?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim: This study aimed to develop a topical antibiotic drug delivery system using aquasomes for enhanced treatment of skin and soft tissue infections (SSTIs). Materials & methods: Cephalothin was loaded into aquasomes using a multi-step process and optimized using design of experiment. The aquasomes were characterized for FT-IR, SEM and zeta potential analysis. Entrapment efficacy, In vitro drug release studies, antibacterial assays and stability study was performed to evaluate the efficacy of the formulated aquasomes. Results & conclusion: The formulated cephalothin-loaded aquasomes exhibited stable properties, controlled drug release and significant antibacterial activity against bacteria. This proves that the developed aquasome-based delivery system has the potential for sustained treatment of SSTIs.
Plain language summary
Cephalothin is a medicine that helps fight bacteria, but it doesn't work well on the skin because it does not come in a gel form. We created a special way, called a magic vehicle, to help the medicine reach the skin better. Infections on the skin and in soft tissues, caused by germs. We found that our new way of giving the medicine is small, strong and fights germs very well. This could be a great way to treat skin infections and help people feel better.
Background
Objective: The study aimed to formulate and characterize a topical antibiotic drug delivery system using aquasomes to enhance the treatment of skin and soft tissue infections (SSTIs).
Materials & methods
Drug selection and loading: cephalothin, a first-generation cephalosporin, was chosen and loaded into aquasomes through a multi-step process.
Aquasome structure: aquasomes are three-layered vesicular nanocarriers with a ceramic core coated with a polyhydroxy compound, specifically trehalose, for drug delivery.
Preparation: the ceramic core was coated with trehalose, followed by the adsorption of cephalothin. A 2-Factor design was utilized to optimize the formulation.
Characterization
Fourier transform infrared spectroscopy (FTIR): FTIR confirmed the presence of functional groups in the calcium phosphate core, trehalose-coated core and cephalothin-loaded aquasomes.
Scanning electron microscopy (SEM): SEM revealed the morphology of the aquasomes and demonstrated the adsorption of cephalothin onto the surface.
Zeta potential analysis: the analysis indicated the electrostatic stability of the aquasomes.
Drug release studies
Controlled release: the aquasomes exhibited controlled and sustained release of cephalothin, with a release rate of 60.72% over approximately 10 h.
Release kinetics: the Higuchi model best fit the release data of the formulation, indicating a diffusion-controlled release mechanism.
Entrapment efficiency: the formulation showed a 35% entrapment efficacy and demonstrated stable properties.
Antibacterial Activity
Efficacy against pathogens: antibacterial assays revealed significant efficacy of cephalothin-loaded aquasomes against Staphylococcus aureus and Escherichia coli.
Comparison with pure drug: cephalothin-loaded aquasomes produced larger zones of inhibition compared with pure cephalothin, highlighting the enhanced antibacterial activity.
Antimicrobial resistance: the study addresses the challenge of antimicrobial resistance by providing a sustained delivery system for existing antibiotics.
Stability studies
Physical stability: the cephalothin-loaded aquasomes demonstrated good stability over a three-month period, evidenced by consistent temperature maintenance, constant size, minimal fluctuations in encapsulation efficiency and unchanged physical appearance.
Discussion & conclusion
Potential for other antibiotics: the aquasome delivery system could be explored for other antibiotics and therapeutic agents to combat antimicrobial resistance in various infections.
Clinical applications: this study provides valuable insights into the development of an effective topical antibiotic delivery system with potential clinical applications in treating SSTIs.
Implications
Enhanced treatment: the innovative use of aquasomes for the sustained release of cephalothin offers a promising approach for improving the efficacy of topical antibiotics.
Future research: further research is suggested to explore the broader application of this delivery system for various antibiotics and to conduct clinical trials to validate its effectiveness in a clinical setting.
1. Background
Skin and soft tissue infections (SSTI) are very commonly caused by bacterial (viral and fungal are less compared with bacterial) that enters through the cuts in the skin and occurs majorly at subcutaneous tissue and connective tissues [Citation1,Citation2]. Staphylococcus aureus, Pseudomonas. and Streptococcus. are the most common infectious agents of SSTI which shows symptoms such as fever, nausea, vomiting and even leads to increase heart beat rates and severe pain [Citation3–5]. Existing diagnosis methods for SSTI includes colony isolation from blood, biopsy, x-ray, Ultrasound, & CT scan; and therapeutics options includes antibiotics course for a period of time [Citation6]. penicillin v, ciprofloxacin, amoxicillin, clindamycin and cephalexin are antibiotics commonly used for mild SSTIs and for severe infections, antibiotics such as penicillin g, vancomycin, cephalothin, ceftriaxone and ceftaroline are generally used [Citation7–10]. Among several types of antibiotics, cephalosporins such as cephalothin, cephalexin, ceftriaxone & ceftaroline are commonly used in SSTI infections [Citation11]. Additionally, the cephalothin (commonly called as Cefalotin) is most used first-class antibiotics that has β-lactam rings and acts against wide range of bacterial infections [Citation12,Citation13]. cephalothin is a potent antibiotic which is a thiophene-2-acetic acid derivative that acts against Staphylococcus and Streptococcus infections in SSTI, and even shows potent activity against resistance bacterial strains [Citation14]. Cephalothin binds with penicillin binding proteins (PBPs) and disrupts the cellular architecture and ultimately inhibit the cell wall synthesis of the infectious bacterial pathogens [Citation15].
