Abstract
Endometrial carcinomas are common malignancies worldwide. Microsatellite instability is often identified in endometrioid endometrial carcinomas (EECs) and in most Lynch syndrome (LS) associated tumours. The authors aimed to identify the number of EECs at a South African public hospital, using the four-mismatch repair immunohistochemical stains, which may suggest possible LS, for the period 2009–2015. Following ethical clearance, 145 cases of archived EEC underwent immunohistochemical testing for MLH1, MSH2, MSH6 and PMS2. Cases demonstrating loss of MLH1 staining were subjected to EpiTYPER for quantitative methylation of the MLH1 promoter region using the Agena MassARRAY® platform. Forty-one (28.28%) cases showed complete loss of tumour nuclei staining for ≥ 1 immunohistochemical stains. Twenty cases (13.79%) showed loss of MLH1/PMS2, 16 cases (11.03%) demonstrated isolated MLH1 loss and one case (0.69%) showed loss of MLH1, PMS2 and MSH6. Two cases showed isolated MSH6 loss and 2 cases showed loss of MSH2 and MSH6. Thirty-seven (90.24%) of 41 mismatch repair-deficient cases showed MLH1 loss. Most (83.78%) of these showed promoter hypermethylation, suggesting a sporadic occurrence of carcinogenesis. The patients from whom the two cases with isolated MSH6 loss, two cases with MSH2/MSH6 loss, and the six cases whereby MLH1 was not explained by methylation should be identified and offered genetic counselling with a view to possible germline mutation assessment as these patients are suspected of having Lynch syndrome. Thus, this study demonstrates a possible 10/145 (6.90%) patients who may have LS and require further testing and suggests a need to screen possible LS patients in the South African population.
Introduction
Carcinomas of the endometrium are commonly encountered tumours in western countries.Citation1–4 The 2019 predicted incidence of endometrial carcinoma in the United States of America is approximately 7%.Citation5 According to the latest published statistics for 2014 by the National Cancer Registry in South Africa, malignancies of the uterine corpus accounted for an approximate incidence of 3% amongst all tumours recorded in females.Citation6
Microsatellite instability (MSI) is a frequent molecular anomaly in endometrioid endometrial carcinoma.Citation7–11 Most cases of microsatellite instability are due to alterations in expression of proteins of the DNA mismatch-repair system; namely MLH1, MSH2, MSH6 and PMS2.Citation11,Citation12 It may be common practice to screen endometrial cancers for loss of these proteins to detect mutations associated with Lynch syndrome, or to determine if a patient is eligible for targeted immunotherapy with pembrolizumab, an anti-PD-1 agent, in some countries.Citation13–16 This is not the case in South Africa. The most common loss is seen in MLH1 as a consequence of sporadic promoter methylation, which, if detected, points towards a sporadic occurrence of the tumour and generally excludes Lynch syndrome.Citation15 Patients in whom methylation is not detected may be referred for genetic counselling with a view to further mutational testing.Citation15
The reported prevalence of Lynch syndrome varies by population and had been shown to range from 0% to 10%.Citation17 For example, Finland has been identified as having well-documented mutations of MLH1.Citation17 Studies by Hampel et al., however, have noted that the incidence of Lynch syndrome identified in endometrial carcinoma is comparable to colorectal carcinoma in patients from the same geographic locale.Citation17 A study by Vergouwe et al. identified that over 20% of colorectal carcinomas in a region of South Africa demonstrated MLH1 or MSH2 loss and, of these, approximately 10.5% were calculated to be a result of germline mutations.Citation18 Their study highlighted the possibility of a high incidence of inherited tumours within the South African population. It is thus possible that a greater incidence of germline mutation associated tumours occur in South African patients. Confirmatory germline mutational assessment is currently not available in public hospitals in the Gauteng province in South Africa. Thus, the cases in this study that have been identified as having mismatch repair deficiencies by immunohistochemistry cannot undergo confirmatory, definitive testing for Lynch syndrome. To the best of the authors’ knowledge, a study assessing for possible Lynch syndrome in endometrial carcinomas in the South African population has not been performed to date.
