?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This work reports a green synthesis of CuO/NiO nanocomposite with different CuO/NiO ratios. The X-ray diffraction (XRD) confirmed the existence of both CuO and NiO. This was also confirmed by energy dispersive X-ray (EDX). Scanning electron microscopy (SEM) reveals that changing the compositions of the nanocomposites resulted in different surface morphologies with different particle shapes, sizes, and levels of aggregation. Nitrogen adsorption-desorption analysis showed microporous structures with pore size in the range of 1.66-1.75 nm and surface area in the range of 82-513 m2g−1. The supercapacitive activity was examined by cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD). The results display that the 1C3N exhibited the highest capacitive performance of 1072 Fg−1 compared to 760, 716, and 260 Fg−1 for 3C1N, 4 N, and 4 C, respectively. Furthermore, the 1C3N electrode shows a cyclic stability of 72% after 4000 cycles, which makes it a promising candidate for supercapacitive applications.
Introduction
The production and utilization of green energy have emerged as an essential need in the current world. The growing global population, shrinking of fossil fuels, and environmental concerns have led to the reevaluation of conventional energy sources [Citation1,Citation2]. In this context, alternative sustainable energy stands out as a promising solution to address the threatening situation caused by these challenges [Citation3]. Accordingly, the demand for efficient energy storage systems has been essential, with the growing emphasis on renewable energy sources and the electrification of various sectors [Citation1,Citation4]. Supercapacitors emerged as promising candidates in this sector due to their high power density, low equivalent series resistance, rapid charge-discharge cycles, longer operational lifetimes compared to traditional batteries, excellent cyclic stability [Citation5], and safe operation conditions [Citation3]. Furthermore, they provide higher specific capacitance and energy density than electric double-layer capacitors due to the multiple oxidation states of their electroactive materials leading to faster faradaic reactions [Citation5,Citation6]. However, it still has some drawbacks such as low energy density [Citation2,Citation7–9].
Based on energy storage mechanisms, supercapacitors are classified into faradic, non-faradic, and hybrid capacitors. Faradic (pseudocapacitors) involve reversible redox reactions, while in non-faradic (electric double-layer capacitors), the energy storage process does not involve a redox reaction between the electrolyte and the electrode [Citation5]. Hybrid supercapacitors combine both faradic and non-faradic processes [Citation3,Citation10]. The active electrode material is considered the most important component in electrochemical supercapacitors. Transition metal oxides show excellent capacitive behavior for pseudocapacitors application as a result of their fast and reversible redox reactions with the electrolyte [Citation11,Citation12] and higher energy density compared to carbon-based materials. However, they show low stability and poor electrical conductivity, which limit commercial applications [Citation13,Citation14].
Consequently, the most challenging task lies in the modification of eco-friendly and cost-effective electrodes to enhance the performance of supercapacitors [Citation1]. The key lies in the development of advanced electrode materials, which depend mainly on the electrochemical characteristics of the electrodes and the techniques employed in producing these materials to ensure high electrical conductivity and specific capacitance to effectively improve the energy density of the supercapacitors [Citation2,Citation3]. Recently, researchers have increasingly turned to sustainable approaches, particularly green synthesis methods, for the production of electrode materials. This novel approach aligns with the global movement towards green and sustainable technologies, reflecting a focused effort to address not only the efficiency of energy storage materials but also their environmental impact [Citation12,Citation15,Citation16].
Copper oxide (CuO) and nickel oxide (NiO) have attracted significant attention in recent years for their exceptional electrochemical properties [Citation17,Citation18]. Nickel oxide nanoparticles (NiO) exhibit attractive characteristics that make them highly recommended for various technological applications. With a unique combination of electrical, optical, and catalytic properties, NiO nanoparticles have gained significant attention in the field of energy storage and conversion. Their tunable properties, high stability and flexibility, well-defined structure, and high surface area contribute to enhanced electrochemical performance, making them excellent candidates for supercapacitors and batteries [Citation17]. On the other hand, copper oxide (CuO) nanoparticles exhibit a rich structure of characteristics that make them highly attractive for a wide range of applications. CuO nanoparticles possess excellent electrical conductivity and a high surface area, making them ideal for application in electronic devices, sensors, energy storage, and catalysts [Citation19]
The combination of metal oxides in a nanocomposite structure shows interactions that can further promote the overall performance of supercapacitors [Citation17]. Mixing of metal oxides improves electrochemical performance by enhancing both the electrochemical active surface area and charge transfer efficiency [Citation19]. Additionally, combining metal oxide nanomaterial with a narrow band gap with that of a broadband gap may result in a composite with enhanced physicochemical properties compared to individual materials. It was reported that the blending of binary and ternary metal oxides induces the formation of oxygen vacancies on the composite metal oxide surface [Citation1]. Consequently, this alters the electronic structure and morphology, thereby promoting enhanced electrochemical performance [Citation15]. In the area of transition metal oxides, the p-type CuO shows a narrow band gap of 1.2 eV, while the p-type NiO exhibits a broader band gap ranging from 3.6 to 4.0 eV [Citation3]. Ahmad et al. synthesized