Abstract
Small bowel obstruction is a common clinical presentation that presents a diagnostic conundrum. Over the last 2 decades, there has been a paradigm shift in the radiological investigation of small bowel obstruction (SBO) and in the indication for and timing of surgical intervention. Cross-sectional imaging (predominantly computed tomography) has largely replaced the widespread use of radiographic small bowel follow-through studies as the imaging modality of choice for SBO. This article illustrates the current imaging modalities available for diagnosis of small bowel obstruction.
Introduction
Intestinal obstruction is a common clinical entity presenting with signs and symptoms that mimic several acute abdominal disorders and is responsible for approximately 20% of all surgical admissions for acute abdominal conditions.Citation1,2 Bowel obstruction may be mechanical or paralytic in origin and may be subdivided into large and small intestinal obstruction. The small bowel (SB) is affected in 60–80% of cases.Citation2
Over the last 2 decades, there has been a paradigm shift in the radiological investigation of small bowel obstruction (SBO) and in the indication for and timing of surgical intervention. The old surgical adage when faced with possible SBO, ‘to never let the sun set or rise on an obstructed bowel’,Citation1 has completely changed with the revolutionary advances in abdominal imaging, forming the cornerstone in guiding clinical decision-making.Citation3 Diagnostic imaging is charged with the multifaceted task of verifying the presence of obstruction and providing relevant information on the site, severity, possible causes, and potential complications of the obstruction.Citation4Imaging addresses the pivotal question of whether to institute a trial of non-surgical treatment or to opt for emergency surgery due to the risk of strangulation.Citation4 The radiological diagnosis of SBO may be made by a variety of imaging techniques including plain radiographs, enteroclysis, and cross-sectional imaging [Computed Tomography (CT) & Magnetic Resonance Imaging (MRI)]. The literature supports the continued use of plain radiographs for initial screening of patients; however, the next step in the imaging pathway depends on the clinical findings and the particular questions that need to be answered in each individual case.
This article reviews the current imaging modalities available for diagnosis in order to assist the clinician in making the most appropriate and cost-effective decision for optimum patient management. Given the escalating costs of healthcare and the advent of newer more advanced and revolutionary imaging modalities; the radiologist needs to guide family practitioners in requesting the most appropriate available diagnostic study to positively influence clinical management, improve patient outcome, lower medical costs, all without compromising a high standard of patient care.
Clinical
Clinically patients present with abdominal pain, distension, nausea, and vomiting,Citation3 which may mimic other abdominal emergencies.