Materials and methods
Following ethical clearance (Clearance certificate number M151051), 145 cases of archived endometrioid endometrial carcinoma for the period 2009–2015 were retrieved from the Department of Anatomical Pathology, University of the Witwatersrand/National Health Laboratory Service at the Charlotte Maxeke Johannesburg Academic Hospital. This department receives cases from a large catchment area of public hospitals and clinics. The samples included biopsies and excision specimens. There were 57 excision specimens out of 145 and the remainder were curettage specimens. These specimens accounted for all cases of endometrioid endometrial carcinoma seen during the period 2009–2015. Our department drains all public hospitals and clinics in the Southern Gauteng area. As such, we receive all pathology specimens from public hospitals in this area. The cases were identified using a SNOMED search on the DISA and LabTrack laboratory information system.
Unfortunately, following curettage, many patients are lost to follow-up in the public hospital setting in South Africa. The cases were not submitted as consults from other pathology departments. The slides were reviewed, and the histological diagnosis confirmed. This study was confined to cases of endometrioid endometrial carcinoma as previous work has shown that microsatellite instability is in essence limited to this subtype of endometrial tumour.Citation19 Age was categorised as < 60 and ≥ 60 years. In general, 50 years of age is generally accepted as the age of menopause; however, an age cut-off of 60 was used as a low percentage of patients (2/145) were under the age of 50.Citation20
Immunohistochemistry
The four mismatch repair antibodies, namely MLH1 (Novocastra, UK), PMS2 (Novocastra, UK), MSH2 (Novocastra, UK) and MSH6 (Novocastra, UK), were used to stain 4 μm deparaffinised sections. A microtome was used to cut tissue sections from paraffin-embedded blocks. The tissue sections were then floated onto slides, which subsequently underwent drying overnight at 60°C. These four immunohistochemical stains had previously been optimised according to departmental standard operating procedure. Staining of tissue sections includes the treatment of primary antibody serum. Each of the tissue sections then underwent staining with the following antibodies: MLH1 (Clone ES05, 1:50), PMS2 (Clone MOR4G, 1:50), MSH2 (Clone 25D12, 1:50) and MSH6 (PU29, 1:50). A mouse linker resulted in increased expression of antigens. The sections were washed with Tris buffered saline (TBS) at pH 7.6. Immunohistochemistry was undertaken using an automated staining machine (DAKO Autostainer Link 48, Denmark). A ready-made solution, the EnVision™ FLEX target Retrieval Solution, High pH, was used for antigen retrieval. The chromogen used was 3,3′ diaminobenzidine hydrochloride solution (DAB, Sigma, USA), which produced a brown pigment. Meyer's haematoxylin was the counterstain used on tissue sections. Appropriate positive and negative control tissue sections were used.
Immunohistochemically stained tumour tissue sections were then assessed in a binary manner. The tumour cells demonstrated either positive staining of their nuclei (irrespective of intensity or volume) or the tumour cells were negative as there was no staining of tumour cells, in the presence of an internal positive control, which took the form of staining of endothelial cells, lymphocytes and stromal cells. As the possibility existed of only a few foci of positive staining nuclei identified in certain cases, implying that the mismatch repair genes remained intact, tissue microarrays had not been performed.
Methylation assessment
MLH1 promoter hypermethylation analysis was performed using MassARRAY EpiTyper analysis, which was undertaken by Agena Bioscience at Inqaba Biotec.
EpiTYPER is a system using DNA methylation analysis technology, which has shown that it is a quantitative method of DNA methylation analysis that provides reliable results. The target sequence of the MLH1 promoter region, (−248 to −178), according to Pérez-Carbonell et al.,Citation21 was entered into the software package, which then identified primers allowing for the best possible DNA coverage. The forward primers were: AGGAAGAGCGGATAGCGATTT and the reverse primers were: TCTTCGTCCCTCCCTAAAACG. A product yield of 187 base pairs was noted and allowed for evaluation of 11 CpG islands. There were 3 CpG targets that could not be assessed due to lower mass cleavage products, which is a known disadvantage of EpiTYPER.Citation22 The remaining 8 CpG sites could be assessed. One (1) μg of genomic DNA was required for bisulphite treatment and a ratio of between 1.7 and 2.0 was recorded for each sample using a Nanodrop spectrophotometer. DNA tubes were stored at 4°C. The forward and reverse primers were reconstituted with nuclease-free water at equimolar concentrations of 100 μM. These were subsequently diluted to a working solution of 1 μM (personal communication, Inqaba Biotec).Citation23 Bisulphite conversion, PCR, in-vitro transcription and cleavage was performed according to the manufacturer's instructions.Citation23 The cleaved products were identified using the MassARRAY compact mass spectrometer and real-time software by Agena. Matrix-assisted laser desorption/ionisation time of flight (MALDI-TOF) mass spectrometry identified patterns that were created from different cleavage products of methylated or unmethylated target regions. The relative amount of methylation was calculated by comparing the signal intensity between mass signals from unmethylated and methylated template DNA. In each case, a percentage of methylation was calculated and depicted in an Epigram.
