Abstract
Aim: Iron deficiency (ID) is associated with heart failure (HF) in a considerable proportion of patients. To improve the quality of life, lower the frequency of hospitalizations, and lower mortality rates of chronic HF patients (HF), this meta-analysis will look into the role of iron supplementation using ferric carboxymaltose (FCM). Methods & results: From inception until 1 October 2023, we conducted a thorough literature search of electronic databases for peer-reviewed publications. Around 5229 HF patients were included, of which 2691 received FCM while 2538 received placebo. Conclusion: FCM reduces HF-related hospitalizations but doesn't improve overall or cardiovascular mortality in those with HF and ID. The overall results support FCM's role in managing iron deficiency in heart failure.
Plain language summary
Heart failure (HF) patients often suffer from iron deficiency (ID), worsening their symptoms and quality of life. Intravenous iron therapy, like ferric carboxymaltose (FCM), has been studied for its benefits in HF. This meta-analysis looked at existing research and found that FCM treatment reduced hospitalizations for HF but didn't significantly impact overall mortality. Although FCM improves patients' lives, more research is needed to understand its long-term effects fully. This study highlights the importance of addressing ID in HF management and supports FCM therapy as a beneficial option for HF patients.
Iron deficiency is prevalent in patients with heart failure (HF), impacting their quality of life and overall prognosis.
Intravenous ferric carboxymaltose (FCM) is recommended to enhance the quality of life in iron-deficient HF patients, particularly those with reduced left ventricular ejection fraction.
FCM treatment is associated with a significant reduction in the combined outcome of hospitalizations for HF and cardiovascular mortality.
While FCM does not appear to reduce overall mortality or cardiovascular mortality in iron-deficient HF patients, it improves symptoms, functional capacity and quality of life.
FCM offers a cost-effective and well-tolerated option for correcting iron deficiency in HF, potentially reducing the disease burden on healthcare systems.
1. Background
Heart failure (HF) is a prevailing condition distinguished by a substantial incidence of iron deficiency (ID), and patients with stable chronic HF have a prevalence rate of more than 50%, which increases even more in those who have previously received treatment for acute HF [Citation1–3]. Irrespective of the degree of anemia, iron-deficient patients with HF often endure a diminished quality of life (QOL) and reduced lifespan [Citation4–7]
Coronary artery disease and myocardial ischemia are the primary underlying causes of HF [Citation8,Citation9]. It has been consistently demonstrated that individuals with HF of ischemic origin face a more unfavorable prognosis compared with their non-ischemic counterparts [Citation10–13]. Iron replacement is paramount in this context, as a body of research consistently points toward the impairment of cardiomyocyte contractility due to iron deficiency. This deficit can be alleviated through iron replenishment [Citation14].
In 2021, guidelines underwent a significant update, firmly recommending the routine use of intravenous iron, specifically ferric carboxymaltose (FCM), to enhance the QOL and daily functionality of iron-deficient HF patients [Citation15]. Numerous well-executed studies substantiate the effectiveness of IV FCM, a nanoparticulate iron-carbohydrate complex designed for macrophage-mediated iron release [Citation16]. This therapy has exhibited noteworthy effects on performance and overall QOL in individuals with HFrEF (heart failure with reduced ejection fraction) and iron deficiency, irrespective of anemia [Citation16]. It is now considered an integral component of outpatient care for HF patients with reduced left ventricular ejection fraction (LVEF). MRI studies have affirmed the cardiac absorption of administered iron [Citation17], improving LVEF within a month of treatment [Citation18].
However, an evident lack of consensus prevails regarding the effectiveness of FCM and its associated factors in reducing the frequency of exacerbations and subsequent hospitalizations, which remains an essential concern for patients with chronic HF. Variables like age, genetic predispositions and the stage of HF have been identified, underscoring the need for further comprehensive research to elucidate the effectiveness of iron carboxymaltose in HF patients, irrespective of the severity. The substantial variations in risk factors and prognoses across different racial and ethnic groups magnify this imperative. In this research, we have undertaken a comprehensive meta-analysis, encompassing the latest available data and incorporating new research to investigate the success rate of iron carboxymaltose therapy in individuals with HF.