CeO2/NiO nanocomposite via a hydrothermal route and found that the nanoflakes combined into flower-like morphology with different oxidation states of Ce and Ni. They reported that the electrochemical performance of CeO2/NiO nanocomposite is higher than that of pure ceria or nickel oxide [Citation20]. Nuamah et al. [Citation6] synthesized Ni/NiO nanocomposite with a remarkable specific capacitance of 2000 Fg−1 and a capacitance retention of 98.6%, after 800 cycles [Citation6]. Mohamed Racik et al. [Citation19] reported an enhancement of the specific capacitance of MnO2/CuO nanocomposite (279.12 Fg−1) compared to that of MnO2 (175.51 Fg−1) and CuO (191.06 Fg−1) [Citation19]. Isacfranklin et al. [Citation17] synthesized NiO-CoO nanocomposite with enhanced specific capacitance compared to that of CoO and NiO with values of 168, 169, and 298 Fg−1respectively. The hybrid electrode showed a 94.30% cyclic performance after 5000 charge-discharge cycles [Citation17]. Yuan et al. [Citation18] prepared NiO/MnO2 nanocomposite hydrothermally and examined its electrochemical performance. They reported an enhancement in the activity of NiO/MnO2 nanocomposite compared to that of NiO and MnO2 [Citation18]. Kanaujiya et al. [Citation13] reported the synthesis of CoMn2O4@MoS2 nanocomposite via the coprecipitation route. The CoMn2O4@MoS2 nanocomposite showed enhanced electrochemical performance compared to that of CoMn2O4 and MoS2. According to them, the interaction between CoMn2O4 and MoS2 resulted in a larger specific surface area with a higher number of oxygen vacancies in the nanocomposite leading to an enhanced supercapacitor performance [Citation13]
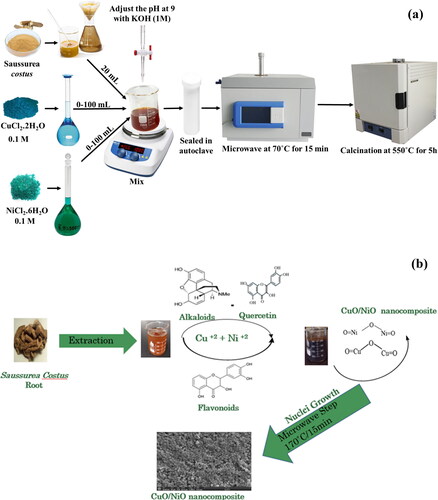
Saussurea costus, commonly known as 'Kuth’ or 'Costus,' is a medicinal plant famous for its phytochemical richness. Utilizing the reducing properties of the plant extract provides a sustainable and eco-friendly substitute for conventional synthesis techniques, Saussurea costus extract is distinguished by its high content of bioactive compounds such as alkaloids, cardiac glycosides, coumarins, flavonoids, phenols, quinones, resins, steroids, tannins and terpenoids [Citation21]. This was also confirmed by [Citation22], who investigated the presence of terpenoids, alkaloids, steroids, flavonoids and resins as mainly phytoconstituents in Saussurea costus, root extract. The phytochemical profile of Saussurea costus root extract was investigated also by [Citation23], using GC-MS analysis. The results confirmed the existence of 69 compounds. The percentages of the phytochemicals were found as follows: alkaloids, 5%; terpenoids, 79%; phenolic compounds, 4%; hydrocarbons, 7%; and sterols, 6% [Citation24]. These compounds highlight its use as a green reducing agent in novel materials synthesis processes, as demonstrated in the synthesis of CuO/NiO2 nanocomposites for supercapacitor energy storage applications. Furthermore, these bioactive compounds in the plant extract function as a capping agent to minimize the aggregation of the nanoparticles. Thus, it helps in maintaining a high specific surface area, which is crucial for supercapacitor applications [Citation25]. A proposed mechanism for the phyto-rule of Saussurea costus extract in the synthesis of CuO/NiO nanocomposites is demonstrated in .
Figure 1. A Schematic diagram of the synthesis procedure that was followed according to the molar ratios is shown in . A proposed mechanism for the rule of the saussurea costus extract in the synthesis of CuO/NiO nanocomposites (b).
Nevertheless, despite extensive research into the nanocomposite fabrication of binary and ternary metal oxide nanomaterials with modified surface chemistry, it still faces many limitations in terms of large-scale production, complicated techniques, and the use of hazardous and toxic chemicals and reagents [Citation3,Citation15]. Consequently, achieving sustainable surface modification of mixed metal oxides for charge storage remains a challenging task within the scientific community. Thus, this work introduces a combination of different approaches to the fabrication of CuO/NiO nanocomposites by utilizing Saussurea costus extract as a capping and reducing agent in combination with the hydrothermal route of synthesis with the aid of microwave in an attempt to minimize chemical consumption, simplify the synthesis conditions such as hydrothermal temperature and time. Merging the potential of Saussurea costus extract aims at expanding eco-friendly synthesis alternatives in energy storage technologies. The findings presented herein not only advance our understanding of CuO/NiO nanocomposites but also emphasize the importance of material design in shaping a more sustainable energy future.
Materials and method
Chemicals
Saussurea costus plant was obtained from the local market of the Hofuf area, Al-Ahsa, KSA. Copper (II) chloride dihydrate (CuCl2.2H2O), Nickel(II) chloride hexahydrate (NiCl2.6H2O), and potassium hydroxide (KOH) were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany). All chemicals and reagents were of analytical grades and used as supplied.
Synthesis of the Saussurea costus extract (SCE)
To prepare the plant extract, 25 g of the plant-dried powder was added to 250 mL of boiling water. The mixture was stirred and boiled for 10 min and then left at room temperature to cool down. Finally, the extract was filtered and kept in the fridge till being used.
Synthesis of the nanocomposite
shows a summary of the synthesis procedure that was followed according to the molar ratios shown in . First, specific volumes of Nickel(II) chloride hexahydrate (0.1 M) and Copper(II) chloride dihydrate (0.1 M) solutions were mixed with a specific volume of the SCE according to Table l. Then KOH (1 M) was added to adjust the pH to 9 with the aid of an Orion star pH meter. The mixture was stirred at 100 rpm and ambient temperature for 15 min to allow precipitation, which can be visually noticed by turning the clear solution into turbid. The mixture was then transferred into sealed autoclaves and treated in the microwave reactor at 170 °C for 1h. The slurry was then centrifuged, and double-washed with distilled water. The final product was then calcined in an oven at 550 °C for 5h.
Table 1. The molar composition of the nanocomposite prepared.