Results
shows that the average age of patients diagnosed with endometrial carcinomas was 65.2 years, with the majority (66.9%) of patients being over the age of 60 years. Most of the endometrial carcinomas in the present study showed grade 2 Fédération Internationale de Gynécologie Obstétrique (FIGO) histological features.
Table 1: Age and pathological distribution of the study samples
Forty-one (28.28%) of the 145 cases demonstrated complete loss of staining of tumour nuclei for one or more immunohistochemical stains in the presence of adequate internal controls such as positive staining of stromal cells, endothelial cells and lymphocytes. There were 20 cases (13.79%) that showed loss of MLH1 and PMS2, whilst 16 cases (11.03%) showed isolated loss of MLH1 staining. A single case (0.69%) showed loss of three markers, namely MLH1, PMS2 and MSH6. There were two cases that demonstrated isolated loss of MSH6 staining and two cases showed loss of staining of both MSH2 and MSH6. Thus, MLH1 demonstrated loss of staining most commonly amongst the four antibodies. One hundred and four (71.72%) cases demonstrated retention of staining of all mismatch repair immunohistochemical markers. demonstrate tumours stained with the four immunohistochemical markers.
Figure 1: Lymphocytes and stromal cell nuclei stain positively (arrows) but the nuclei of tumour cells are negative. 4 μm MLH1 stained section. Original magnification: 200x.
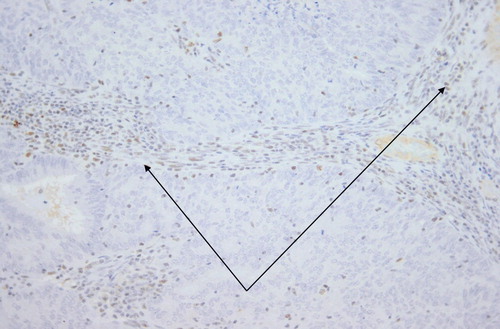
Figure 2: Lymphocytes and stromal cell nuclei stain positively (arrows). Nuclei of tumour cells are negative. 4 μm PMS2 stained section. Original magnification: 200x.
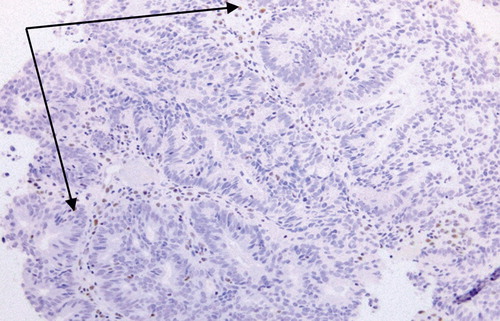
Figure 3: There is positive staining of tumour nuclei. 4 μm MSH2 stained section. Original magnification: 200x.
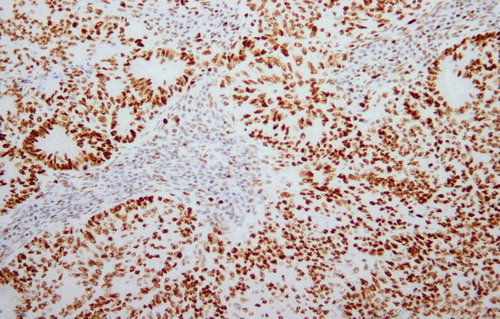
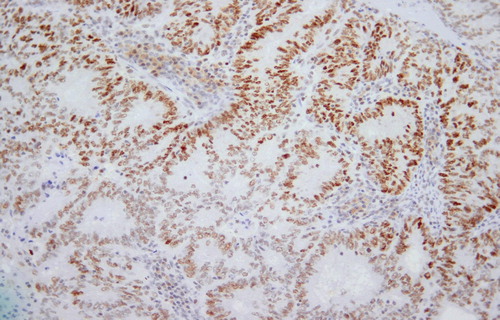
Figure 4: Positive staining of tumour nuclei is demonstrated. 4 μm MSH6 stained section. Original magnification: 200x.
demonstrates a statistically significant association between the tumour grade and the absence of IHC staining (p = 0.002). Of the 41 patients with an abnormal/loss of IHC staining, 22% had a grade 1 tumour, whilst 65.9% of the 41 cases with abnormal immunohistochemical staining showed grade 2 histological features. Approximately double the proportion of cases had abnormal immunohistochemical staining with grade 2 histological features than those with normal IHC staining. Of the 41 cases with abnormal IHC staining, 12.2% were grade 3 tumours.