2. Materials & methods
2.1. Data sources & search strategy
This systematic review and meta-analysis is reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA) guidelines [Citation19]. From inception until 2023, a thorough literature search was conducted through PubMed, Embase, and Cochrane CENTRAL databases. Online libraries, including www.clinicaltrialresults.org and www.clinicaltrials.gov, as well as articles and presentations from significant cardiology sessions were also identified to find grey literature. There were no limitations on the publication's timeline and only articles for which the full text was available in English were considered. Two independent investigators (BS Rangwala and MA Shafique) conducted a thorough systematic review using relevant keywords outlined in the supporting information ().
Table 1. Search strategy.
2.2. Study selection
All the articles were screened by two independent investigators (BS Rangwala and MS Mustafa). The studies were screened based on title and extract and then further evaluated based on full text. When there were differing opinions, a third reviewer (MA Shafique) helped settle it. We selected randomized controlled trials for our analysis and excluded review articles, case reports, case series, cross-sectional studies, editorials, commentaries and preclinical studies. After the author debate and consensus, the inclusion criteria were determined. There was no cap on the size of studies considered for inclusion.
2.3. Data extraction & outcomes of interest
Two authors (MA Shafique and MS Mustafa) independently extracted data from the selected studies. The baseline characteristics that were removed include the first author's last name, year of publication and sample size. Our outcomes of interest for this meta-analysis were cardiovascular mortality, events for all-cause mortality, number of patients hospitalized for HF, at least one serious adverse event, subjects with cardiac events, treatment discontinuation, hemoglobin levels and injection site conditions. There were variations in the main results of each trial. The clinical primary goal for AFFIRM-AHF was a composite of cardiovascular death and recurring HF hospitalizations while in other trials, functional capacity and QOL were the primary outcomes. Using the updated Cochrane Risk-of-Bias tool for Randomised Controlled Trials (ROB 2.0), the quality of randomized controlled trials was evaluated [Citation20].
2.4. Statistical analysis
For dichotomous outcomes, odds ratios (ORs) were computed; for recurrent occurrences, rate ratios (RRs) were employed. The Higgins I2 statistic was used to evaluate heterogeneity [Citation21]. In light of the variability observed in research designs and patient demographics, the primary analysis employed a random-effect model [Citation22]. Because there were less than ten included papers, publication bias was not evaluated [Citation23]. Statistical significance was defined at 5% for this investigation. For all other analyses, Review Manager (Version 5.3; Cochrane Collaboration, Oxford, UK) was utilized.
3. Results
3.1. Literature search
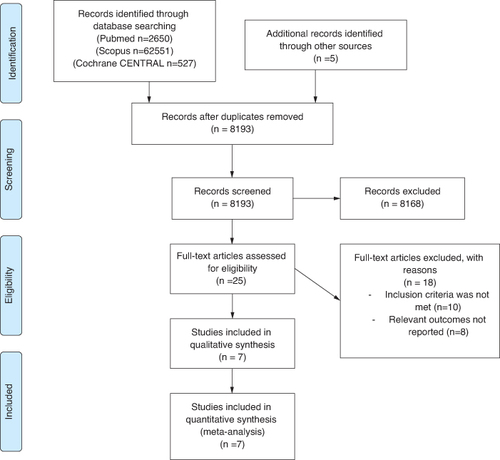
The PRISMA flow diagram () summarizes the process of searching for relevant literature and choosing studies. After 8193 studies were found through the search, 10 matched the predetermined eligibility criteria and were included in the meta-analysis.
3.2. Study characteristics
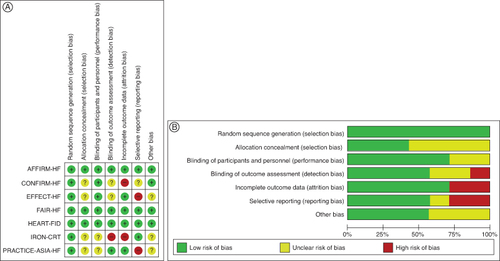
After thorough selection, the study included 5229 patients, of which 3235 were males and 1994 were females. All trials only enrolled HF patients with decreased LVEF, with a cut-off for LVEF ranging from 35 to 50% at baseline. The majority of the included trials did not specify the target hemoglobin. But maintaining iron levels over the typical cut-off was the aim. provides the demographics of the included studies' baseline populations. FAIR-HF, AFFIRM-AHF and Heart-FID of our included studies had an overall low risk of bias and of high quality as determined by the Cochrane Risk of Bias tool. However, Effect-HF, Iron-CRT and Practice-Asia-HF are of moderate-to-low quality ().