Over the last 50 years, the aetiology of SBO has changed from predominantly hernias (Figures and b) to adhesions (Figure ), Crohn’s disease (Figure1d) and malignancies as the top three causes of SBO in Western society.Citation3,4 Hernias remain the predominant cause of SBO in some developing countries,Citation3 with TB and HIV forming a significant contributory factor in the developing world.
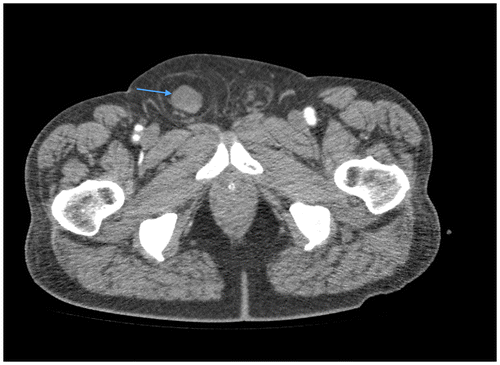
Figure 1a: Sagittal post-contrast CT showing an umbilical hernia with herniation of small bowel loops through a midline defect in the abdominal wall
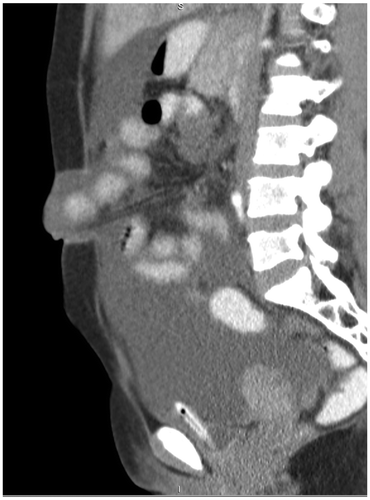
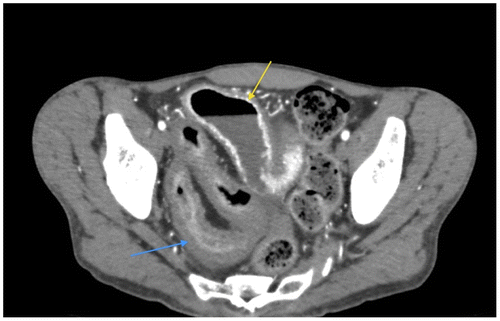
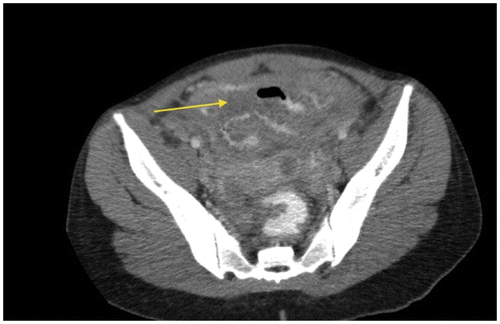
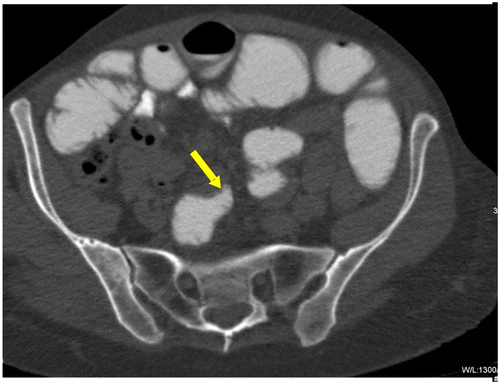
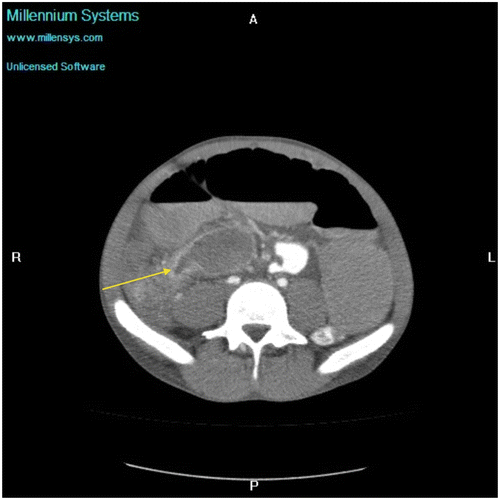
Figure 1c: Axial CT showing SBO with abrupt transition point (yellow arrow) with no associated mass in keeping with an adhesive band, confirmed at surgery
Radiological investigations
Plain radiographs
Despite advances in modern radiology, plain radiographs remain the pivotal starting point in the diagnostic algorithm of SBO. The acute abdominal series encompasses an erect and supine abdominal radiograph and an erect chest radiograph. Conventional radiography is used to triage patients and assist the clinician in formulating an individualized management plan. Given the non-specific signs and symptoms of SBO, plain radiographs are a bedside test that may potentially rule out other causes of an acute abdomen such as renal calculi, pancreatitis, and appendicitis. The literature shows that abdominal radiography in conjunction with clinical examination is diagnostic in only 50–60% of cases of SBO.Citation3−6 SBO is suggested by the findings of small bowel distension with absent colonic gas; however, due to the great interpreter variability and confusion over diagnostic terms, emphasis has been placed on the consistency of the terms used by radiologists to describe intestinal bowel patterns when reviewing plain abdominal films.Citation3
The following terminology has been advocated to describe SB gas patternsCitation3–5: as can be seen in Table : SMALL BOWEL GAS PATTERNS.
Table 1: Small bowel gas patterns
The presence of ≥ 2 air fluid levels, differential air fluid levels in the same loop of bowel more than 2 cm in height and a mean air–fluid level of > 25 mm in width on erect abdominal radiographs is highly suggestive of high grade obstruction.Citation1,4,5 The cause of SBO is often unidentifiable on plain films. Rarely pathologies such as gallstone ileus, inguinal hernias, gossypibomas, ingested foreign bodies and worm bolus may be diagnosed on plain filmCitation6 (Figures and ). Features consistent with strangulation such as pneumatosis intestinalis, gas in the portal veins and thickened oedematous folds are rarely seen.Citation6 An erect chest radiograph/lateral decubitus abdominal film may show pneumoperitoneum if SBO is complicated by perforation.