Antimicrobial resistance (AMR) has become a major burden and chronic health problem worldwide due to the over usage of antibiotics [Citation16]. Accumulating evidences indicates that the antibiotics used against the skin and soft tissue infections are also becoming resistant and thus it has to be properly addressed [Citation17,Citation18]. Instead of looking for new antibiotics development which consumes time and requires huge amount of funds, the existing antibiotics can be delivered specially to the site of infection with controlled release manner [Citation19]. Aquasomes are 3-layered vesicular nano carriers used to specially delivery the drugs to the site of target which has been used in various diseases such as cancer, cardiovascular diseases and infections [Citation20,Citation21]. In general, the aquasomes consists of ceramic core which coated with polyhydroxy compound to which the drug molecule will be loaded [Citation22]. Though the cephalothin exhibits poor soluble property and it can be overcome by loading them into the aquasomes to increase their bioavailability and therapeutic efficacy [Citation23]. In the present study, we have formulated a cephalothin loaded aquasomes and characterized it through Scanning Electron Microscope (SEM) analysis, Fourier Transform Infrared Spectroscopy (FT-IR) analysis, followed by zeta potential and particle size analysis. Further, they were tested for their antibacterial potential against strains such as respectively.
2. Materials & methods
2.1. Chemicals & Reagents
The drug was purchased from Orchid HealthCare Pvt. Ltd Chennai, Tamil Nadu. Calcium chloride, disodium hydrogen phosphate, trehalose was purchased from Sisco Research Laboratories Pvt. Ltd Chennai, Tamil Nadu.
2.2. Preparation of aquasomes
Aquasomes are prepared by spinal method [Citation24], calcium phosphate is used as backbone (core material), coating it with trehalose and finally adsorption of the drug.
In the first step of the aquasome formulation, a ceramic core was prepared using the co-precipitation technique with sonication. This involved slowly adding a 0.75M disodium hydrogen phosphate solution in water to a 0.25M calcium chloride solution under sonication for 2 h, leading to the reaction:
Subsequently, in the second step, the ceramic core (200 mg) was coated with trehalose (0.06M) through sonication for 90 min. The resulting mixture was filtered, freeze-dried and stored at 2°C. Moving on to the third step, drug loading was performed by dispersing the coated core particles in drug solution. Aquasomes are colloidal drug delivery systems that can improve drug stability and bioavailability. The drug concentration of 200 μg/ml may ensure effective antimicrobial activity against a broad spectrum of bacteria. The preparation of the aquasomes involved loading a cephalothin solution (200 μg/ml) cephalothin solution with a 7.2 pH buffer. The dispersion was left overnight at 2°C for a 24-h drug adsorption process, followed by lyophilization to obtain drug-loaded aquasomes. These distinguished steps collectively constitute the formulation process of aquasomes [Citation25–28].
2.3. Statistical analysis
Design expert 13 software was used to optimize the formulation. Thirteen runs were conducted to optimize the experimental design, using a 2-level-factors Central Composite Design (CCD). The factors are calcium phosphate mixture (gm) and trehalose (mg) coded as Y1 and Y2, respectively. The experimental factors and levels are presented in . The obtained data were analyzed using CCD to fit a quadratic polynomial equation, correlating the Design of Experiment (DOE) variables (Y1 and Y2) to the equation.
Table 1. Factor and levels of formulation.
Where A represents the predicted response, α0 stands for the intercept, the linear coefficient denotes α1 and α2. while (α1,1), (α1,2), (α1,2) denotes the quadratic coefficient [Citation29].
2.4. FTIR analysis
The Fourier transform infrared spectroscopy (FTIR) analysis was performed for calcium phosphate core, trehalose coated core and cephalothin loaded aquasome using Shimadzu FTIR analyzer. Potassium bromide pellet method was followed by mixing the samples with KBr in 1:100 ratios and subjected to FTIR analyzer. Happ-Genzel apodization was employed and the spectra were recorded between 4500 to 400 cm-1 ranges, with a resolution of 4 cm-1 [Citation30].
2.5. SEM analysis
The morphological characteristics of the calcium phosphate core, trehalose coated core and cephalothin loaded aquasome were analyzed using Hi-Resolution Scanning Electron Microscope (HR-SEM), Thermosceintific Apreo S. Alongside, the quantitative analysis of elements present in the samples were also analyzed using energy-dispersive spectroscopy (EDS). Everhart–Thornley detector (ET detector) was used for the analysis with a voltage of 20 kV [Citation31,Citation32].
2.6. Zeta potential & particle size analysis
The zeta potential and particle size of the calcium phosphate core, trehalose coated core and cephalothin loaded aquasome were determined using Zeta Sizer Malvern/Nano ZS-90. Dynamic light scattering (DLS) technique was employed to determine the mean particle size and zeta potential. For zeta potential analysis, the dispersant refractive index was set at 1.33, Viscosity cP (centipoise) was set at 0.88, dispersant dielectric constant was set at 78.5, temperature was set at 25°C and measurement position was set at 2 mm respectively. For particle size analysis, same dispersant refractive index and Viscosity cP (centipoise)ranges was followed. While, the material refractive index was set at 1.59 and material absorption was set at 0.01 respectively [Citation33].