Table 2. Baseline Characteristics.
3.3. Meta-analysis results
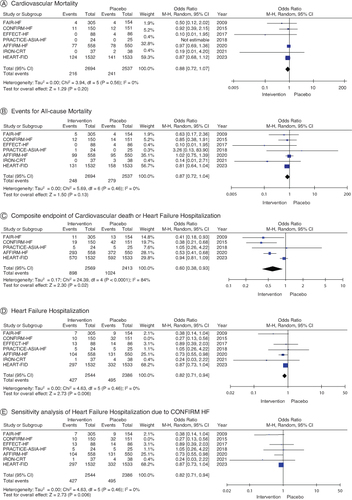
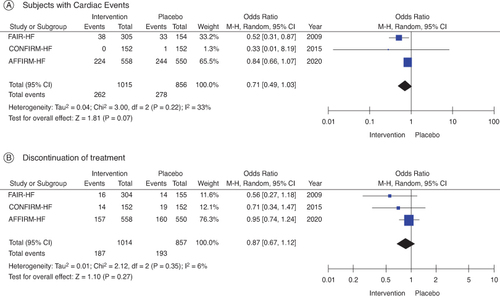
The effects of FCM and placebo on clinical outcomes in HF patients with iron insufficiency are contrasted in (). FCM supplementation in HF patients with iron deficiency exhibited no appreciable effect on cardiovascular mortality (OR: 0.88; 95% CI: 0.72–1.07; p = 0.56; A) in contrast to placebo, according to seven studies that examined the effect of FCM on cardiovascular mortality. Comparing FCM supplementation to placebo, all-cause mortality in iron-deficient HF patients was not significantly affected (OR: 0.87; 95% CI: 0.72–1.04; p = 0.46), according to the studies that assessed the effect of the supplement on overall mortality (B). Nonetheless, patients receiving FCM treatment had a significantly lower proportion of patients encountering the composite end point of hospitalization for HF or cardiovascular death (OR: 0.60; 95% CI: 0.38–0.93; I2 = 84%; p = 0.0001; C). Furthermore, there were significantly fewer hospitalizations for HF in the FCM arms during follow-up (OR: 0.66; 95% CI: 0.48–0.91; I2 = 54%; D). Sensitivity analyses of research employing IV FCM in hospitalizations for HF (OR: 0.82; 95% CI: 0.1–0.94; I2 = 0%; E), omitting the trial that contributed the most to heterogeneity; CONFIRM HF demonstrated comparable outcomes. According to three studies, FCM did not significantly increase the risk of subjects experiencing serious cardiac events (OR: 0.71; 95% CI: 0.49–1.03; I2: 33%; p = 0.22; A). Moreover, compared with placebo, it was not found to increase events upon treatment discontinuation (OR: 0.87; 95% CI: 0.67–1.12; p = 0.35; B).
3.4. Other results
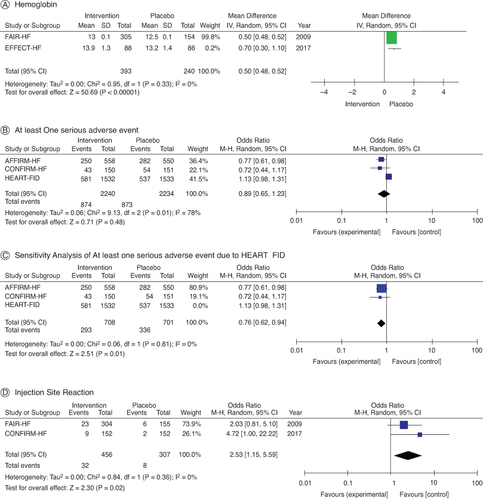
Hemoglobin levels were shown to be greater following IV iron delivery (MD: 0.50; 95% CI: 0.48–0.52; I2 = 0%) in comparison to the control group (A). FCM did not result in any significant adverse effects (OR: 0.89; 95% CI: 0.65–1.23; I 2 = 78%; p = 0.01), according to three trials that only reported one profound adverse impact (B). However, HEART FID demonstrated fewer occurrences in FCM arms compared with placebo after sensitivity analyses of at least one significant side effect (OR: 0.76; 95% CI: 0.62–0.94; I2 = 0%) (C) excluded the trial with the most significant contribution to heterogeneity. The injection site reaction did not differ between the IV FCM and control groups (OR: 2.53; 95% CI: 1.25–5.594; I2 = 0%; D).