Electrode fabrication and electro-testing
In this work, all the electrochemical measurements are done on Nickel foam substrate (Ni foam) as a current collector due to its porous structure and its outstanding bone which extends good mechanical stability. The electrochemical activity of the fabricated electrodes was examined using a half-cell assembly with a 2 M KOH electrolyte. The reference electrode was Ag/AgCl (3 M KCl) and the counter electrode was platinum. A slurry containing 10% PVDF, 10% carbon black, and one of the nanocomposites (4 C, 3C1N, 1C3N, and 4 N) in the NMP solvent as the active material (1 mg) and then was dried in an oven at 80 °C. Electrochemical measurement was carried out in 2 M KOH aqueous electrolytic solution using Galvanostatic charge/discharge (GCD) and cyclic voltammetry (CV) analysis. Cyclic voltammetry was performed at different scan rates over the potential range of 0–0.55 V, whereas the galvanostatic charge-discharge measurements were carried out at different current loads in the same 0–0.5 V potential range.
Characterization
Nitrogen adsorption-desorption was used to measure the BET surface area, pore size, and pore volume of the samples using NOVA 4200e, Quantachrome Instruments, B Beach, FL, USA. To explore the crystallinity of the samples, X-ray diffraction with a Cu-detector over the 2Ɵ range of 10-80˚ was performed with XRD-7000, Shimadzu, Tokyo, Japan. Thermal gravimetric analysis (TGA) was used to investigate the thermal stabilities of the samples with TGA-51 Thermogravimetric Analyzer, Shimadzu, Tokyo, Japan. The surface structure and morphology were studied with scanning electron microgram (SEM) imaging using Thermo Scientific, Quattro S, USA. The particle size distribution of the nanocomposite was measured with dynamic light scattering (DLS) analysis using Thermo Scientific, Quattro S, USA. FTIR analysis for the wavenumber range of 4000-4000 cm−1 was used to select the active functional groups available on the surface with Thermo Scientific, Quattro S, USA. The electrochemical properties of the electrode materials were analyzed using cyclic voltammetry and galvanostatic charge-discharge techniques, performed on a Nova Auto Lab (Metrohm) electrochemical workstation.
The capacitance, power density, and energy density calculation
The specific capacitance was calculated using the given formula;
Where Cs represent the calculated specific capacitance in Fg−1, dt represent the discharge time, dV represent the operating potential window, I represents the applied current, and m corresponds to the mass of the coated active materials over the electrodes [Citation26].
Where ∆V represent the voltage drop in the linear portion of the discharge curve, Cs represent the specific capacitance calculated from the symmetric assembly cell, and ∆t corresponds to the discharge time [Citation27].
Results and discussion
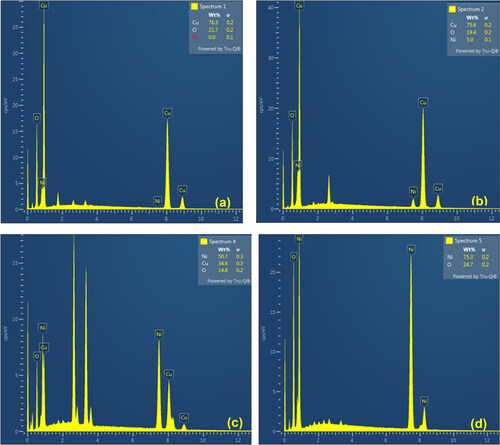
The SEM micrographs of the nanocomposites are shown in at two different magnifications. The 4 C images in show spherical particles with different sizes and high level of aggregation. This aggregation is mainly because of the nano-size crystals which leads to large surface energy, and thus particles tend to aggregate to this surface energy [Citation28]. This aggregation of the particles resulted in a very microporous structure as will be discussed later leading to hindered diffusion since most of the surface area will be within the porous structure. A similar observation of CuO nanoparticles prepared by coprecipitation at room temperature was reported by [Citation29]. Mohamed Racik et al. [Citation19] prepared spherical CuO nanoparticles by hydrothermal approach and reported that the spherical structure promotes the bonding between the electrolyte and the active electrode, and thus facilitates the transfer of electrons [Citation19]. In a previous contribution to our research group, CuO with bipyramidal morphology was prepared hydrothermally with the aid of Acacia nilotica pod extract but without a microwave [Citation21,Citation30]. show the morphology of 3C1N. It seems that the particles are mainly spherical with a narrow particle size distribution compared to 4 C. Similar to 4 C, 3C1N shows some aggregation resulting in diffusion limitation. By increasing the percentage of NiO in sample 1C3N, the morphology tends to be of polygonal shapes with sharp edges and flat surfaces with some aggregation as shown in . This morphology is expected to minimize diffusion resistance and thus improve the electrochemical performance as reported elsewhere for cubic shape morphology since most of the surface area is external and not within the pores as in the case of 4 C and 3C1N [Citation11]. Chatterjee et al. [Citation3] reported an aggregated nanostructures consisted of parallel nanosheets for a nanocomposite with CuO/NiO of 1. They attributed the high degree of ion diffusion between the electrolyte and the electrode and thus the high capacitance to this morphology [Citation3]. For sample 4 N, the morphology is shown in (g& h) and it reveals irregular shapes with a wide range of particle size distribution but a lower level of aggregation. Yuan et al. [Citation18] synthesized NiO by a two-step method via chemical deposition and hydrothermal and reported nanoflake morphology [Citation18]. The successful combination of NiO and CuO is confirmed by the EDS analysis shown in . Moving from 4 C to 4 N through 3C1N and 1C3N resulted in increasing the content of Ni and decreasing that of Cu. Furthermore, for all samples only Cu, Ni, and O appear without any traces of Cl in the precursor salt revealing the successful conversion of CuCl2.2H2O and NiCl2.6H2O into CuO and NiO, respectively. It worth mentioning that the composition of 3C1N and 1C3N obtained by EDS analysis is not matching with the precursor’s ratios used in synthesis. This is mainly related to different rate of precipitation of Cu(OH)2 (Ksp = ) and Ni(OH)2 (Ksp =
). The one with lowest solubility will tend to precipitate faster than the other and thus, the mass ratio of Cu/Ni (15:1) in sample 3C1N as shown in is higher than the precursors ratio of 3.3:1. Same analysis is applicable to sample 1C3N.