Table 2: Comparison of age and tumour grades of immunohistochemically detected loss of staining and those with retained nuclear staining
The proportion of cases with retained IHC staining in FIGO grades 1 and 3 endometrial carcinomas was approximately four times more than those demonstrating loss of staining for their respective tumour grades. Grade 2 tumours showed a moderately increased proportion of IHC staining compared with tumours that had loss of IHC staining. One of the 37 cases that showed MLH1 loss that underwent hypermethylation analysis had insufficient DNA for further analysis. There were 31 (83.78%) cases that demonstrated methylation levels of more than 10% and there were 5 cases (13.51%) that had very low levels of methylation and were thus regarded as not being methylated.Citation24
demonstrates that there were no statistically significant differences in the age of those who harboured methylated tumours versus those who did not (p = 0.532).
Table 3: Comparison of age and tumour grades of hypermethylated and unmethylated tumours
More than two-thirds of methylated cases demonstrated grade 2 histological features in contrast to unmethylated cases.
For comparison with the present study, only numbers of endometrioid endometrial carcinoma in other studies are shown as some of these studies examined all histological subtypes of endometrial carcinoma, whilst other studies focused only on endometrioid endometrial carcinomas ().Citation2,Citation17,Citation25–33 The present study shows that a high percentage of MMR-deficient cases of endometrioid endometrial carcinoma are due to MLH1 promoter methylation, similar to studies by other authors. The cases that are unmethylated raise concern for a possible underlying germline mutation, which in many of the western studies have been investigated.
Table 4: Comparison of some previously reported endometrial carcinomas in the literature with the present study
Discussion
Hampel et al. have shown that sensitivity of family history is not a useful method of screening for possible patients with Lynch syndrome.Citation34 In a country such as South Africa, which is multiculturally rich, it is possible that language differences may be a limiting factor in acquiring an in-depth, extensive history of tumours in family members. This may then fare poorly as a screening utensil for Lynch syndrome. Ryan and co-workers as well as Garg and colleagues have noted that approximately 50% of germline mutation carriers with endometrial carcinoma do not demonstrate histological features that have been associated with inherited mutations.Citation35,Citation36 The histological features include location of tumours in the lower uterine segment, tumour infiltrating lymphocytes, heterogeneity of tumour and peri-tumoural lymphocytes.Citation35,Citation36 Shia has also stated that histological findings may not serve as a useful screening tool.Citation37 In the present study, the histological features could not be assessed in each case as not all patients underwent hysterectomy. This may be ascribed to many patients being lost to follow-up after their initial curettage. It is therefore not possible to discuss findings in the local population. However, by adopting an endometrial carcinoma screening programme, anatomical pathologists are placed in a position whereby they may be able to identify possible patients with Lynch syndrome in South Africa.
Methylation analysis by EpiTYPER demonstrated that the majority of cases (83.78%) of the abnormal/deficient MMR stains could be attributed to hypermethylation. This is consistent with findings of studies undertaken in western societies.Citation2,Citation38 Identification of hypermethylation in these patients suggests a sporadic occurrence of carcinogenesis and, as such, these patients would not undergo additional molecular tests.