2.7. In vitro drug release of drug-loaded aquasomes
In the in vitro drug release studies, a Franz diffusion cell was employed to assess the release of the aquasome formulation. The cellophane membrane, fitted between the donor and receptor compartments, served as the application site for the aquasome formulation. The dissolution medium utilized was a phosphate buffer with a pH of 7.2. To maintain a consistent temperature of 37°C, water circulation in the outer jacket of the cell was employed. The entire setup was placed on a magnetic stirrer, ensuring continuous stirring using a magnetic bead. At specified time intervals, 5 ml samples were withdrawn, diluted to 10 ml with the same solvent and replaced with fresh dissolution media to sustain sink conditions. Spectrophotometric analysis at 237 nm (nanometer) was performed on the withdrawn samples to calculate the percentage cumulative drug release (%CDR). The drug release study was conducted in triplicate for consistency [Citation34].
2.8. Drug release kinetics
In order to understand kinetic principles, data obtained from in vitro drug release studies are plotted in a variety of kinetics models. MS Excel statistical functions are used to process the data for regression analysis. Zero Order and First Order Kinetics models, Higuchi's model and Korsmeyer-Peppas models are used to analyze in vitro drug release data [Citation35].
2.9. Entrapment efficacy
To determine the percentage entrapment efficacy (% EE) of the drug in aquasomes, 1 ml of formulation centrifuged at 15,000 rpm for 1 hour to separate entrapped from un-entrapped drug. After supernatant removal, the sediment was spectrophotometrically analyzed at 237 nm using a UV spectrophotometer to quantify the loaded drug, The cephalothin loaded aquasome was determined by the following formula [Citation36].
2.10. Antibacterial activity
A diffusion assay, designed to evaluate the antibacterial effectiveness of nanoparticles containing a therapeutic compound, commenced with the cultivation of bacterial cultures in sterile nutrient broth at 37°C within a shaking incubator. Subsequently, a bacterial culture suspension was evenly distributed over sterile Mueller-Hinton agar plates, allowing a five-minute interval for optimal culture absorption. To assess the antibacterial potential of the nanoparticles, precisely measured 5 mm wells were created in the agar plates using a gel puncture technique. Subsequently, drug-loaded aquasomes and pure drug for both bacterial species were placed inside the wells. The prepared plates underwent a 24-hour incubation period at 37°C. After incubation, zones of inhibition, reflective of bacterial growth suppression, were measured in centimetres. For comparative purposes, cephalothin (at a concentration of 10 mg/ml) served as a positive control to gauge the experimental results [Citation37].
2.11. Stability study
According to ICH q1c guidelines aquasome stability was observed over 3 months in the stability chamber. Cephalothin-loaded aquasomes were prepared and stored in amber glass vials. Samples were subjected to stability testing at 4°C ± 0.20°C for durations of 1, 2 and 3 months. The following parameters were monitored at each time point: particle size, percentage encapsulation efficiency (%EE) and physical appearance. The physiological changes in drug-loaded formulations were thoroughly investigated [Citation38].
3. Results
3.1. Characterization of cephalothin loaded aquasomes
3.1.1. Optimization of formulation
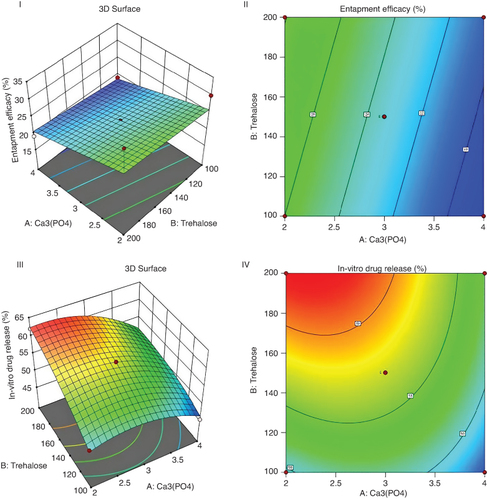
Thorough statistical analysis was conducted on the aquasome formulation to optimize its performance in terms of entrapped efficacy and in vitro drug release. Two key factors, Factor 1 (A:Ca3(PO4)) and Factor 2 (B:Trehalose), were investigated across 13 runs to elucidate their effects on the responses. ANOVA was used to assess the significance of the model statistically. The Table presented sum of squares (SS), mean square (MS), degrees of freedom (DF), p-value, F-value and ANOVA coefficients. The model's significance was determined using the Fisher distribution (F-value and p-value). For entrapped efficacy, the model indicated significance (p = 0.0391), with Factor 1 (A: Ca3(PO4)) exhibiting a notable effect (p = 0.0157), whereas Factor 2 (B: Trehalose) did not significantly affect entrapped efficacy (p = 0.4297). However, Lack of Fit was significant (p < 0.0001), suggesting potential areas for model refinement. Regarding in vitro drug release, the model was highly significant (p < 0.0001), with both Factor 1 (A: Ca3(PO4)) and Factor 2 (B: Trehalose) significantly influencing the response. Moreover, interactions (AB) and squared terms (A2, B2) also played significant roles. Lack of Fit remained significant (p < 0.0001), indicating the need for further model refinement. Analysis of the experimental data revealed that higher levels of Factor 1 (A: Ca3(PO4)) generally corresponded to higher levels of entrapped efficacy and in vitro drug release. Conversely, the effects of Factor 2 (B: Trehalose) were less consistent across the runs. The experimental results provide the importance of Factor 1 (A: Ca3(PO4)) and Factor 2 (B: Trehalose) in influencing both entrapped efficacy and in vitro drug release. The data provide valuable information into optimizing the aquasome formulation to achieve desired drug encapsulation and release characteristics. Response surface plot and counter plot of formulation shown in .