The crystallinity of the fabricated nanocomposites was determined by XRD which is presented in . The diffraction peaks of 4 C at 32.73°, 35.78°, 38.97°,49.14°, 54.10°, 58.37°, 61.10°, 66.64°, 68.51°, 72.87°, and 75.83° resemble the (110), (-111), (111), (-202), (020), (202), (-113), (-311), (220), (311) and (-222) planes of monoclinic CuO (JCPDS card No. 80-1268) [Citation3]. The diffraction peaks of 4 N at 37.45°, and 43.65° are associated with (111), and (200) planes of face-centered-cubic NiO (JCPDS card No. 78-0429), respectively [Citation3,Citation20,]. The diffraction peaks of 3C1N and 1C3N nanocomposites at 36.20°, 49.60°, 54.2°, 59.10°, 61.10°, 66.29°, 68.80°, 72.65°, 75.43° correspond to the (-111), (-202), (020), (202), (-113), (-311), (220), (311), (-222) planes of monoclinic CuO (JCPDS card No. 80- 1268), while the peaks at 37.2°, and 43.29°Correspond to (111) and (200) planes of face-centered-cubic NiO (JCPDS card No. 78-0429), respectively [Citation3,Citation20,] The peak appears in 4 C, 1C3N, and 3C1N at 28° is related to monoclinic Cu2O phase (xrd reference).
Figure 4. XRD analysis (a), TGA analysis (b), FTIR analysis (c), DLS, and BET analysis (d) for 4 C, 3C1N, 1C3N, and N.
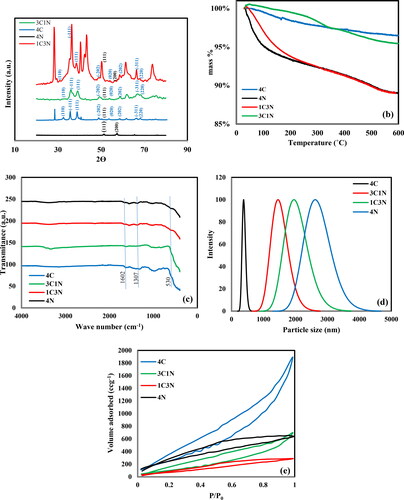
The thermal stability of the nanocomposites was tested in the temperature range of 20-600 °C with a temperature ramp of 2 °C/min as shown in . The surface area of the nanocomposite plays a crucial role in determining the amount of adsorbed water and accordingly the sample behavior upon TGA treatment. The high surface area of 513 m2g−1 for sample 4 C (] is expected to provide more active sites for water molecules to be adsorbed onto the surface, and thus it is expected for this sample to show the highest mass loss. However, as the temperature rises during TGA, a slow and gradual release of adsorbed water, results in a total mass loss of 5% compared to a total loss of 10% for 4 N and 1C3N which have a lower surface area of 148 and 82 m2g−1, respectively. This behavior sheds light on the influence of the pore size on the adsorption-desorption of water molecules. Smaller pores may restrict the diffusion of water molecules, leading to a smaller amount of adsorbed water. Consequently, these materials may exhibit a delayed and more gradual mass loss during TGA. On the other hand, larger pores allow for easier adsorption-desorption of water, resulting in a quicker and more pronounced mass loss during TGA. Furthermore, most of the surface area is located within the pores and if the pore size is small then not all the surface area will be accessible for water molecules. This interaction between surface area and pore size is critical in understanding the overall behavior of mass loss in TGA. Materials with a combination of high surface area and a well-defined pore structure often exhibit enhanced water adsorption-desorption characteristics. The surface area provides adsorption sites, while the pore size influences the accessibility and ease of adsorption-desorption. Understanding this mass loss of adsorbed water in TGA is essential for understanding the electrochemical behavior of the electrodes as will be discussed later.
Table 2. The specific surface area (BET), pore size, and pore volume obtained by nitrogen adsorption-desorption.
displays the FTIR spectra for all prepared nanocomposites in the range 4000-400 cm−1. All the samples show some common peaks 3400 (wide peak), 1600, 1300, and 530 cm−1are related to the stretching vibration of the OH group, the bending vibrations of adsorbed water molecules and OH groups on the surface, bending vibrations of Cu–O–H and Ni-O-H, and the Cu–O and Ni-O bond, respectively [Citation31]. shows the dynamic light scattering measurement of the particle size distribution for the four samples. The 4 C displays the smallest particle size indicating either a lower rate of particle growth or a lower tendency of aggregation, this small particle size resulted in the highest surface area as will be discussed next. On the other hand, 4 N which is made of NiO, shows the largest particle size. For 3C1N and 1C4N the particle size is related to the percentage of NiO, the higher its content, the larger the particle size. shows the nitrogen adsorption-desorption isotherms for the four samples. According to the IUPAC classification, they all have type IV isotherm with a hysteresis loop. The nitrogen adsorption-desorption results presented in and Figures S1–S4 (supplementary material) show that all samples have microporous structures with pore diameters less than 2 nm. These microporous structures hinder molecular diffusion within the pores due to limited accessibility and constrained pathways. The critical interaction between pore size, surface area, and diffusion limitations is obvious in applications such as supercapacitors. The significance of pore size in controlling supercapacitor performance is highlighted by the results. Despite 4 C boasting the highest surface area at 513 m2g−1, its electrochemical performance is notably lower at 260 Fg−1 compared to 3C1N and 1C3N, with surface areas of 189 m2g−1 and 82 m2g−1, respectively. However, 3C1N and 1C3N demonstrate higher capacitance values of 760 Fg−1 and 1072 Fg−1. Worth mentioning is the case of 4 N, with a surface area of 148 m2g−1, yet showing a capacitance of 716 Fg−1. This trend suggests that pore size significantly influences performance in coincidence with surface area. Samples with larger pore sizes (1.75) beat others, emphasizing the importance of accessibility to active sites. The microporous structure’s potential to cause diffusion-limited performance highlights the need for a balanced combination of surface area and pore size for optimal supercapacitor functionality in microporous materials.
