total aminoglycoside dose consumptions, tissue accumulation-related toxicity and were associated with profoundly lower hospital costs (Crist et al., Citation1987). Continuous TDM service quality optimisation, together with appropriate TDM programmes are important to achieve optimal treatment outcomes with gentamicin TDM.
The findings of this quality audit need to be interpreted in light of its strengths and limitations. The evaluation was conducted by clinical pharmacists with pharmacokinetic training using evidence-based criteria, which assures the internal validity of this work. The relatively small sample size was anticipated a priori and thus all potential records were captured during the one-year study time frame. The use of a universal sampling technique assured that all possible gentamicin TDM records were captured. This chart review highlights the incomplete documentation practices; there are many ‘undetermined’ findings in several parts of the results, calling into question the documentation quality in our institution. There are no TDM-specific forms in our institution which may explain this finding. The influence of other co-administered medications impacting gentamicin concentration was not considered in this study due to suboptimal documentation in the medical records. Given that the local clinical practice is guided mostly by expatriates from Middle Eastern or Asian training backgrounds, TDM practices in Qatar could be generalisable to other Middle Eastern settings (Al-Sulaiti et al., Citation2020; Kheir et al., Citation2015). Therefore, the findings of this work may be of regional value. The appropriateness of laboratory analytical factors was not assessed and needs to be explored in future audits. The relatively high rate of missing important clinical data in the medical records resulted in the inability to comprehensively evaluate the impact of inappropriate TDM practices on clinical safety and efficacy outcome measures. Future studies exploring the relationship between gentamicin TDM quality and clinical and economical outcomes are needed.
Conclusion
This study highlights the prescence of inappropriate sampling time and post-analytical actions related to gentamicin TDM services at a tertiary hospital in Qatar. Future studies are needed to determine setting-specific reasons behind inappropriate TDM practices and assess associated clinical outcomes. Moreover, there is a need to establish ways to improve the quality of TDM and adhere to best practices.
Acknowledgments
The authors are grateful to Qatar University and Qatar National Research Fund for supporting this project. We thank Dr. Oraib Amjed Abdallah for reviewing this manuscript. Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors cannot share the data related to this study unless with the permission of Hamad Medication Corporation (HMC) due to data sharing institutional agreements.
Additional information
Funding
References
- Al-Lanqawi, Y., Capps, P., Abdel-hamid, M., Abulmalek, K., Phillips, D., Matar, K., Sharma, P., & Thusu, A. (2007). Therapeutic drug monitoring of gentamicin: evaluation of five nomograms for initial dosing at Al-Amiri Hospital in Kuwait. Medical Principles and Practice, 16(5), 348–354. https://doi.org/10.1159/000104807
- Al-Sulaiti, F. K., Nader, A., El-Mekaty, E., Elewa, H., Al-Badriyeh, D., El-Zubair, A., Saad, M. O., & Awaisu, A. (2020). Vancomycin therapeutic drug monitoring service quality indices and clinical effectiveness outcomes: A retrospective cohort and clinical audit. JACCP: Journal of the American College of Clinical Pharmacy, 3(4), 778–785. https://doi.org/10.1002/jac5.1223
- Al Za'abi, M., Al Muqbali, J., & Al-Waili, K. (2015). Sampling time and indications appropriateness for therapeutically monitored drugs at a teaching university hospital in Oman. Saudi Pharmaceutical Journal, 23(4), 458–462. https://doi.org/10.1016/j.jsps.2014.11.005
- Batton, D. G., Barrington, K. J., & Wallman, C. (2006). Prevention and management of pain in the neonate: an update. Pediatrics, 118(5), 2231–2241. https://doi.org/10.1542/peds.2006-2277
- Bauer, L. A. (2008). Applied clinical pharmacokinetics. McGraw Hill.