Table 2. Response surface regression: analysis of variance.
Figure 1. Response surface plot and counter plot of relationship between A:ca3(po4) and B:trehalose on entrapment efficacy(I & II) and in vitro drug release (III & IV) of formulation.
Indicates the magnitude of entrapped efficacy (%), Contour lines: Represent levels of drug release (%) corresponding to different combinations of Factor 1 and Factor 2.
ca3(po4): Calcium phosphate, color scale.
3.1.2. FTIR spectrum
Fourier transform infrared (FTIR) analysis is a significant tool used to identify the functional groups present in the sample, which thus determines the structural changes of the function groups [Citation39]. FTIR spectra of the calcium phosphate core, trehalose coated core and cephalothin loaded aquasome was shown in the Supplementary Figure–S1 and also, the FTIR peaks along with their wavelength and functional groups were shown in the . From the FTIR results, it is observed that the calcium phosphate core showed peak stretching's that corresponds to C–I, –CH2 rock, C–O, –NH3+ rock, –CH3 bend, C=C, –OH; trehalose coated core showed peak stretching's that corresponds to C–I, C–Br, C–C–N, –CH2 rock, C–O, –NH3+ rock, –CH2 twist; and cephalothin loaded aquasome showed peak stretching's that corresponds to C–Br, C–C-N, –CH2 rock, C–O, –NH3+ rock, –CH2 twist, C–N respectively. The peak stretch at 3200–3400 cm-1 ranges correspond to the –OH group and around 2250 cm-1 ranges corresponds to the C–N group [Citation40]. Likewise, we could able to find the similar peak stretches at 3226.91 cm-1 for calcium phosphate core and 2355.68 cm-1 for cephalothin loaded aquasome respectively. All calcium phosphate core, trehalose coated core and cephalothin loaded aquasome showed below 500 cm-1 range indicating that they might have a C–I or C–Br bonds and also shows peaks around 980 cm-1 range indicating –CH2 rock, around 1050 cm-1 range indicating C–O, around 1120 cm-1 range indicating NH3+ rock and 1330 cm-1 range indicating –CH2 twist, respectively.
Table 3. FTIR peaks of calcium phosphate core, Trehalose coated core and cephalothin-loaded aquasome.
3.1.3. SEM analysis
Scanning electron microscopy (SEM) analysis provides a detailed and magnified view of a formulation and used to identify their size, shape, chemical composition and other physicochemical properties [Citation41]. At 50 μm field range the SEM images was captured, and the horizontal field of view (HFW) for calcium phosphate core, trehalose coated core and cephalothin loaded aquasome was observed at 1 μm respectively. The magnified morphological view was shown in the . From the SEM analysis, the average size of the particles of calcium phosphate core, trehalose coated core and cephalothin loaded aquasome was observed at 0.5 to 1 μm. A similar observation was reported by Damera DP et al., 2019, in which the hydroxyapatite (HAP) core was coated with Cellobiose to which the bovine serum albumin (BSA) was loaded. These average size of BSA Loaded Aquasomes was observed to be between 200 and 1000 nm. From our SEM analysis we could observe the dense occurrence in the cephalothin loaded aquasome indicating the cephalothin antibiotic was adsorbed in the prepared aquasome.
Figure 2. SEM (left panel) and EDX (right panel) analysis of calcium phosphate core (A), trehalose coated core (B) and Cephalothin loaded aquasome (C).
SEM, which shows the presence of spherical calcium phosphate core in the nano range.
μm: Micrometer; KeV: Kilo-electron volt; SEM: Scanning electron microscopy.
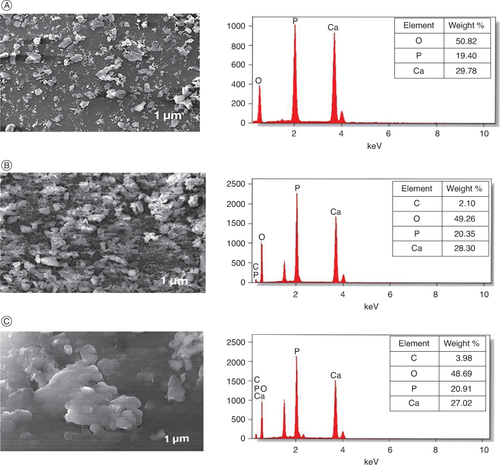
Energy-dispersive x-ray spectroscopy (EDS) analysis provides a better understanding of elements present in formulation and also provides their relative abundance in the whole composition [Citation42]. It has a huge impact on the development of nanoparticles, which evaluates the detrimental effects produced by the nanoparticles at molecular level and also helps in the identification of chemical composition of the drug delivering nanoparticle to various diseases [Citation43,Citation44]. The EDS analysis of calcium phosphate core, trehalose coated core and cephalothin loaded aquasome along with their weight % among overall composition was shown in the . From our analysis, we could able to observe that calcium phosphate core has elements such as O, P, Ca; while trehalose coated core and cephalothin loaded aquasome has elements such as O, P, Ca and C-N. This indicates the coated material and the antibiotic loaded aquasomes has the carbon support in their structure with 2.1 and 3.9 weight % respectively. Also, the atom% of C in trehalose coated core, and cephalothin loaded aquasome was observed to be 3.7 and 7 respectively, indicating the antibiotic loaded aquasome has more carbon support when loaded on the coated core.