Table 3. Reported nanocomposites and their characteristics and performance in supercapacitors.
Electrochemical measurement
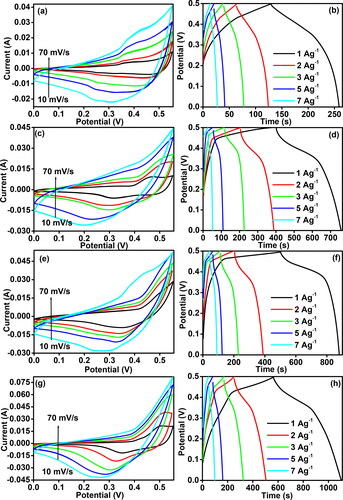
Cyclic voltammetry curves were prepared for the four electrodes to investigate their capacitance and oxidation-reduction behaviour. represents the comparative CV curves of 4 C, 3C1N, 1C3N, and 4 N using a scan rate of 10 mVs−1 in the potential range of 0.0–0.55 V. The CV profiles show the anodic and cathodic peaks, confirming the occurrence of the redox reaction during the electrochemical energy storage procedure. Furthermore, these profiles confirm the pseudo capacitance behaviour of the electrochemical energy storage process. The cyclic voltammetry curves exhibit distinct redox peaks which are characteristic of the electrochemical behavior of nickel oxide and copper oxide. These peaks correspond to the oxidation and reduction processes intrinsic to these materials. For NiO, the peaks typically represent the Ni(II)/Ni(III) transitions, while for CuO, they correspond to the Cu(I)/Cu(II) redox reactions. These electrochemical activities are critical for their application in energy storage technologies, as they directly influence the charge storage capabilities of the electrode materials. shows that the CV curve of 1C3N occupies the largest area compared to 4 C, 3C1N, and N under the same scanning conditions, suggesting the best electrochemical capacitance performance among all electrodes. In , the CD curves depict the performance of 4 C, 3C1N, 1C3N, and 4 N within the 0-0.5 V potential range, measured at a current density of 1 Ag−1. Notably, the CD duration followed the order 1C3N> 3C1N> 4 N > 4 C. Moreover, as presented in , 1C3N exhibited the highest specific capacitive value for a current density of 1 Ag−1. shows an inverse relationship between the specific capacitance and the current density for all electrodes. Increasing the current density decreases the diffusion of the ionic species over the electrode surface and thus decreases specific capacitance. It also shows that 1C3N retained 750 Fg−1 even at a higher current load of 7 Ag−1, with almost 68% of the capacitance retained as compared to that measured at 1 Fg−1. display the CV curves of 4 C, 4 N, 3C1N, and 1C3N, respectively at different scan rates within the range 10-70 mVS−1. For each electrode, the CV curve shows an increase in the electrochemical response with increasing scan rates. Furthermore, increasing the scan rate shifted the oxidation peaks to the positive potential and the reduction peaks to the negative potential confirming the pseudocapacitive behaviour of the electrodes. It also highlighted the charge-diffusion polarization in the pseudocapacitive electrodes, which is taking place during charging and discharging cycles. As ions are consumed or produced at the surface of the electrode during charge/discharge cycle, a concentration gradient is created leading to ionic diffusion from the high concentration to low concentration region to continue the redox reactions. However, if the rate of ionic diffusion is slower than the rate of consumption or production of ions at the electrode-electrolyte interface, a concentration gradient develops, leading to polarization. This polarization results in a drop in voltage across the electrode and decrease the rate of charge transfer and thus, lower the efficiency of the pseudocapacitive electrode [Citation11].
Figure 5. CV Curves at 10 mVs−1(a), CD curves at 1 Ag−1 (b), the specific capacitance at various scan rates (c), and (d) bar graph of 4 C, 3C1N, 1C3N, and 4 N electrodes.
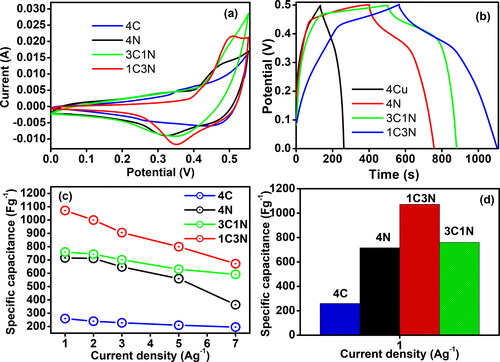
Figure 6. CV Curves of 4 C (a), CV curves of 4 N (c), CV curves of 3C1N (e) and CV curves of 1C3N (g) at different scan rates, CD graph of 4 C (b), CD graph of 4 N (d), CD graph of 3C1N (f) and CD graph of 1C3N (h) at different current density.
In general, 1C3N displayed excellent capacitive performance and cyclic stability compared to 4 C, 3C1N, and 4 N due to its polygonal-like shape and larger pore size which facilitate the approach of active sites for intercalation with electrolyte ions during the electrochemical reaction. It is well known that energy storage capacity of nanocomposites is strongly depending on their size, shape, surface area, and pore size. Smaller nanoparticles with high surface area is expected to have high energy storage capacity by providing more active sites for charge exchange. Furthermore, the size of nanoparticles can also affect the length of the diffusion path within the pores. Smaller nanoparticles with shorter diffusion paths shows faster charge/discharge rates and thus higher energy storage performance. Furthermore, pore size distribution can be a limiting factor in many cases if it was small enough to add diffusion limitation to the transfer process within the pores as in the case of sample 4 C which is having higher surface area compared to 1C3N. However, because of smaller particle size of 1.66 nm compared to 1.75 nm for 1C3N, the performance of 1C3N was better. The shape of nanoparticles can also influence the electrical conductivity of the nanocomposite. For instance, one-dimensional nanostructures like nanowires or nanotubes can provide efficient electron transport pathways, leading to improved charge transfer rate and higher energy storage capacity. Overall, controlling the size and shape of nanoparticles within nanocomposite can modify their structural and electrochemical properties for energy storage application.