An article by Hashmi et al. has documented isolated MLH1 loss in a cohort of patients with colorectal carcinoma.Citation39 In their study, they noted that the cases demonstrating isolated MLH1 loss would undergo methylation analysis and BRAF mutational assessment. The identification of any degree of nuclear staining was interpreted as positive in the present study. Thus, even faint, focal staining of tumour nuclei was interpreted as MMR proficient. As such, this accounts for the identification of PMS2 positive staining despite loss of MLH1. This could possibly be attributed to missense mutations as noted by Shia, who stated that missense mutations may culminate in inactive mutant proteins that may still retain their antigenicity.Citation40 However, as mutational analysis had not been performed, it is not possible to state this with certainty. Furthermore, the clone of antibody used for PMS2 at the time that this study was performed is not a clone that has provided the best results as indicated by NordiQCCitation41 and, as such, this may have resulted in an overly sensitive stain. Whilst the clones for the antibodies PMS2 (clone MOR4G), MSH2 (clone 25D12) and MSH6 (clone PU29) have not been assessed to be the best clones for their respective antibodies in assessments by NordiQC, these antibodies have been optimised for diagnostic use in our laboratory and have demonstrated adequate staining of internal controls such as endothelial cells, stromal cells and lymphocytes in control and test tissue samples.Citation41–43 Nevertheless, the results of the present study suggest that the possibility of PMS2 staining, although slight, may still exist in the absence of MLH1 staining.
Of the IHC diagnosed MMR-deficient tumours in the present study, the mean age at diagnosis was 65.3 (± 9.7) years (), which is higher than that noted in studies by Buchanan et al. and Kato et al., but is fairly similar to that seen by McConechy et al.Citation44–46 Of the IHC-deficient cases, grade 2 histological features were seen in 65.9% of samples in the present study, whilst the minority of tumours (12.2%) showed grade 3 features. Grade 2 histological features were also identified as being the most common subtype in a study by Bruegl et al. and McConechy et al.Citation46, Citation47 This may be due to microsatellite unstable endometrial carcinomas being coupled to higher grade and stage.Citation48 In contrast, a study undertaken by Buchanan et al. demonstrated that grade 1 histological features were the most commonly occurring subtype.Citation44 The present study suggests a need to screen possible Lynch syndrome patients in the South African population. It is suggested that screening be performed in patients below 70 years of age as most patients in the current study were over 60 years of age and therefore, if screening is only for patients under the age of 60 years, many patients with possible Lynch syndrome may not be detected. In an attempt to curb the cost of tests, biopsies from such patients may undergo initial IHC testing for only MSH6 and PMS2, as suggested by Cho et al.Citation15 Should abnormal/loss of staining in MSH6 and/or PMS2 IHC stains be documented, these may be followed by immunohistochemical testing of MSH2 and/or MLH1. Many pathology laboratories have access to immunohistochemical tests and the cost-effectiveness of IHC testing has been demonstrated. These factors together with a sensitivity and specificity of 83% and 89% respectively for Lynch syndrome are pointers to IHC being a useful screening tool.Citation15 Subsequent reflex testing for promoter methylation may then be performed when indicated. Loss of staining of specific immunohistochemical markers may then facilitate testing of specific genes, should such tests be available.Citation15 Identification of mismatch-proficient versus mismatch-deficient tumours may facilitate consideration of alternative treatment options as tumour cells of mismatch repair-proficient tumours are sensitive to methylating agents whereas mismatch repair-deficient tumours are not.Citation49 In addition, with the option of targeted therapy being available, the presence of mismatch-repair mutations allows for consideration of use of an antibody directed against programmed cell death (PD-1), which has shown favourable results.Citation11,Citation50
Conclusion
In total, 41/145 cases demonstrated mismatch repair deficiency by immunohistochemistry, of which 37 (90.24%) showed MLH1 loss. Most (83.78%) MLH1 deficient cases demonstrated promoter hypermethylation, suggesting sporadic carcinogenesis. The two cases with isolated MSH6 loss, two cases with MSH2 and MSH6 loss, and six cases with deficient MLH1staining, unexplained by methylation, are suspected of having Lynch syndrome.Citation51 Thus, a possible 10/145 (6.90%) patients may have LS requiring further testing. Two studies suggested a greater incidence of germline mutation associated tumours in South African patients compared with the Western world.Citation18,Citation52 Our study aims to highlight the possibility of Lynch syndrome associated endometrial carcinomas and envisages that state patients with suspected germline tumours, requiring mutational assessment, may be offered tumour specific therapy.
Disclosure statement
No conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan 1;68(1):7–30.
- Peterson LM, Kipp BR, Halling KC, et al. Molecular characterization of endometrial cancer: a correlative study assessing microsatellite instability, MLH1 hypermethylation, DNA mismatch repair protein expression, and PTEN, PIK3CA, KRAS, and BRAF mutation analysis. Int J Gynecol Pathol. 2012 May;31(3):195–205.