4. Discussion
The primary findings of this meta-analysis reveal a noteworthy decrease in the combined outcome of cardiovascular mortality and recurrent hospitalizations when comparing iron-deficient HF patients treated with FCM to those who received a placebo. However, there is no observed favorable impact on overall mortality or cardiovascular mortality in iron-deficient HF patients.
HF is a chronic condition and a severe public health issue affecting approximately 37.7 million people worldwide [Citation24]. It significantly affects the quality of patients' lives with symptoms including dyspnea, fluid retention, chronic fatigue, and reduced exercise tolerance and is a leading cause of frequent hospitalizations [Citation3]. Moreover, increased morbidity and mortality are associated with HF, imposing significant strain on healthcare systems [Citation3,Citation24]. Management of HF requires a multifaceted approach, including combination drug therapy and ID correction [Citation25]. ID is a frequent comorbidity in HF, impacting around 40–70% of HF patients, irrespective of their left ventricular function [Citation26,Citation27]. It is linked to reduced exercise capacity and elevated mortality rates [Citation28]. It is worth mentioning that over 40% of patients with chronic HF who do not exhibit anemia or abnormalities in their hematological parameters display insufficient iron reserves, as indicated by laboratory tests [Citation26].
The pivotal role of iron as a trace element in regulating oxygen levels, supporting oxidative metabolism in skeletal and cardiac tissues, and facilitating mitochondrial function is believed to establish a connection between the pathophysiology of HF and iron deficiency [Citation29]. Changes in mitochondria in HF may be associated with a deficit in mitochondrial iron, possibly due to iron's role as a cofactor in numerous mitochondrial enzymes involved in oxidative phosphorylation or antioxidant defense. Consequently, ID has the potential to exacerbate mitochondrial dysfunction linked to HF. Iron also assumes a critical role in sustaining cellular energy and tissue metabolism, extending its influence beyond the hematopoietic system [Citation30]. Cardiomyocytes, given their higher energy demands, are particularly susceptible to reduced iron supply and utilization, potentially impacting contractility [Citation31].
Factors that may lead to the depletion of iron reserves in HF patients include poor absorption and gastrointestinal bleeding, which can be triggered or worsened by using anticoagulants, antiplatelet drugs or non-steroidal anti-inflammatory medications [Citation32]. Furthermore, increasing levels of inflammatory cytokines and systemic chronic inflammation are typical in HF patients, which causes the liver to produce more hepcidin. Functional ID occurs when hepcidin inhibits the absorption of iron from the gastrointestinal tract and the release of iron from its storage sites [Citation32,Citation33].
Research has shown that even among HF patients adhering to guideline-recommended medical therapy at target doses, readmission rates remain elevated, underscoring the need to explore additional factors for enhancing these patients' overall clinical condition and prognosis [Citation34]. Given ID's crucial contribution to the pathophysiology of HF leading to poor outcomes and increased mortality, it must be corrected during the management of HF. Various approaches for addressing ID in HF have been proposed. Restoring ID through increased erythropoiesis does not provide benefits to HF patients [Citation32], and attempts to rectify ID with oral supplementation have shown little efficacy in HF [Citation27], as evidenced by the IRON-HF [Citation35] and IRONOUT-HF trials [Citation36]. In contrast, Intravenous FCM has demonstrated the ability to enhance functional capacity, alleviate symptoms and enhance the QOL for HF patients with ID through multiple clinical trials [Citation27,Citation37].