Figure 3a: Abdominal radiograph showing tubular air lucency (blue arrow) resembling bowel overlying the left inguinal region in keeping with an inguinal hernia
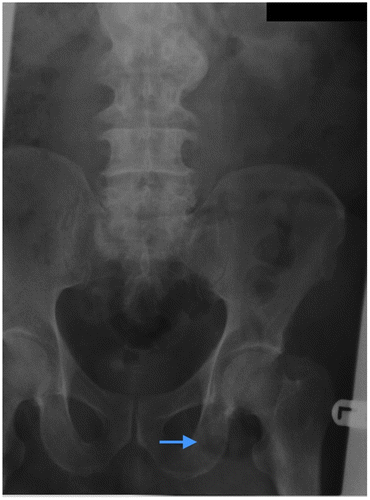
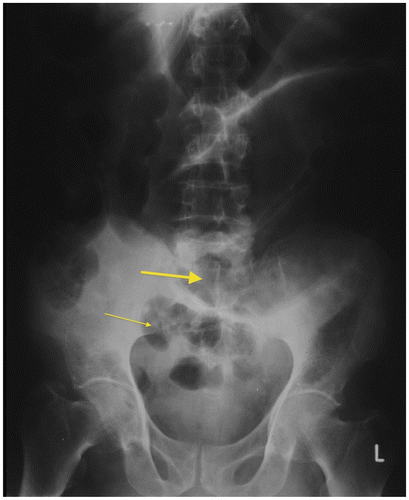
Figure 3b: Plain radiograph of surgically-confirmed ileosigmoid knot showing dilated sigmoid colon with the apex in the left upper quadrant and the point of convergence overlying the right iliac fossa (thick arrow) with dilated small bowel loops in the pelvis (thin arrow)
Significant limitations in the diagnostic accuracy and specificity of plain film radiography are clearly documented in the literature.Citation1 Despite these limitations, the recognition of an unequivocal SBO pattern, the high sensitivity in detecting high grade obstruction, the widespread availability of plain radiographs, and its cost-effectiveness result in conventional radiography forming the mainstay in the evaluation of suspected SBO.Citation6,7
Advantages: Cost-effective, easily available. If an unequivocal SBO pattern is diagnosed, urgent surgical management may be commenced. Serial imaging may be performed to assess progression.
Limitations: Maybe normal in early obstruction. May be non-diagnostic in low grade obstruction. Rarely demonstrates aetiology.
Multi-detector computed tomography (MDCT)/CT enterography/CT enteroclysis (CTE)
Given the significant limitations in the diagnostic accuracy and specificity of plain film radiography, cross-sectional imaging like CT has revolutionized the assessment of SBO. CT has effectively replaced contrast studies as the imaging modality of choice for suspected SBO. Advances in MDCT with multiplanar (MPR) and 3-Dimensional (3D) reformat capabilities allows the demonstration of pathological processes involving the bowel wall, bowel lumen, mesentery, mesenteric vessels, and peritoneal cavity.Citation7 MDCT is superb in confirming the presence of, determining the site, level, and cause of SBO, and in demonstrating complications, for example infarction and perforation.Citation5 Thus, CT has become an important tool in the pre-operative assessment of SBOCitation,4,5 providing an anatomical road-map for surgery, especially for high grade partial and complete SBO.Citation5 Controversy exists regarding the use of oral contrast in the CT assessment of SBO; however, current teaching advocates using IV contrast only in the acute setting, as the retained intraluminal fluid provides a natural negative contrast agent allowing for accurate bowel assessment. Some authors advocate unenhanced CT for the assessment of SBO, especially when renal impairment or contrast allergies exist; however, further peer evaluation is required.
The speed of helical MDCT and its ability to reveal the cause of obstruction makes it invaluable in the acute setting.Citation4 CT has proven useful in triaging patients into operative versus non-operative treatment groups by differentiating the ileus from mechanical obstruction, and by revealing the more serious complications of closed loop obstruction and strangulation, which necessitate urgent surgical intervention. CT findings modified patient management in 21% either by changing conservative to surgical management (18%) or vice versa.Citation4
CT answers the following five specific questions that impact clinical management.