3.1.4. Zeta potential & particle size determination
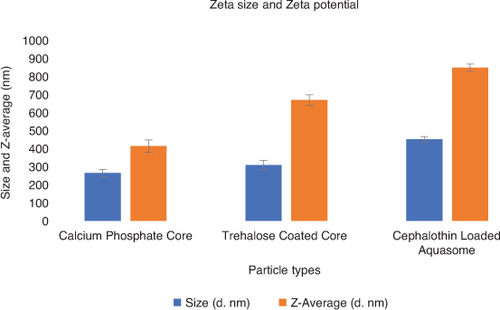
Zeta potential determines the stability of the particle and is governed by the electrostatic force whether by attraction of repulsion between the particles [Citation45]. The zeta potential and particle size analysis of the calcium phosphate core, trehalose coated core and cephalothin loaded aquasome was determined using Zeta Sizer Malvern/Nano ZS-90 and the results were shown in the Supplementary Figure–S2. Also the zeta potential analysis values such as Zeta Potential (mV), Zeta Deviation (mV), Conductivity (mS/cm) and the particle size analysis values such as Z-Average (d. nm), Polydispersity index (PDI), Intercept, Size (d. nm), St Dev (d. nm) were tabulated in the & Supplementary Table–S1 respectively. In general, the zeta potential can be positive or negative but should not be zero to stay away from the aggregation of particles in the environment [Citation46,Citation47]. The zeta Potential and conductivity of calcium phosphate core was observed as -6.66 mV and 3.95 mS/cm; while the trehalose coated core showed -10.3 mV and 0.948 mS/cm, and cephalothin loaded aquasome showed -14.6 mV and 0.282 mS/cm respectively. Interestingly, our results didn't show any zero values indicating particle aggregation is not present and also indicates the stability of the cephalothin loaded aquasome as showed in the Supplementary Figure–S2.
Table 4. Particle size of calcium phosphate core, Trehalose coated core and cephalothin-loaded aquasome.
This phenomenon can indeed be attributed to the surface modification of the particles during the coating and loading processes toward a stronger repulsive force between the particles. The surface modification through trehalose coating and cephalothin loading enhances the stability of the aquasome, which may leads to increase in Zeta potential. From our results, the size and Z-Average of calcium phosphate core was observed to be 265 d. nm and 414 d. nm; while for trehalose coated core it was observed as 309 d. nm and 670 d. nm, and for cephalothin loaded aquasome it was observed as 452 d. nm and 849 d. nm respectively. Thus, it is clearly indicating that the particle size was increased to 452 d. nm in the cephalothin loaded aquasome as showed in the .
3.2. Drug release studies of cephalothin-loaded aquasomes
The in vitro drug release was investigated through Franz diffusion cells employing cellophane membranes, revealing a drug release rate of 60.72% over a period of approximately 10 h for the drug-loaded aquasomes Supplementary Figure–S3. This result showed that the formulated aquasomes exhibited a controlled and sustained release of the drug. Based on the analysis of drug release kinetics, the coefficient of determination (R2) values for zero-order, first-order, Higuchi and Korsmeyer release models were determined to be 0.970, 0.950, 0.993 and 0.613, respectively. The high R2 value of 0.993 obtained for the Higuchi model indicates a strong correlation between the observed and predicted drug release profiles. Hence, it can be concluded that the release of cephalothin from the aquasome formulation follows Higuchi release kinetics.
3.3. Antibacterial potential of cephalothin-loaded aquasomes
The well diffusion method was employed to assess the antibacterial efficacy of drug-loaded aquasomes against both Gram-positive (Staphylococcus aureus, S. aureus) and Gram-negative (Escherichia coli, E. coli) bacteria. The antibacterial activity, as indicated by the zone of inhibition, showed impact of cephalothin-loaded aquasomes, with a zone of 38 ± 3 mm against Staphylococcus aureus and 20 ± 5 mm against Escherichia coli shown in Supplementary Table–S2. The experimental results states that Staphylococcus aureus was more susceptible to the drug-loaded aquasomes compared with Escherichia coli. This susceptibility of Staphylococcus aureus attributed to factors such as cell wall plasmolysis or the separation of cytoplasm from the cell wall. Additionally, the outer membrane of Gram-negative bacteria influences molecular permeability, potentially contributing to the observed variation in susceptibility between Gram-positive and Gram-negative bacteria.
3.4. Stability study
The stability study was carried out by maintaining the temperature within the specified range of 4°C ± 0.20°C throughout the three-month experimental period. The size of cephalothin-loaded aquasomes remained constant, and minimal fluctuations was observed over time. The percentage of encapsulation efficiency (%EE) remained slightly unchanged indicating that cephalothin has effective entrapment efficacy and the physical appearance of the samples remained constant throughout the study, there were no visible signs of aggregation or decay as shown in .
Table 5. Result of stability study.