Previous meta-analyses have demonstrated the capacity of FCM to improve symptoms, oxygen kinetics, functional capacity and QOL physical performance in HF patients by addressing various functional impairments in HF [Citation38,Citation39]. This meta-analysis also emphasizes the benefits of FCM in reducing hospitalizations and enhancing active status. A lower frequency of hospitalizations not only positively impacts the QOL of HF patients but also reduces the disease burden on healthcare systems [Citation3]. It is a cost-effective method of correcting ID [Citation40] with satisfactory patient acceptance and tolerability [Citation41].
In a clinical trial examining the impact of FCM on the extent of diastolic function impairment, reactive vasodilation and oxidative stress biomarkers, it was observed that FCM enhanced cardiac performance, particularly in patients with notable diastolic dysfunction. This improvement was associated with enhanced endothelial function and a reduction in oxidative stress among the patients. Thus, restoring ferritin levels with FCM can help mitigate these issues and improve exercise tolerance in patients of HF with preserved ejection fraction [Citation42].
A study examining the impact of FCM in HF patients with iron deficiency who also had impaired kidney function, while not included in the analysis due to distinct inclusion criteria, yielded valuable results demonstrating that FCM offers benefits to HF patients, irrespective of the presence of moderate-to-severe kidney issues [Citation43]. Patients in the study had varying levels of kidney function at the beginning, and eventually, FCM resulted in similar benefits across all kidney function levels. The main advantage of FCM treatment was reduced hospitalizations and improved QOL up to 24 weeks after treatment. However, FCM did not affect the overall risk of death from any cause. These outcomes corroborate the conclusions of our meta-analysis and indicate that FCM can be a beneficial therapeutic option for individuals with HF and iron deficiency, regardless of their kidney function.
Although our analysis did not reveal any mortality-lowering benefit of FCM compared with the placebo group, it is essential to bear in mind that the duration of these trials was relatively brief. Longer-term studies are necessary to ascertain potential mortality advantages. A point estimate in favor of FCM implies that it could offer advantages in terms of reducing mortality, likely due to its influence in decreasing hospitalizations related to advancing HF or cardiovascular causes.
4.1. Strengths
Earlier meta-analyses have examined the impact of FCM on decreasing mortality related to all cardiovascular causes in iron-deficient patients of HF and the frequency of hospitalizations. However, these previous analyses needed more statistical power because they had fewer events and smaller sample sizes [Citation44]. Our meta-analysis incorporates data from four more randomized clinical trials, including the IRON-CRT [Citation45], HEART-FID [Citation46], PRACTICE-ASIA-HF [Citation47] and EFFECT-HF [Citation48] trials, significantly expanding the sample and eliminating the low-quality studies. This meta-analysis exclusively includes clinical trials, providing robust data. Moreover, all the studies included are high quality and have a low risk of bias, thus strengthening the validity of our results.
4.2. Limitations
Although there was no heterogeneity among the trials, the results regarding mortality did not achieve statistical significance, likely due to the relatively short trial duration and the low number of mortality events. More clinical trials of longer duration are required to investigate these outcomes further. The analysis focused on studying the frequency of HF-related hospitalizations. However, it did not include an examination of factors such as the duration of hospitalization, the underlying causes of hospitalization and comorbidities leading to hospitalization. Moreover, this analysis did not include an assessment of the disease symptoms and QOL after treatment with FCM.
5. Conclusion
FCM significantly decreases hospitalizations related to HF. However, it does not demonstrate any positive impact on mortality rates from all causes or cardiovascular issues in patients with HF and concomitant iron deficiency. These results support the role of FCM in the guidelines for treating ID in HF by highlighting its effectiveness as a therapeutic option [Citation49] .
Author contributions
The conceptualization was done by MA Shafique and V Zuhaira. The literature and drafting of the manuscript were conducted by BS Rangwala, V Zuhaira, MA Shafique, MS Mustafaa, SMF Zaidib, F Danisha, AW Khana and FU Rehmanb. The editing and supervision were performed by BS Rangwala and MA Shafique. All authors have read and agreed to the final version of the manuscript.