(a) Is the sb obstructed?
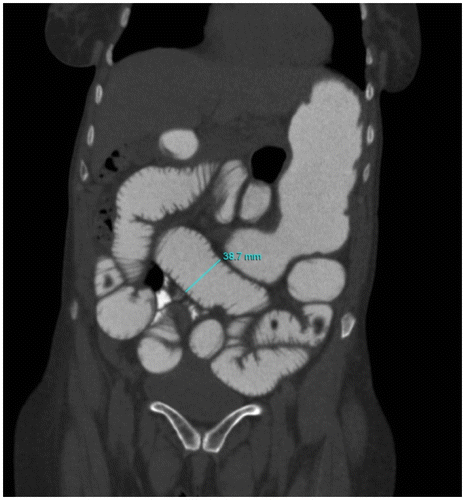
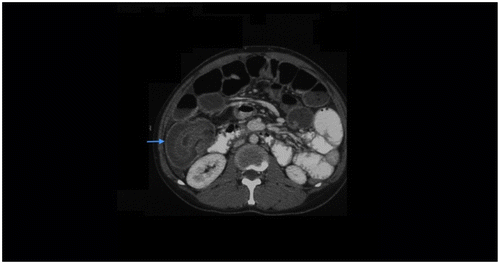
CT criteria for SBO: Presence of dilated proximal (> 2.5 cm from outer wall to outer wall) (Figure ) and collapsed or normal caliber distal loopsCitation1,8.
(b) Where is the transition point [tp]?
The transition point (TP) is determined by identifying a caliber change between the dilated proximal and collapsed distal SB loopsCitation1,8 (Figure ). A systematic retrograde approach starting from the rectum and proceeding proximally to the caecum, ileum, and jejunum is advocated,Citation1,8 to identify whether the small or large bowel is involved and to identify the transition zone. The transition point may be described as absent, abrupt [marked prestenotic dilatation and obvious transition] or gradual/discrete [no/minimal prestenotic dilatation].
Figure 5: Coronal CT abdomen showing an abrupt beak-like transition point in the right iliac fossa with mural hyperenhancement (arrow) secondary to Crohn’s disease
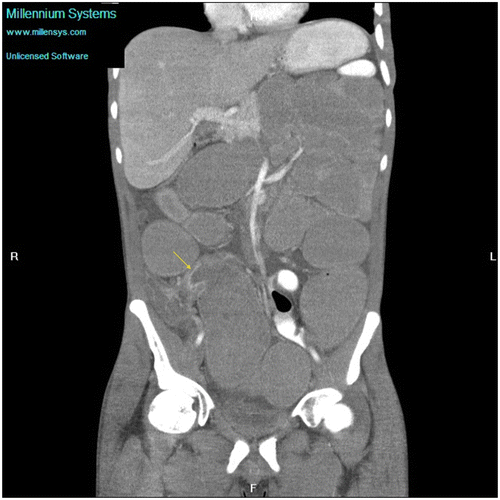
Figure 6a: Axial CT in a known haemophiliac showing SBO secondary to a large hyperdense haematoma (arrow)
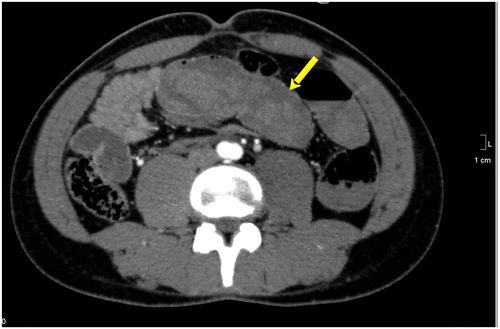
Figure 6b: Axial CT showing gross SBO with a target sign in the right iliac fossa secondary to active Crohn’s disease
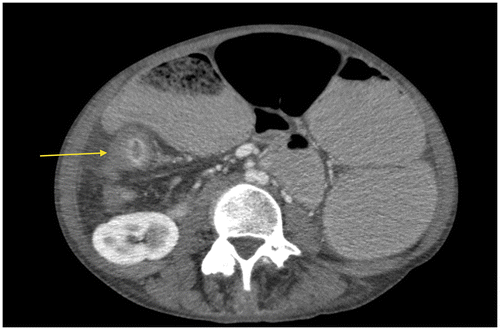
Figure 6c: Coronal CT showing gross small bowel dilatation (yellow arrow) with extensive mesenteric adenopathy (blue arrow) secondary to tuberculosis
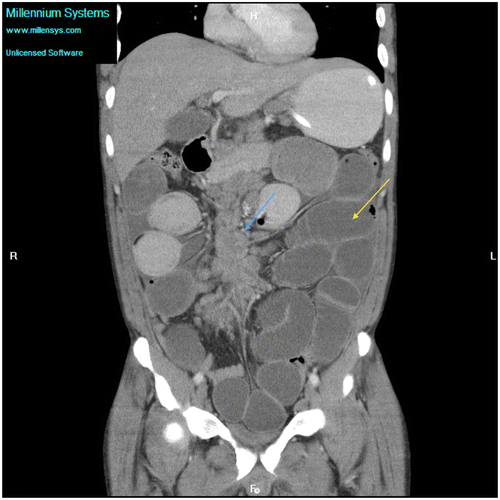
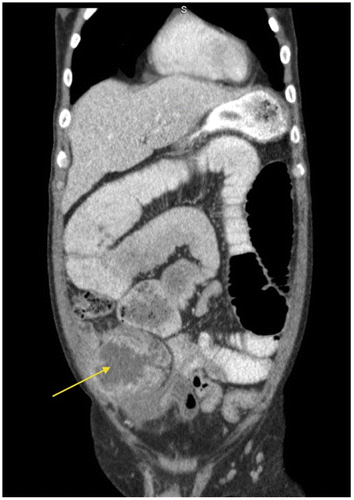
Figure 6d: Coronal CT showing marked small bowel obstruction secondary to a tumour in the right iliac fossa (arrow)
(c) What is the cause of the obstruction?
The rule of thumb is that the answer to this question is almost always at the TP as can be seen in Table : SBO CAUSES.
Table 2: Causes of SBO
Given the scourge of Human Immunodeficiency Virus (HIV) and Mycobacterium Tuberculosis (TB) infection in the developing world, and South Africa specifically, the impact of HIV and TB as a causative agent of SBO requires a special mention.
Mycobacterium tuberculosis (TB) infection in SBO
Tuberculous small bowel infection leading to obstruction is reportedly more prevalent in developing countries. TB is rampant in our environment, leading to increased mortality and morbidity.Citation9 Concomitant HIV infection, with the development of multidrug and extremely drug resistant strains, significantly compounds the clinical problem, increasing mortality and the risk of post-operative sepsis. Abdominal TB has a chronic indolent course and is usually prevalent in poor socio-economic groups with poor healthcare access, which often leads to delays in diagnosis, greater morbidity, and a greater healthcare burden.