The combination of CuO and NiO may enhance the nanocomposite 's performance compared to the individual components. Mixing of NiO with CuO may facilitate charge transfer, improve the electronic structure, and the reaction kinetics, leading to enhanced catalytic, optical, or electrical properties compared to individual counterparts. Furthermore, morphology of 3C1N and 1N3C, such as particle size and shape are different from that of CuO and NiO nanoparticles. The morphological changes induced by NiO may lead to more efficient utilization of active sites, and enhanced accessibility of reactants, which enhance the performance. The size and shape of nanocomposites critically influence their energy storage capacity primarily through effects on surface area, ion transport pathways, electrical conductivity, and packing density. Nanocomposites with larger surface areas offer more active sites for energy storage, while unique shapes can facilitate quicker and more efficient ion transport, enhancing the device’s performance. Moreover, the electrical properties and how these particles assemble can also play a significant role in optimizing the energy density and efficiency of energy storage devices. These factors collectively determine how effectively a nanocomposite can be used in applications such as batteries and supercapacitors. Furthermore, the interfacial interaction between CuO and NiO phases in the nanocomposite plays a crucial role in charge transfer between CuO and NiO nanoparticles, leading to synergistic effects improving the 1C3N and 3C1N performance over 4 C and 4 N as shown in . Also, by controlling the ratio of CuO to NiO, it is possible to modify the nanocomposite characteristics to achieve a certain goal. This tuning ability provides a significant advantage of nanocomposites over CuO and NiO nanoparticles. Thus, the better performance of 1C3N and 3C1N nanocomposites compared to 4 C and 4 N nanoparticles despite 4C’s and 4N’s higher surface area compared to 1C3N can be related to synergistic effects, improved morphological structure, and CuO/NiO interfacial interactions. All these factors contribute to the improved performance of 1C3N and 3C1N over 4 C and 4 N.
shows the Galvanostatic Charge-Discharge (GCD) curves of 4 C, 4 N, 3C1N, and 1C3N, respectively, at current densities of 1, 2, 3, 5 and 7 A g−1. The GCD curves for all electrode materials show that the charge/discharge period becomes longer by decreasing the current density. It also shows a signal similarity in all profiles, proposing a uniform electrochemical behaviour. These curves show a notable symmetry, confirming a high level of electrochemical reversibility through the materials [Citation4,Citation32]. Moreover, each charge-discharge curve shows a distinct charge-discharge stage, emphasizing the incidence of well-defined electrochemical processes. The plateau potential observed in the discharge curve aligns consistently with the reduction peak observed in the CV curves shown in . This alignment shows the electrode material’s efficiency in redox reactions and highlights its worthy electrochemical performance [Citation4] Overall, these findings together confirm the favourable electrochemical characteristics and strong redox capabilities of the investigated electrode material.
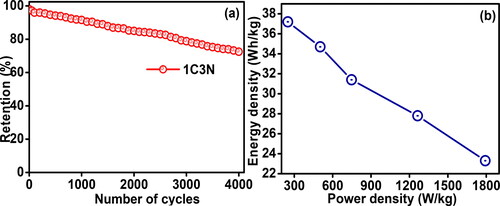
One major requirement for commercial application is the super capacitive performance stability, and thus the cyclic performance of the 1C3N electrodes was tested over 4000 cycles and the result is presented in . The stability of the 1C3N electrodes was tested for consecutive charge/discharge cyclic with a constant current rate. shows that the stability of 1C3N a slow and linear drop in the capacity of 1C3N is observed over the 4000 cycles because of the active site saturation during successive charge/discharge cycles. Moreover, the retention of 72% after 4000 cycles for 1C3N reveals excellent cycling stability compared to the previously reported CuO/NiO-based electrode reported elsewhere [Citation3]. This excellent stability of 1C3N is related to its polygonal shape morphology. Furthermore, the power Vs. energy density performance of the 1C3N electrode was analyzed through the Ragone plot shown in . shows a linear inverse relation between the energy density and the power density, which is like the result reported elsewhere [Citation11].
Conclusions
In summary, a facile green synthesis route was followed for the microwave-aided fabrication of CuO/NiO nanocomposite with Saussurea costus extract serving as a reducing and capping agent. The impact of changing the ratio of the precursor salts in the synthesis mixture on structural, morphological, and electrochemical activity was systematically investigated using different characterization techniques. Among the four samples, 1C3N (specific capacitance ∼1072 Fg−1) showed superior capacitive performance and cyclic stability (72% after 4000 cycles) compared to 4 C (specific capacitance ∼260 Fg−1) and 4 N (specific capacitance ∼716 Fg−1). These results highlight the advantage of mixing metal oxide to improve the performance of the resulting nanocomposite. The cubic-like morphology with sharp edges and flat smooth surface of 1C3N reduces diffusion times for electrolytic ions and electrons and lowers internal and charge transfer resistance during the electrochemical process. The 1C3N electrode’s excellent supercapacitive performance was achieved through a facile and green synthesis method, making it a very promising candidate for supercapacitor energy storage applications. It is worth mentioning that the particle dimension and the surface morphology can be optimized to improve the accessibility of the active sites within the porous structure through suitable modification of the synthesis conditions for more efficient performance.