4. Discussion
Skin and soft tissue infections (SSTI) are common and frequent which are caused by bacteria such as Staphylococcus, Pseudomonas. and Streptococcus. occurring at the connective tissues. cephalothin (or Cefalotin) is a first-generation antibiotic belonging to cephalosporin class is commonly used against SSTIs due to their potent activity even against resistant bacterial strains. However, its therapeutic efficacy toward topical application is limited by its poor solubility [Citation48]. Aquasome a 3-layered vesicular nano carriers consisting of ceramic core that can be coated with polyhydroxy compound to which the drug molecule will be loaded is significant strategy to overcome the solubility issue, as well as it improves the bioavailability of the drug and ultimately increase their therapeutic effect [Citation49]. In this study, we developed a cephalothin-loaded aquasome and subjected it to optimized using design of experiments. The aquasome was characterized using Fourier transform infrared spectroscopy (FT-IR), scanning electron microscope (SEM), zeta potential, zeta particle size analysis and percentage entrapment efficacy. Additionally, we conducted in vitro antibiotic release studies, assessed antibacterial activities, and evaluated short-term stability [Citation50]. Our results revealed that the cephalothin loaded aquasome has a size range from 10 to 1000 nm have functional groups that enables significant bonding, and zeta potential value that enables electrostatic interactions in the biological environment. Thus, we conclude that the formulated cephalothin loaded aquasome is stable and significant against bacterial infections. While our study focuses particle size analysis and formulation design, we have included a comprehensive stability study section to cover other important aspects of the experiment's integrity and performance. However, limited assessment of antimicrobial activity of clinically relevant antimicrobial agents, in animal models or clinics experiments.
5. Conclusion
This study aimed to enhance the bioavailability and solubility of cephalothin for treating SSTIs by using a sustained release approach at the infection site. Aquasomes were formulated and optimized using a 2-factorial design approach in experimental design. FTIR analysis confirmed the presence of functional groups for bond formation in the cephalothin-loaded aquasome. SEM analysis revealed a formulation size ranging from 0.1 to 1 μm, while zeta potential analysis confirmed values conducive to electrostatic interactions in biological environments. Entrapment efficacy was determined to be 35%. Moreover, drug release studies demonstrated sustained release of cephalothin from the aquasome formulation, following (Matrix Diffusion-Controlled Release) Higuchi kinetics (R2 = 0.993), indicating a strong correlation between observed and predicted release profiles. Additionally, the formulation exhibited significant antibacterial potential against both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria, with greater susceptibility observed in Staphylococcus aureus. Stability studies over a three-month period at 4°C ± 0.20°C revealed satisfactory physical and chemical stability of the cephalothin-loaded aquasomes. These findings support the potential utility of the formulation for further pharmaceutical applications. Future directions of this study include in vivo investigations using animal models or clinical trials to validate the efficacy and safety of cephalothin-loaded aquasomes. Exploring the formulation's performance against a broader range of bacterial strains, including resistant ones, would enhance its applicability. Furthermore, long-term stability assessments, biocompatibility evaluations and comparative studies with other antibiotics could provide comprehensive insights. Optimization of drug loading capacity could further advance the potential of these aquasomes for treating skin and soft tissue infections. In conclusion, our study focused on developing cephalothin-loaded aquasomes and optimizing their formulation, highlighting their promising potential as effective treatment strategies for SSTIs. Further research is warranted to fully realize their clinical applicability.
6. Future perspective
Future research on cephalothin-loaded aquasomes should prioritize the following areas to enhance their efficacy for treating skin and soft tissue infections (SSTIs):
Clinical trials: conduct extensive clinical trials to validate safety, efficacy, optimal dosage and application protocols in human subjects.
Antimicrobial testing: broaden testing to include multi-drug resistant strains to provide a comprehensive efficacy profile.
Combination therapy: explore combination therapies with other antibiotics to leverage potential synergistic effects.
Long-term stability studies: conduct rigorous stability studies under various environmental conditions to ensure robustness during storage and transportation.
In vivo studies: perform detailed in vivo studies using animal models to assess pharmacokinetics, biodistribution and therapeutic performance.
Scale-up and manufacturing: address challenges associated with large-scale production to ensure consistency, quality and cost–effectiveness.
Exploration of other drugs: investigate the aquasome system for delivering other antibiotics or therapeutic agents to broaden its application scope.
Author contributions
B Shanmugam made the conception and wrote the manuscript. UM Srinivasan reviewed and corrected the work.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No funded writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study did not involve any human or animal subjects. Therefore, no ethical approval was required. All experimental procedures were conducted in compliance with standard laboratory practices and safety regulations.
Supplementary Materials
Download MS Word (228.4 KB)Acknowledgments
We thank SRM college of Pharmacy, SRMIST for providing me the full support throughout the research.
Supplemental material
Supplementary data for this article can be accessed at https://doi.org/10.1080/20565623.2024.2367849
References
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol J Can Mal Infect Microbiol Medicale. 2008;19:173–184. doi:10.1155/2008/846453
- Tognetti L, Martinelli C, Berti S, et al. Bacterial skin and soft tissue infections: review of the epidemiology, microbiology, aetiopathogenesis and treatment: a collaboration between dermatologists and infectivologists. J Eur Acad Dermatol Venereol JEADV. 2012;26:931–941. doi:10.1111/j.1468-3083.2011.04416.x
- Sunderkötter C, Becker K. Frequent bacterial skin and soft tissue infections: diagnostic signs and treatment. J Dtsch Dermatol Ges J Ger Soc Dermatol JDDG. 2015;13:501–524; quiz 525–526. doi:10.1111/ddg.12721
- Moffarah AS, Al Mohajer M, Hurwitz BL, et al. Skin and Soft Tissue Infections. Microbiol Spectr. 2016;4:1–16. doi:10.1128/microbiolspec.DMIH2-0014-2015
- Esposito S, Pagliano P, De Simone G, et al. Epidemiology, aetiology and treatment of skin and soft tissue infections: final report of a prospective multicentre national registry. J Chemother Florence Italy. 2022;34:524–533. doi:10.1080/1120009X.2022.2075170
- Ramakrishnan K, Salinas RC, Agudelo Higuita NI. Skin and soft tissue infections. Am Fam Physician. 2015;92:474–483.