It has been well documented that TB is the most common infective cause of SBO worldwide.Citation10 In a Tanzanian study performed by Chalya et al.,Citation9 22.4% of patients developed SBO due to TB infection. This was comparable to a study performed by Ali et al.Citation11 in Pakistan, which attributed 21.8% of cases of SBO to TB.
Intestinal TB usually presents in one of 3 main formsCitation9:
| • | Ulcerative (undermining ulcers with their long axis perpendicular to the intestinal long axis) | ||||
| • | Hypertrophic/Ulcerohypertrophic (transmural granulomatous thickening) | ||||
| • | Fibrous Stricturing Form | ||||
The commonest site of involvement is the ileocaecal region, possibly due to an increased physiological stasis and abundance of Peyer’s patches at this site.Citation9,10 Other sites of involvement include lymph nodes, ileum, caecum, peritoneum, and abdominal viscera. SBO may be caused by endoluminal narrowing in hypertrophic ileocaecal TB, by stricture/adhesion formation, or by extrinsic bowel wall compression from adjacent adenopathy. CT plays an important role in diagnosis. Imaging findings include:
| • | Circumferential mural thickening of the caecum and ilium; | ||||
| • | Asymmetric thickening of the ileocaecal valve and medial caecal wall; | ||||
| • | Mesenteric adenopathy (low density centres commonly due to caseative necrosis); | ||||
| • | Ascites; | ||||
| • | Omental/Mesenteric masses; | ||||
| • | Peritoneal thickening and enhancement; | ||||
| • | Chronic tuberculous peritonitis can cause mesenteric retraction and fibrosis leading to mechanical obstruction; and | ||||
| • | Bowel perforation, which is rare. | ||||
HIV and SBO
Gastrointestinal (GI) involvement in HIV and Acquired Immune Deficiency Syndrome (AIDS) is common.Citation12 The most life-threatening HIV associated GI complications include SBO and perforation, which are relatively uncommon and are usually related to opportunistic infections and AIDS-related malignancies. Clinicians should be especially cognisant of this, given the high HIV seroprevalence rate in sub-Saharan Africa, which may lead to unusual causes of SBO. Due to the immunocompromised state, clinical presentation may be chronic and indolent, leading to delays in diagnosis and management as with TB.