- Ben Romdhane, H., Ben Fredj, N., Chaabane, A., Ben Aicha, S., Chadly, Z., Ben Fadhel, N., Boughattas, N., & Aouam, K. (2019). Interest of therapeutic drug monitoring of aminoglycosides administered by a monodose regimen. Néphrologie & Thérapeutique, 15(2), 110–114. https://doi.org/10.1016/j.nephro.2018.08.004
- Crcek, M., Zdovc, J., & Kerec Kos, M. (2019). A review of population pharmacokinetic models of gentamicin in paediatric patients. Journal of Clinical Pharmacy and Therapeutics, 44(5), 659–674. https://doi.org/10.1111/jcpt.12850
- Crist, K. D., Nahata, M. C., & Ety, J. (1987). Positive impact of a therapeutic drug-monitoring program on total aminoglycoside dose and cost of hospitalization. Therapeutic Drug Monitoring, 9(3), 306–310. https://doi.org/10.1097/00007691-198709000-00010
- Dandachi, I., Chaddad, A., Hanna, J., Matta, J., & Daoud, Z. (2019). Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the Middle East using a one health approach. Frontiers in Microbiology, 10(1941). https://doi.org/10.3389/fmicb.2019.01941
- du Toit, M., Burger, J. R., Rakumakoe, D. M., & Rheeders, M. (2019). Standards of aminoglycoside therapeutic drug monitoring in a South African private hospital: perspectives and implications. Ghana Medical Journal, 53(1), 8–12. https://doi.org/10.4314/gmj.v53i1.2
- Flannigan, C., Kilpatrick, S., Redpath, J., & Hogan, M. (2010). 481 Can a gentamicin specific chart reduce neonatal medication errors? Pediatric Research, 68(1), 245–246. https://doi.org/10.1203/00006450-201011001-00481
- Gilbert, D. N., Eliopoulos, G. M., Chambers, H. F., Saag, M. S., & Pavia, A. (2019). The Sanford guide to antimicrobial therapy 2019. Antimicrobial Therapy, Inc.
- Hartman, S. J. F., Brüggemann, R. J., Orriëns, L., Dia, N., Schreuder, M. F., & de Wildt, S. N. (2020). Pharmacokinetics and target attainment of antibiotics in critically ill children: a systematic review of current literature. Clinical Pharmacokinetics, 59(2), 173–205. https://doi.org/10.1007/s40262-019-00813-w
- HMC: Hamad Medical Corporation. (2006–2020). Antimicrobial prescribing policy. Hamad Medical Corporation.
- HMC: Hamad Medical Corporation. (2016). Annual report (Annual report). Hamad Medical Corporation.
- Ho, K. K., Thiessen, J. J., Bryson, S. M., Greenberg, M. L., Einarson, T. R., & Leson, C. L. (1994). Challenges in comparing treatment outcome from a prospective with that of a retrospective study: assessing the merit of gentamicin therapeutic drug monitoring in pediatric oncology. Therapeutic Drug Monitoring, 16(3), 238–247. https://doi.org/10.1097/00007691-199406000-00003
- Hodiamont, C. J., Janssen, J. M., de Jong, M. D., Mathôt, R. A., Juffermans, N. P., & van Hest, R. M. (2017). Therapeutic drug monitoring of gentamicin peak concentrations in critically ill patients. Therapeutic Drug Monitoring, 39(5), 522–530. https://doi.org/10.1097/FTD.0000000000000432
- Ismail, R., Sarriff, A., & Abdul Rahman, A. F. (1990). Therapeutic drug monitoring for gentamicin in Hospital Universiti Sains Malaysia. Medical Journal of Malaysia, 45(1), 57–64.