- Garcia-Dios DA, Lambrechts D, Coenegrachts L, et al. High-throughput interrogation of PIK3CA, PTEN, KRAS, FBXW7 and TP53 mutations in primary endometrial carcinoma. Gynecol Oncol. 2013 Feb 1;128(2):327–34.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015 Jan 1;65(1):5–29.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan 1;69(1):7–34.
- The South African National Cancer Registry. Cancer in South Africa (2014) Johannesburg. [cited 2019 March 1]. http://www.nicd.ac.za/index.php/centres/national-cancer-registry/
- Arabi H, Guan H, Kumar S, et al. Impact of microsatellite instability (MSI) on survival in high grade endometrial carcinoma. Gynecol Oncol. 2009 May;113(2):153–8.
- Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013 May 2;497(7447):67–73.
- Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013 Jan 1;62(1):111–23.
- Yeramian A, Moreno-Bueno G, Dolcet X, et al. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2013 Jan 24;32(4):403–13.
- Wadee R, Grayson W. A potpourri of pathogenetic pathways in endometrial carcinoma with a focus on Lynch Syndrome. Ann Diagn Pathol. 2019 Apr 1;39:92–104.
- Bell DW, O’Hara AJ. The genomics and genetics of endometrial cancer. Adv Genomics Genet. 2012 Mar;33–47.
- Dillon JL, Gonzalez JL, DeMars L, et al. Universal screening for Lynch syndrome in endometrial cancers: frequency of germline mutations and identification of patients with Lynch-like syndrome. Hum Pathol. 2017 Dec;70:121–8.
- Watkins JC, Yang EJ, Muto MG, et al. Universal screening for mismatch-repair deficiency in endometrial cancers to identify patients with Lynch Syndrome and Lynch-like Syndrome. Int J Gynecol Pathol. 2017 Mar;36(2):115–27.
- Cho KR, Cooper K, Croce S, et al. International society of gynecological pathologists (ISGyP) endometrial cancer project: guidelines from the special techniques and ancillary studies group. Int J Gynecol Pathol. 2019 Jan;38(Iss 1 Suppl 1):S114–22.
- Mills AM, Longacre TA. Lynch Syndrome screening in the gynecologic tract: current state of the art. Am J Surg Pathol. 2016 Apr;40(4):e35–44.
- Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66(15):7810–7.
- Vergouwe F, Boutall A, Stupart D, et al. Mismatch repair deficiency in colorectal cancer patients in a low-incidence area. S Afr J Surg 2013;51(1):16–21. DOI:107196/SAJS1314.2013;51(1):6.
- Zighelboim I, Goodfellow PJ, Gao F, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007 May 20;25(15):2042–8.
- Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011 Sep;38(3):425–40.
- Pérez-Carbonell L, Alenda C, Payá A, et al. Methylation analysis of MLH1 improves the selection of patients for genetic testing in Lynch Syndrome. J Mol Diagn. 2010 Jul;12(4):498–504.
- Olkhov-Mitsel E, Bapat B. Strategies for discovery and validation of methylated and hydroxymethylated DNA biomarkers. Cancer Med. 2012 Oct;1(2):237–60.
- Quantitative Methylation Analysis Epityper Protocol from Agena.pdf.
- Suchiman HED, Slieker RC, Kremer D, et al. Design, measurement and processing of region-specific DNA methylation assays: the mass spectrometry-based method EpiTYPER. Front Genet. 2015 Sep 17 [cited 2018 Aug 24];6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4585020/
- Bartley AN, Luthra R, Saraiya DS, et al. Identification of cancer patients with Lynch Syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res. 2012 Feb 1;5(2):320–7.
- Bruegl AS, Djordjevic B, Urbauer DL, et al. Utility of MLH1 methylation analysis in the clinical evaluation of Lynch Syndrome in women with endometrial cancer. Curr Pharm Des. 2014;20(11):1655–63.
- Bruegl AS, Ring KL, Daniels M, et al. Clinical challenges associated with universal screening for Lynch Syndrome–associated endometrial cancer. Cancer Prev Res. 2017 Feb;10(2):108–15.
- Cornel KMC, Wouters K, Van de Vijver KK, et al. Gene promoter methylation in endometrial carcinogenesis. Pathol Oncol Res. 2018 Nov 14 [cited 2019 Feb 17]; Available from: http://link.springer.com/10.1007/s12253-018-0489-2
- Hashmi AA, Mudassir G, Hashmi RN, et al. Microsatellite instability in endometrial carcinoma by immunohistochemistry, association with clinical and histopathologic parameters. Asian Pac J Cancer Prev. 2019 Sep 1;20(9):2601–6.