- Bartoszko JJ, Mertz D, Thabane L, et al. Antibiotic therapy for skin and soft tissue infections: a protocol for a systematic review and network meta-analysis. Syst Rev. 2018;7:138. doi:10.1186/s13643-018-0804-8
- Dryden M. Novel techniques in the treatment of skin and soft tissue infection. Curr Opin Infect Dis. 2022;35:72–78. doi:10.1097/QCO.0000000000000818
- Hamner M, Nedved A, Austin H, et al. Improving duration of antibiotics for skin and soft-tissue infections in pediatric urgent cares. Pediatrics. 2022;150:1–8. doi:10.1542/peds.2022-057974
- Gillet Y, Lorrot M, Cohena R, et al. Antibiotic treatment of skin and soft tissue infections. Arch Pediatr Organe Off Soc Francaise Pediatr. 2017;24:S30–S35. doi:10.1016/S0929-693X(17)30516-X
- Godzeski CW, Brier G, Pavey DE. Cephalothin, a new cephalosporin with a broad antibacterial spectrum. I. In vitro studies employing the gradient plate technique. Appl Microbiol. 1963;11:122–127. doi:10.1128/am.11.2.122-127.1963
- Nguyen HM, Graber CJ. Cephalothin susceptibility testing as class representative for oral cephalosporins: is it time to move on? Diagn Microbiol Infect Dis. 2013;76:483–485. doi:10.1016/j.diagmicrobio.2013.03.022
- Rugani K de S, Kogawa AC, Salgado HRN. Review for analytical methods for the determination of sodium cephalothin. Crit Rev Anal Chem. 2019;49:187–194. doi:10.1080/10408347.2018.1506697
- do Nascimento PA, Kogawa AC, Salgado HRN. Cephalothin: review of characteristics, properties, and status of analytical methods. J AOAC Int. 2021;104:1593–1608. doi:10.1093/jaoacint/qsaa163
- Sarkar P, Yarlagadda V, Ghosh C, et al. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. MedChemComm. 2017;8:516–533. doi:10.1039/C6MD00585C
- Kazimoto T, Abdulla S, Bategereza L, et al. Causative agents and antimicrobial resistance patterns of human skin and soft tissue infections in Bagamoyo, Tanzania. Acta Trop. 2018;186:102–106. doi:10.1016/j.actatropica.2018.07.007
- Makwela AB, Grootboom WM, Abraham V, et al. Antimicrobial management of skin and soft tissue infections among surgical wards in South Africa: findings and implications. Antibiot Basel Switz. 2023;12:275. doi:10.3390/antibiotics12020275
- Kaliyeva SS, Lavrinenko AV, Tishkambayev Y, et al. Microbial landscape and antibiotic susceptibility dynamics of skin and soft tissue infections in Kazakhstan 2018–2020. Antibiot Basel Switz. 2022;11:659. doi:10.3390/antibiotics11050659
- Valladales-Restrepo LF, Aristizábal-Carmona BS, Giraldo-Correa JA, et al. Antibiotic management of uncomplicated skin and soft tissue infections in the real world. Microorganisms. 2023;11:1369. doi:10.3390/microorganisms11061369
- Kulkarni S, Prabhakar B, Shende P. Aquasomes: advanced vesicular-based nanocarrier systems. Curr Pharm Des. 2022;28:2404–2414. doi:10.2174/1381612828666220728112741
- Ramalingam PS, Balakrishnan P, Rajendran S, et al. Identification of dietary bioflavonoids as potential inhibitors against KRAS G12D mutant—novel insights from computer-aided drug discovery. Curr Issues Mol Biol. 2023;45:2136–2156. doi:10.3390/cimb45030137
- Banerjee S, Sen KK. Aquasomes: a novel nanoparticulate drug carrier. J Drug Deliv Sci Technol. 2018;43:446–452. doi:10.1016/j.jddst.2017.11.011
- Rojas-Oviedo I, Salazar-López RA, Reyes-Gasga J, et al. Elaboration and structural analysis of aquasomes loaded with indomethacin. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2007;32:223–230. doi:10.1016/j.ejps.2007.07.008
- Shirtode S, Unde E, Mule S, et al. An overview on aquasomes. Int J Creat Res Thoughts. 2022;10:641–649.
- Popat Jadhav P, Hanmantrao Aloorkar N. A review on aquasome. Int J Creat Res Thoughts. 2021;9:2320–2882.
- Patel Chirag J, Tyagi S, Patel P, et al. Aquasomes: a potential approach for novel drug delivery. Pharmatutor. 2012.