- Kadambari, S., Heath, P. T., Sharland, M., Lewis, S., Nichols, A., & Turner, M. A. (2011). Variation in gentamicin and vancomycin dosage and monitoring in UK neonatal units. Journal of Antimicrobial Chemotherapy, 66(11), 2647–2650. https://doi.org/10.1093/jac/dkr351
- Kheir, N., Awaisu, A., Gad, H., Elazzazy, S., Jibril, F., & Gajam, M. (2015). Clinical pharmacokinetics: perceptions of hospital pharmacists in Qatar about how it was taught and how it is applied. International Journal of Clinical Pharmacy, 37(6), 1180–1187. https://doi.org/10.1007/s11096-015-0183-3
- Krzyżaniak, N., Pawłowska, I., & Bajorek, B. (2016). Review of drug utilization patterns in NICUs worldwide. Journal of Clinical Pharmacy and Therapeutics, 41(6), 612–620. https://doi.org/10.1111/jcpt.12440
- Lee, J., Yoon, S., Shin, D., Han, H., An, H., Lee, J., Lim, K. S., Yu, K. S., & Lee, H. (2014). Predictive performance of gentamicin dosing nomograms. Drug Design, Development and Therapy, 8, 1097–1106. https://doi.org/10.2147/DDDT.S66981
- Lim, W. X. S., Chua, W. B. B., Chua, J. M., Lee, Q., Chan, J. W., Sultana, R., & Poh, B. H. (2020). A retrospective review of the efficiency of first-dose therapeutic drug monitoring of gentamicin, amikacin, and vancomycin in the pediatric population. The Journal of Clinical Pharmacology, 60(1), 7–15. https://doi.org/10.1002/jcph.1509
- Mijović, G., Čizmović, L., Vuković, M. N., Stamatović, S., & Lopičić, M. (2020). Antibiotic consumption in hospitals and resistance rate of Klebsiella pneumoniae and Escherichia coli in Montenegro. Acta Clinica Croatica, 59(3), 469–479. https://doi.org/10.20471/acc.2020.59.03.11
- Ohnishi, K., & Mikamo, H. (2013). Results of a questionnaire survey on the use of gentamicin sulfate injection. Kansenshogaku Zasshi, 87(3), 357–367. https://doi.org/10.11150/kansenshogakuzasshi.87.357
- Reynolds, L. F., Mailman, T. L., & McMillan, D. D. (2012). Gentamicin in neonates at risk for sepsis – Peak serum concentrations are not necessary. Paediatrics & Child Health, 17(6), 310–312. https://pubmed.ncbi.nlm.nih.gov/23730168
- Roberts, J. A., Norris, R., Paterson, D. L., & Martin, J. H. (2012). Therapeutic drug monitoring of antimicrobials. British Journal of Clinical Pharmacology, 73(1), 27–36. https://doi.org/10.1111/j.1365-2125.2011.04080.x
- Saddi, V., Preddy, J., Dalton, S., Connors, J., & Patterson, S. (2017). Variation in gentamicin dosing and monitoring in pediatric units across New South Wales. Pediatric Quality & Safety, 2(2), e015. https://doi.org/10.1097/pq9.0000000000000015
- Singu, B. S., Mubita, M., Thikukutu, M. M., Mufenda, J. K., McKenzie, S. B., & Verbeeck, R. K. (2018). Monitoring of gentamicin serum concentrations in obstetrics and gynaecology patients in Namibia. International Journal of Clinical Pharmacy, 40(3), 520–525. https://doi.org/10.1007/s11096-018-0626-8
- Sweileh, W. M. (2009). A prospective comparative study of gentamicin- and amikacin-induced nephrotoxicity in patients with normal baseline renal function. Fundamental & Clinical Pharmacology, 23(4), 515–520. https://doi.org/10.1111/j.1472-8206.2009.00702.x
- Valitalo, P. A., van den Anker, J. N., Allegaert, K., de Cock, R. F., de Hoog, M., Simons, S. H., Mouton, J. W., & Knibbe, C. A. (2015). Novel model-based dosing guidelines for gentamicin and tobramycin in preterm and term neonates. Journal of Antimicrobial Chemotherapy, 70(7), 2074–2077. https://doi.org/10.1093/jac/dkv052