Financial disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Supplementary Materials
Download PDF (124 KB)Supplemental material
Supplementary data for this article can be accessed at https://doi.org/10.1080/20565623.2024.2367956
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Cohen-Solal A, Damy T, Terbah M, et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail. 2014;16(9):984–991. doi:10.1002/ejhf.139
- Van Aelst LNL, Abraham M, Sadoune M, et al. Iron status and inflammatory biomarkers in patients with acutely decompensated heart failure: early in-hospital phase and 30-day follow-up. Eur J Heart Fail. 2017;19(8):1075–1076. doi:10.1002/ejhf.837
- Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71(7):782–793. doi:10.1016/j.jacc.2017.12.027
- von Haehling S, Ebner N, Evertz R, et al. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019;7(1):36–46. doi:10.1016/j.jchf.2018.07.015
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31(15):1872–1880. doi:10.1093/eurheartj/ehq158
- Rangel I, Gonçalves A, de Sousa C, et al. Iron deficiency status irrespective of anemia: a predictor of unfavorable outcome in chronic heart failure patients. Cardiology. 2014;128(4):320–326. doi:10.1159/000358377
- Sama IE, Woolley RJ, Nauta JF, et al. A network analysis to identify pathophysiological pathways distinguishing ischaemic from non-ischaemic heart failure. Eur J Heart Fail. 2020;22(5):821–833. doi:10.1002/ejhf.1811
- Severino P, D'Amato A, Pucci M, et al. Ischemic heart disease and heart failure: role of coronary ion channels. Int J Mol Sci. 2020;21(9):3167. doi:10.3390/ijms21093167
- Gajanana D, Shah M, Junpapart P, et al. Mortality in systolic heart failure revisited: ischemic versus non-ischemic cardiomyopathy. Int J Cardiol. 2016;224:15–17. doi:10.1016/j.ijcard.2016.08.316
- Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119(24):3070–3077. doi:10.1161/CIRCULATIONAHA.108.815944
- Silverdal J, Sjöland H, Bollano E, et al. Prognostic impact over time of ischaemic heart disease vs. non-ischaemic heart disease in heart failure. ESC Heart Fail. 2020;7(1):264–273. doi:10.1002/ehf2.12568
- Vedin O, Lam CSP, Koh AS, et al. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail. 2017;10(6):e003875. doi:10.1161/CIRCHEARTFAILURE.117.003875
- Bakogiannis C, Briasoulis A, Mouselimis D, et al. Iron deficiency as therapeutic target in heart failure: a translational approach. Heart Fail Rev. 2020;25(2):173–182. doi:10.1007/s10741-019-09815-z
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
- Martin-Malo A, Borchard G, Flühmann B, et al. Differences between intravenous iron products: focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail. 2019;6(2):241–253. doi:10.1002/ehf2.12400
- Núñez J, Miñana G, Cardells I, et al. Noninvasive imaging estimation of myocardial iron repletion following administration of intravenous iron: the myocardial-IRON trial. J Am Heart Assoc. 2020;9(4):e014254. doi:10.1161/JAHA.119.014254
- Del Canto I, Santas E, Cardells I, et al. Short-term changes in left and right ventricular cardiac magnetic resonance feature tracking strain following ferric carboxymaltose in patients with heart failure: a substudy of the myocardial-IRON trial. J Am Heart Assoc. 2022;11(7):e022214. doi:10.1161/JAHA.121.022214
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi:10.1016/j.jclinepi.2009.06.006
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
- Higgins J, Thompson S, Deeks J, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327:557–560. doi:10.1136/bmj.327.7414.557
- Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi:10.1002/jrsm.12
- Sterne JAC, Egger M, Moher D, Boutron I. Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editors. Cochrane Handbook for Systematic Reviews of Interventions Version. 2017. www.training.cochrane.org/handbook
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–378. doi:10.1038/nrcardio.2016.25
- Stretti L, Zippo D, Coats AJS, et al. A year in heart failure: an update of recent findings. ESC Heart Fail. 2021;8(6):4370–4393. doi:10.1002/ehf2.13760