Author contributions
Conceptualization, E.D., A.T., and N.P.; methodology, E.D., A.T., and N.P.; formal analysis, E.D., A.T., N.P., and M.R.E.; data curation, E.D., A.T., N.P., and M.R.E.; writing—original draft preparation, E.D.; writing—review and editing, E.D., and N.P.; project administration, E.D. All authors have read and agreed to the published version of the manuscript.
Supplementary materials.docx
Download MS Word (222.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is available upon request.
Additional information
Notes on contributors
Enshirah Da’na
Enshirah Da’na is an associate professor in chemical engineering department, Faculty of Engineering Technology, Al-Balqa Applied University.
Nazish Parveen
Nazish Parveen Assistant professor in the department of chemistry,King Faisal University, Alahsa, Saudi Arabia.
Amel Taha
Amel Taha Assistant professor in department of chemistry,King Faisal University, Alahsa, Saudi Arabia.
Mohamed R. El-Aassar
Mohamed R. El-Aassar Full professor in Department of Chemistry, College of Science, Jouf University, Sakaka 2014, Saudi Arabia.
References
- Chen GZ. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int Mater Rev. 2017;62(4):173–202. doi: 10.1080/09506608.2016.1240914.
- Krishnasamy K, Purushothaman KK. Preparation and characterisation of MnS@ Mn3O4/C nanoflakes for hybrid supercapacitor applications. Mater Technol. 2022;37(1):1–8. doi: 10.1080/10667857.2020.1810923.
- Chatterjee S, Ray A, Mandal M, et al. Synthesis and characterization of CuO-NiO nanocomposites for electrochemical supercapacitors. J Mater Eng Perform. 2020;29(12):8036–8048. doi: 10.1007/s11665-020-05261-3.
- Sidhu NK, Rastogi AC. Vertically aligned ZnO nanorod core-polypyrrole conducting polymer sheath and nanotube arrays for electrochemical supercapacitor energy storage. Nanoscale Res Lett. 2014;9(1):453. doi: 10.1186/1556-276X-9-453.
- Payami E, Teimuri-Mofrad R. A novel ternary Fe3O4@Fc-GO/PANI nanocomposite for outstanding supercapacitor performance. Electrochim Acta. 2021;383:138296. doi: 10.1016/j.electacta.2021.138296.
- Nuamah RA, Noormohammed S, Sarkar DK. Pulsed reverse potential electrodeposition of carbon-free Ni/Nio nanocomposite thin film electrode for energy storage supercapacitor electrodes. Coatings. 2021;11(7):780. doi: 10.3390/coatings11070780.
- Kumar R, Abdel-Galeil MM, Matsuda A, et al. One step synthesis Pd/NiO@rGO/CNTs nanocomposite for energy storage as supercapacitor application. J Phys: Conf Ser. 2020;1461(1):012109. doi: 10.1088/1742-6596/1461/1/012109.
- Nguyen VT, Ting JM. A redox-additive electrolyte and nanostructured electrode for enhanced supercapacitor energy Density. ACS Sustain Chem Eng. 2020;8(49):18023–18033. doi: 10.1021/acssuschemeng.0c05891.
- Shi HH, Naguib HE. Fabrication and characterization of polyaniline-graphene nanoplatelets composite electrode materials for hybrid supercapacitor applications. Behav Mech Multifunct Mater Compos. 2015;9432:94320U. doi: 10.1117/12.2083934.
- Sookhakian M, Basirun WJ, Teridi MAM, et al. Prussian blue-nitrogen-doped graphene nanocomposite as hybrid electrode for energy storage applications. Electrochim Acta. 2017;230:316–323. doi: 10.1016/j.electacta.2017.02.022.
- Aljaafari A, Parveen N, Ahmad F, et al. Self-assembled cube-like copper oxide derived from a metal- organic framework as a high- performance electrochemical supercapacitive electrode material. Sci Rep. 2019;9(1):9140. doi: 10.1038/s41598-019-45557-6.
- Çıplak Z, Yıldız A, Yıldız N. Green preparation of ternary reduced graphene oxide-Au@polyaniline nanocomposite for supercapacitor application. J Energy Storage. 2020;32(June):101846. doi: 10.1016/j.est.2020.101846.
- Kanaujiya N, Kumar N, Singh M, et al. CoMn2O4 nanoparticles decorated on 2D MoS2 frame: a synergetic energy storage composite material for practical supercapacitor applications. J Energy Storage. 2021;35(January):102302. doi: 10.1016/j.est.2021.102302.
- Tuichai W, Karaphun A, Ruttanapun C. Ag nanomaterials deposited reduced graphene oxide nanocomposite as an advanced hybrid electrode material for asymmetric supercapacitor device. J Alloys Compd. 2020;849:156516. doi: 10.1016/j.jallcom.2020.156516.
- Shaheen I, Shahzad Ahmad K, Zequine C, et al. Functionalization of MoO3[Sbnd]NiMoO4 nanocomposite using organic template for energy storage application. J Storage Mater. 2020;29:101309. doi: 10.1016/j.est.2020.101309.
- Teimuri-Mofrad R, Hadi R, Abbasi H, et al. Synthesis, characterization and electrochemical study of carbon nanotube/chitosan-ferrocene nanocomposite electrode as supercapacitor material. J Electron Mater. 2019;48(7):4573–4581. doi: 10.1007/s11664-019-07226-2.
- Isacfranklin M, Yuvakkumar R, Ravi G, et al. Hybrid NiO-CoO nanocomposite for high energy supercapacitor applications. Ceram Int. 2021;47(6):8486–8489. doi: 10.1016/j.ceramint.2020.11.215.
- Yuan YF, Lin JX, Zhang D, et al. Freestanding hierarchical NiO/MnO2 core/shell nanocomposite arrays for high-performance electrochemical energy storage. Electrochim Acta. 2017;227:303–309. doi: 10.1016/j.electacta.2017.01.002.