Although rare, the literature documents opportunistic infections (e.g. cytomegalovirus, Mycobacterium Avium Intercellulare, Histoplasmosis, and Cryptococcus) causing SBO.Citation12,13
Cytomegalovirus (CMV) may lead to intussusceptionCitation14 or inflammatory polypoidal mass lesions/pseudotumour formation causing SBO. HIV infected patients are also susceptible to a higher rate of reactivation TB which usually occurs when the CD4 count drops to less than 400.Citation12
AIDS related GI malignancies may be complicated by obstruction. Kaposi’s sarcoma is the commonest GI AIDS defining neoplasm. Typically it presents with submucosal masses that may lead to intussusception.Citation12,14 CT is non-specific and may reveal focal submucosal nodules/large masses with mural thickening.
Non Hodgkins B cell lymphoma is the second most common AIDS-associated neoplasm. The terminal ileum is frequently affected.Citation12 SBO may be complete/partial and may be due to luminal occlusion/extrinsic luminal compression by lymph node masses or secondary to lymphoma acting as a lead point for intussusception.Citation15 CT evaluation of lymphoma is mandatory for staging and follow-up of disease progression.
The literature also demonstrates that adult intussusception is commoner in HIV positive patients due to an increased incidence of small bowel pathology. GI manifestations that may predispose to intussusception include lymphoma, Kaposi’s sarcoma, CMV infection, and lymphoid hyperplasia.Citation14
(d) How severe is the obstruction?
Complete versus partial SBO depends on the degree of distal collapse, proximal bowel dilatation, and the transit of ingested oral contrast.Citation1,8 Passage of contrast through the TP into the collapsed bowel indicates partial SBO.Citation8
(e) Is it simple or complicated SBO?
In simple obstruction the blood supply to the bowel is intact.
Complications include closed loop obstruction (a characteristic 180 degree semi-circular orientation of dilated bowel loops around the mesentery), which is diagnosed when a variable length of bowel is partially/completely occluded both proximally and distally. In a closed loop /incarcerated SB, a ‘C’ or ‘U’ shaped or radial configuration of fluid filled dilated bowel loops may be seen with prominent stretched mesenteric vessels converging towards the point of torsion.Citation1,8 On CT, a ‘beak sign’ is seen as a fusiform tapering at the torsion site.Citation1,8 ‘Whirl sign’ is convergence of mesenteric vessels around a fixed point of obstructionCitation1 (Figure ).
Figure 7: Axial CT appearance of the whirl sign (arrow) due to convergence of vessels around a fixed point of obstruction
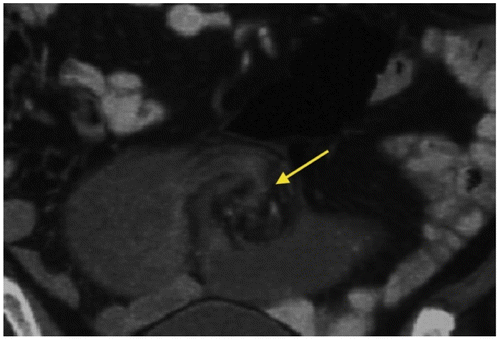
Strangulation refers to obstruction with vascular compromise which occurs in ± 10% of patients, especially where there is a delay in diagnosis and surgical management.Citation1,7Venous outflow obstruction is the commonest cause of ischaemia, followed by arterial occlusion.Citation8 CT findings suggestive but not specific for strangulation include circumferential mural thickening, increased mural attenuation, a halo or ‘target/bulls-eye’ (Figure ) appearance due to bowel oedema, mesenteric fat stranding, pneumatosis intestinalis, and portal venous gas.Citation1,6–8 A specific finding is lack of mural enhancement, asymmetric, or even delayed enhancement.Citation1 Localized fluid and mesenteric haemorrhage may occur.Citation1,7 Radiology cannot distinguish between reversible or irreversible ischaemia/bowel infarction.Citation7 Curved multiplanar reformats along the closed loop axis help identify the pivot point and torsion of mesenteric vessels.Citation8 SBO may lead to perforation (Figure ).