- Kim J, Kong J, Yang W, et al. DNA mismatch repair protein immunohistochemistry and MLH1 promotor methylation testing for practical molecular classification and the prediction of prognosis in endometrial cancer. Cancers. 2018 Aug 21;10(9):279.
- Nagle CM, O’Mara TA, Tan Y, et al. Endometrial cancer risk and survival by tumor MMR status. J Gynecol Oncol. 2018 [cited 2018 Nov 10];29(3). Available from: https://synapse.koreamed.org/DOIx.php?id=10.3802/jgo.2018.29.e39
- Stelloo E, Jansen AML, Osse EM, et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol. 2016 Oct 13;28:96–102.
- Woo YL, Cheah PL, Shahruddin SI, et al. The immunohistochemistry signature of mismatch repair (MMR) proteins in a multiethnic asian cohort with endometrial carcinoma. Int J Gynecol Pathol. 2014 Nov;33(6):554–9.
- Hampel H, Panescu J, Lockman J, et al. Comment on: screening for Lynch Syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2007 Oct 1;67(19):9603.
- Ryan P, Mulligan AM, Aronson M, et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer. 2012;118(3):681–8.
- Garg K, Leitao MM, Kauff ND, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol. 2009 Jun;33(6):925–33.
- Shia J, Holck S, DePetris G, et al. Lynch syndrome-associated neoplasms: a discussion on histopathology and immunohistochemistry. Fam Cancer. 2013 Jun;12(2):241–60.
- Metcalf AM, Spurdle AB. Endometrial tumour BRAF mutations and MLH1 promoter methylation as predictors of germline mismatch repair gene mutation status: a literature review. Fam Cancer. 2014 Mar;13(1):1–12.
- Hashmi AA, Ali R, Hussain ZF, et al. Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological parameters. World J Surg Onc. 2017 Dec;15(1):116.
- Shia J, Ellis NA, Klimstra DS. The utility of immunohistochemical detection of DNA mismatch repair gene proteins. Virchows Archiv. 2004 Nov;445(5):431–41.
- Nordic Immunohistochemical Quality Control, PMS2 run 53 2018 [Internet]. 2018 [cited 2018 Nov 19]. Available from: http://www.nordiqc.org/downloads/assessments/104_84.pdf
- Nordic Immunohistochemical Quality Control, MSH2 run 50 2017. 2018.
- Nordic Immunohistochemical Quality Control, MSH6 run 52 2018 [Internet]. 2018 [cited 2018 Nov 19]. Available from: http://www.nordiqc.org/downloads/assessments/101_83.pdf
- Buchanan DD, Tan YY, Walsh MD, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014 Jan 10;32(2):90–100.
- Kato M, Takano M, Miyamoto M, et al. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol. 2015;26(1):40.
- McConechy MK, Talhouk A, Li-Chang HH, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2015 May;137(2):306–10.
- Bruegl AS, Kernberg A, Broaddus RR. Importance of PCR-based tumor testing in the evaluation of Lynch Syndrome–associated endometrial cancer. Adv Anat Pathol. 2017;24(6):372–8.
- Karamurzin Y, Rutgers JKL. DNA mismatch repair deficiency in endometrial carcinoma. Int J Gynecol Pathol. 2009 May;28(3):239–55.
- Wielders EA, Hettinger J, Dekker R, et al. Functional analysis of MSH2 unclassified variants found in suspected Lynch syndrome patients reveals pathogenicity due to attenuated mismatch repair. J Med Genet. 2014 Apr 1;51(4):245–53.
- Kunitomi H, Banno K, Yanokura M, et al. New use of microsatellite instability analysis in endometrial cancer. Oncol Lett. 2017 Sep;14(3):3297–301.
- Wang Y, Wang Y, Li J, et al. Lynch syndrome related endometrial cancer: clinical significance beyond the endometrium. J Hematol Oncol. 2013 Mar 25;6:22.
- Blokhuis MM, Pietersen GE, Goldberg PA, et al. Lynch syndrome: the influence of environmental factors on extracolonic cancer risk in hMLH1 c.C1528T mutation carriers and their mutation-negative sisters. Fam Cancer. 2010 Sep;9(3):357–63.