- Mohamed Racik K, Manikandan A, Mahendiran M, et al. Fabrication of manganese oxide decorated copper oxide (MnO2/CuO) nanocomposite electrodes for energy storage supercapacitor devices. Phys E: Low-Dimens Syst Nanostruct. 2020;119(February):114033. doi: 10.1016/j.physe.2020.114033.
- Ahmad N, Ali Alghamdi A, Al-Abdulkarim HA, et al. Structural, morphological, and electrochemical performance of CeO2/NiO nanocomposite for supercapacitor applications. Appl Sci (Switzerland). 2021;11(1):411. doi: 10.3390/app11010411.
- Nanocomposites. 2022. Characterization Zno-cuo. “Acacia Nilotica Pods’ Extract Assisted-Hydrothermal Synthesis.” 1–12.
- Choulhary GP. Phytochemical and pharmacological study of Saussurea Lappa Clarke: a review. Eur J Pharm Med Res. 2015;2(7):120–125.
- Idriss H, Siddig B, González-Maldonado P, et al. Inhibitory activity of Saussurea costus extract against bacteria, candida, herpes, and SARS-CoV-2. Plants (Basel). 2023; 12(3):460. doi: 10.3390/plants12030460.
- Abdallah EM, Qureshi KA, Ali AMH, et al. Evaluation of some biological properties of saussurea costus crude root extract. Biosci Biotech Res Comm. 2017;10(4):601–611. doi: 10.21786/bbrc/10.4/2.
- You B, Li N, Zhu H, et al. Graphene oxide-dispersed pristine CNTs support for MnO2 nanorods as high performance supercapacitor electrodes. ChemSusChem. 2013;6(3):474–480. doi: 10.1002/cssc.201200709.
- Chodankar NR, Dubal DP, Gund GS, et al. A symmetric MnO2/MnO2 flexible solid state supercapacitor operating at 1.6 V with aqueous gel electrolyte. J Energy Chem. 2016;25(3):463–471. doi: 10.1016/j.jechem.2016.01.020.
- Sanger AAK, Kumar A, Jain PK, et al. Silicon carbide nanocauliflowers for symmetric supercapacitor devices. Ind Eng Chem Res. 2016;55(35):9452–9458. doi: 10.1021/acs.iecr.6b02243.
- Srivastava PC. 2014. Realizing NiO nanocrystals from a simple chemical method realizing NiO nanocrystals from a simple chemical method. (August 2010). doi: 10.1007/s12034-011-0142-0.
- Yadav MS. Fabrication and characterization of supercapacitor electrodes using chemically synthesized CuO nanostructure and activated charcoal (AC) based nanocomposite. J Nanopart Res. 2020;22(10):303. doi: 10.1007/s11051-020-05027-x.
- Cuo S, Nanocomposites Z. 2022. Applied sciences catalytic degradation of anionic organic dye on greenly.
- Da’na E, Taha A, Afkar E. Green synthesis of iron nanoparticles by acacia nilotica pods extract and its catalytic, adsorption, and antibacterial activities. Appl Sci (Switzerland). 2018;8(10):1922. doi: 10.3390/app8101922.
- Huang YY, Lin LY. Synthesis of ternary metal oxides for battery-supercapacitor hybrid devices: influences of metal species on redox reaction and electrical conductivity. ACS Appl Energy Mater. 2018;1(6):2979–2990. doi: 10.1021/acsaem.8b00781.
- Yadav MS, Sinha AK, Singh MN. Electrochemical behaviour of ZnO-AC based nanocomposite electrode for supercapacitor. Mater Res Express. 2018;5(8):085503. doi: 10.1088/2053-1591/aad2c4.
- Le Z, Liu F, Nie P, et al. Pseudocapacitive sodium storage in mesoporous single-crystal-like TiO2-graphene nanocomposite enables high-performance sodium-ion capacitors. ACS Nano. 2017;11(3):2952–2960. doi: 10.1021/acsnano.6b08332.
- Nayak AK, Das AK, Pradhan D. High performance solid-state asymmetric supercapacitor using green synthesized graphene-WO3 nanowires nanocomposite. ACS Sustain Chem Eng. 2017;5(11):10128–10138. doi: 10.1021/acssuschemeng.7b02135.
- Ansarinejad H, Shabani-Nooshabadi M, Ghoreishi SM. Enhanced supercapacitor performance using a Co3O4@Co3S4 nanocomposite on reduced graphene oxide/Ni foam electrodes. Chem Asian J. 2021;16(10):1258–1270. doi: 10.1002/asia.202100124.
- Singh S, Sahoo RK, Shinde NM, et al. Synthesis of Bi2O3-MnO2 nanocomposite electrode for wide-potential window high performance supercapacitor. Energies. 2019;12(17):3320. doi: 10.3390/en12173320.
- Yu F, Tiong VT, Pang L, et al. Kostya (Ken) Ostrikov, and Hongxia Wang. 2019. “Flower-like Cu 5 Sn 2 S 7/ZnS nanocomposite for high performance supercapacitor. Chin Chem Lett. 2019;30(5):1115–1120. doi: 10.1016/j.cclet.2019.01.004.
- Alamro T, Ram MK. Polyethylenedioxythiophene and molybdenum disulfide nanocomposite electrodes for supercapacitor applications. Electrochim Acta. 2017;235:623–631. doi: 10.1016/j.electacta.2017.03.102.
- Zhang Y, Chang C R, Dong Jia X, et al. Influence of metallic oxide on the morphology and enhanced supercapacitive performance of NiMoO4 electrode material. Inorg Chem Commun. 2020;112(October 2019):107697. doi: 10.1016/j.inoche.2019.107697.
- Sohouli E, Teymourinia H, Ramazani A, et al. Preparation of high-performance supercapacitor electrode with nanocomposite of CuO/NCNO flower-like. Sci Rep. 2023;13(1):16221. doi: 10.1038/s41598-023-43430-1.