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Loncar G, Obradovic D, Thiele H, et al. Iron deficiency in heart failure. ESC Heart Fail. 2021;8(4):2368–2379. doi:10.1002/ehf2.13265
- Melenovsky V, Petrak J, Mracek T, et al. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail. 2017;19(4):522–530. doi:10.1002/ejhf.640
- Dunn LL, Suryo Rahmanto Y, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17(2):93–100. doi:10.1016/j.tcb.2006.12.003
- Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol. 2005;6(4):345–351. doi:10.1038/nrm1620
- Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81(3):412–419. doi:10.1093/cvr/cvn301
- Jankowska EA, von Haehling S, Anker SD, et al. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34(11):816–829. doi:10.1093/eurheartj/ehs224
- Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Hematol. 2009;122(2–3):78–86. doi:10.1159/000243791
- Eivazi M, Mutharasan RK, Cleveland E, et al. Outcome of titrating guideline directed medical therapy in heart failure patients at 90-day post-hospital discharge. Journal of Cardiac Failure. 2019;25:S138. doi:10.1016/j.cardfail.2019.07.399
- Beck-da-Silva L, Piardi D, Soder S, et al. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168(4):3439–3442. doi:10.1016/j.ijcard.2013.04.181
- Lewis GD, Malhotra R, Hernandez AF, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. 2017;317(19):1958–1966. doi:10.1001/jama.2017.5427
- McDonagh T, Damy T, Doehner W, et al. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail. 2018;20(12):1664–1672. doi:10.1002/ejhf.1305
- Butler J, Khan MS, Friede T, et al. Health status improvement with ferric carboxymaltose in heart failure with reduced ejection fraction and iron deficiency. Eur J Heart Fail. 2022;24(5):821–832. doi:10.1002/ejhf.2478
- Dhoot S, Mittal S, Singh SP, et al. Effect of ferric-carboxy maltose on oxygen kinetics and functional status in heart failure patients with iron deficiency. Future Sci OA. 2020;6(5):Fso467. doi:10.2144/fsoa-2019-0156
- Bourguignon S, Faller M, Champs FO, et al. Budget impact of intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency in France. ESC Heart Fail. 2019;6(3):559–569. doi:10.1002/ehf2.12432
- McDonagh T, Macdougall IC. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail. 2015;17(3):248–262. doi:10.1002/ejhf.236
- Mollace A, Macrì R, Mollace R, et al. Effect of ferric carboxymaltose supplementation in patients with heart failure with preserved ejection fraction: role of attenuated oxidative stress and improved endothelial function. Nutrients. 2022;14(23):5057. doi:10.3390/nu14235057
- Macdougall IC, Ponikowski P, Stack AG, et al. Ferric carboxymaltose in iron-deficient patients with hospitalized heart failure and reduced kidney function. Clin J Am Soc Nephrol. 2023;18(9):1124–1134. doi:10.2215/CJN.0000000000000223
- Khan MS, Usman MS, von Haehling S, et al. Ferric carboxymaltose for the treatment of iron-deficient heart failure patients: a systematic review and meta-analysis. ESC Heart Fail. 2020;7(6):3392–3400. doi:10.1002/ehf2.13146
- Martens P, Dupont M, Dauw J, et al. The effect of intravenous ferric carboxymaltose on cardiac reverse remodelling following cardiac resynchronization therapy-the IRON-CRT trial. Eur Heart J. 2021;42(48):4905–4914. doi:10.1093/eurheartj/ehab411
- Harrington J, Mentz RJ, Rockhold FW, et al. Baseline characteristics of patients in the randomized study to investigate the efficacy and safety of ferric carboxymaltose as treatment for heart failure with iron deficiency: HEART-FID trial. Am Heart J. 2023;266:25–31. doi:10.1016/j.ahj.2023.08.005
- Yeo TJ, Yeo PSD, Hadi FA, et al. Single-dose intravenous iron in Southeast Asian heart failure patients: a pilot randomized placebo-controlled study (PRACTICE-ASIA-HF). ESC Heart Fail. 2018;5(2):344–353. doi:10.1002/ehf2.12250
- van Veldhuisen DJ, Ponikowski P, van der Meer P, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136(15):1374–1383. doi:10.1161/CIRCULATIONAHA.117.027497
- Geng Q, Li S, Wang Z, et al. Efficacy and safety of combined neprilysin and RAS inhibition in heart failure: a meta-analysis of randomized controlled trials. Int J Cardiol. 2019;293:159–164. doi:10.1016/j.ijcard.2019.05.048