Figure 8a: Axial CT showing bulls-eye or target appearance in which there are alternating layers of high and low attenuation due to mural hyperenhancement and submucosal oedema (arrow)
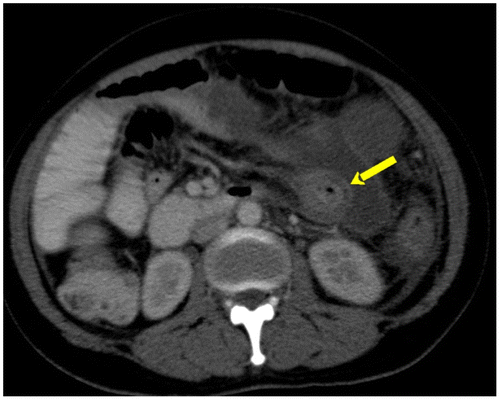
Figure 8b: SBO in a known patient with tuberculosis complicated by perforation. Extraluminal contrast filled collection (yellow arrow)
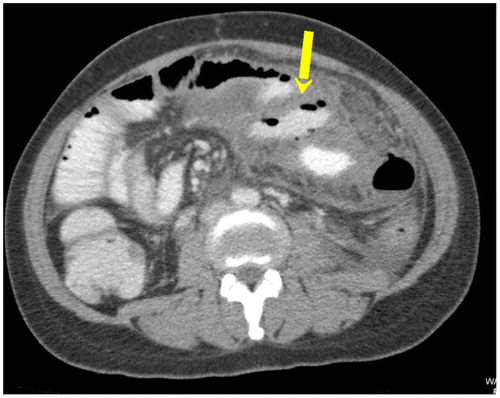
Figure 9a: Axial CT enterography using water showing gross SBO with an abrupt transition point (arrow) in the right iliac fossa secondary to a stricture from tuberculosis
Advantages: Abdominal CT is fast, readily available, non-invasive, requires minimal technical expertise, and provides a global overview of the abdomen and alimentary tract.Citation4 There is increased diagnostic confidence with the use of 3D and multiplanar reformats. This accurately assesses for complications which impacts on patient management, and distinguishes SBO from conditions that may mimic SBO (ileus, scleroderma, and ischaemia).
Limitations: Mild partial low grade or intermittent obstruction with an indiscernible TP may be overlooked on CT due to inadequate luminal distension. Long tube suctioning and vomiting may lead to bowel decompression, which may be misleading. Motion/streak artefacts/retained residual barium in the gastrointestinal tract can obscure detection of CT features.Citation16
CT Enteroclysis (CTE) is a hybrid technique that encompasses the advantages of CT and enteroclysis (EC) in a single study.Citation5 It offers better control of small bowel distension as it involves naso-intestinal intubation with controlled continuous administration of positive/neutral enteric contrast in conjunction with CT acquisition. By challenging the SB distensibility with continuous infusion, CTE offers improved sensitivity and specificity over standard barium EC and CT exams in the evaluation of suspected intermittent or low grade SBO.Citation3,5 3D acquisition capabilities allow evaluation of enteric and extra-intestinal structures and EC allows for improved bowel distensibility with better recognition of mucosal detail and subtle TP. CTE is favoured over traditional EC as the problem of overlapping bowel loops can be overcome and a global 3D overview of intestinal and extra-intestinal pathology is provided in a single examination.Citation4,5 Studies show a greater sensitivity and specificity (89% and 100%, respectively) with CTE than when using CT alone (50% and 94%, respectively) in patients with suspected partial SBO.Citation3,4 CTE has emerged as a promising tool in the diagnostic algorithm of SBO.Citation4
Advantages: This is a single ‘all in one’ study for the evaluation of enteric and extra-intestinal structures with adequate bowel distension, which allows improved recognition of mucosal detail and subtle TP. There is a high negative predictive value.