- Patel S, Aundhia C, Seth A, et al. Aquasomes: a novel approach in drug carrier system. Eur J Pharm Med Res [Internet]. 2018. [ cited 2023 May 18]; Available from: https://www.researchgate.net/publication/324794429_AQUASOMES_A_NOVEL_APPROACH_IN_DRUG_CARRIER_SYSTEM
- Mesariya S, Joshi K, Jain H, et al. Aquasomes — a self-assembled nanotechnology system. ChemInform. 2012;43:no–no. doi:10.1002/chin.201230277
- Ojewumi ME, Ehinmowo AB, Ekanem GP, et al. Central composite design for solvent extraction of oil from neem (Azadirachta indica) seed. IOP Conf Ser Mater Sci Eng. 2021;1107:012109. doi:10.1088/1757-899X/1107/1/012109
- Elangovan S, Arumugam S. Purification, characterization, and biological activities of melanin pigment isolated from Indian squid Uroteuthis duvaucelii. Aquac Int. 2023;31:3095– 3108. doi:10.1007/s10499-023-01158-9
- Damera DP, Kaja S, Janardhanam LSL, et al. Synthesis, detailed characterization, and dual drug delivery application of BSA loaded aquasomes. ACS Appl Bio Mater. 2019;2:4471–4484. doi:10.1021/acsabm.9b00635
- Gigli V, Piccinino D, Avitabile D, et al. Laccase mediator cocktail system as a sustainable skin whitening agent for deep eumelanin decolorization. Int J Mol Sci. 2022;23:1–12. doi:10.3390/ijms23116238
- İlhan M, Gültekin HE, Rençber S, et al. Chapter 12 - aquasomes: a novel platform for drug delivery. Syst Nanovesicular Drug Deliv. 2022;191–206. doi:10.1016/B978-0-323-91864-0.00020-6
- Abdul Wahid A. Development and characterization of nanoemulsion gel for topical drug delivery of nabumetone. Int J Pharm Pharm Res. 2016;7:126.
- Rajak P, Islam R, Karmakar A, et al. Aquasomal drug delivery system: a special emphasis on the formulation techniques and applications. Ars Pharm Internet. 2023;64:359–375. doi:10.30827/ars.v64i4.28264
- Waghule T, Rapalli VK, Singhvi G, et al. Design of temozolomide-loaded proliposomes and lipid crystal nanoparticles with industrial feasible approaches: comparative assessment of drug loading, entrapment efficiency, and stability at plasma pH. J Liposome Res. 2021;31:158–168. doi:10.1080/08982104.2020.1748648
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi:10.1016/j.jpha.2015.11.005
- Bajaj S, Singla D, Sakhuja N. Stability testing of pharmaceutical products. J Appl Pharm Sci. 2012;2:129–138.
- Wang R, Wang Y. Fourier transform infrared spectroscopy in oral cancer diagnosis. Int J Mol Sci. 2021;22:1–21. doi:10.3390/ijms22031206
- Sofronia AM, Baies R, Anghel EM, et al. Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater Sci Eng C Mater Biol Appl. 2014;43:153–163. doi:10.1016/j.msec.2014.07.023
- Ural N. The significance of scanning electron microscopy (SEM) analysis on the microstructure of improved clay: an overview. Open Geosci. 2021;13:197–218. doi:10.1515/geo-2020-0145
- Avula A, Galor A, Blackwelder P, et al. Application of scanning electron microscopy with energy-dispersive x-ray spectroscopy for analyzing ocular surface particles on Schirmer strips. Cornea. 2017;36:752–756. doi:10.1097/ICO.0000000000001173
- Scimeca M, Bischetti S, Lamsira HK, et al. Energy Dispersive X-ray (EDX) microanalysis: a powerful tool in biomedical research and diagnosis. Eur J Histochem EJH. 2018;62:2841. doi:10.4081/ejh.2018.2841
- Voinov MA, Sosa Pagán JO, Morrison E, et al. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J Am Chem Soc. 2011;133:35–41. doi:10.1021/ja104683w
- Clogston JD, Patri AK. Zeta potential measurement. Methods Mol Biol Clifton NJ. 2011;697:63–70. doi:10.1007/978-1-60327-198-1_6
- Pochapski DJ, Carvalho dos Santos C, Leite GW, et al. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir. 2021;37:13379–13389. doi:10.1021/acs.langmuir.1c02056
- Kaledin LA, Tepper F, Kaledin TG. Pristine point of zero charge (p.p.z.c.) and zeta potentials of boehmite's nanolayer and nanofiber surfaces. Int J Smart Nano Mater. 2016;7:1–21. doi:10.1080/19475411.2016.1148077
- Nascimento PA do, AC K HRNS. Development and validation of an innovative and ecological analytical method using high performance liquid chromatography for quantification of cephalothin sodium in pharmaceutical dosage. J Chromatogr Sep Tech. 2018;09:1–8. doi:10.4172/2157-7064.1000394
- Kommineni S, Ahmad S, Vengala P, et al. Sugar coated ceramic nanocarriers for the oral delivery of hydrophobic drugs: formulation, optimization and evaluation. Drug Dev Ind Pharm. 2012;38:577–586. doi:10.3109/03639045.2011.617884
- Vengala P, Subrahmanyam C, Gangaraju M. In vitro and in vivo evaluation of piroxicam loaded ceramic nanoparticles. Int J Pharma Sci Res. 2016;7:303–3098.