Limitations: Poor patient tolerance due to placement of a naso-enteric tube, lack of availability, cost, high radiation dose, and dependence on radiologist skill, as it is a technically demanding procedure.
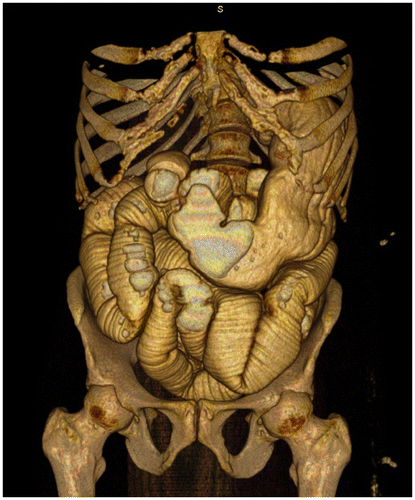
CT Enterography (Figures 9a and b) is a robust new technology that involves the ingestion of large amounts of neutral oral contrast followed by post-IV contrast MDCT imaging; it is expected to largely replace barium studies for SB investigation.Citation18
Figure 9b: 3D reconstruction of CT enterography showing marked SBO. Manipulation on the workstation allows accurate evaluation of the transition point
Advantages: Luminal distension improves reliability of the investigation. It is a rapid, readily available technique that does not entail naso-intestinal intubation, is not operator-dependent, and is more tolerable for patients.Citation18 It is less technically demanding compared to enteroclysis.
Limitations: It’s clinical usefulness in diagnosing intermittent/low grade SBO is unestablished. There may be poor patient tolerance for ingestion of large volumes of contrast in the acute setting. There is less control of bowel wall distension compared to enteroclysis. It utilizes ionizing radiation, unlike MRI.
Magnetic resonance imaging (MRI)
Whilst MRI has not traditionally been the investigation of choice for imaging SBO due to long scan times, poor image quality, exorbitant cost and lack of availabilityCitation3–5,19; increasingly faster sequences (e.g. heavily T2 weighted HASTE) has allowed faster scan times of approximately 10 min.Citation3 This has led to a rising popularity of MRI for the diagnosis and follow-up of SBO, especially given the global awareness of reducing radiation exposure. MRI may be performed with or without luminal distension; however, in the clinical setting of SBO two requirements for a successful study include very fast sequences and adequate luminal distension. As with CT, bowel distension using MRI can be achieved by passive oral contrast ingestion (MR Enterography) or active naso-intestinal infusion (MR enteroclysis). As a result, MRI and MR Enteroclysis (MRE) is emerging as an all-in one modality with the potential to revolutionize SB assessment through its direct multiplanar imaging capabilities, lack of ionizing radiation, and excellent soft tissue resolution.Citation2,19,20 The limitation of MRI without enteroclysis/enterography, as with CT, is in patients with intermittent/low grade obstruction due to poor luminal distension.
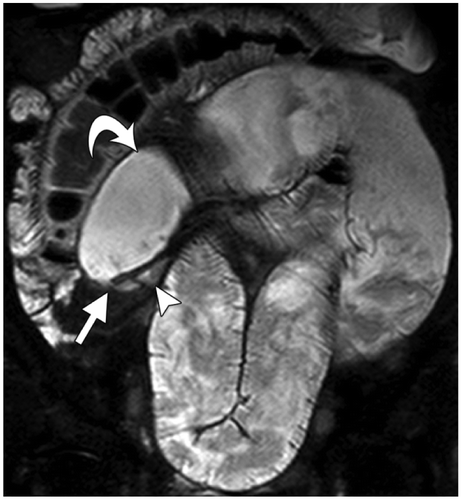
MR Enterography involves the oral administration of polyethylene glycol without nasointestinal intubation followed by serial MRI scanning.Citation2 Luminal contrast increases the reliability of the study with regard to grading of SBO, measurement of luminal diameter and mural thickness.Citation2 As with CT, a transition point and cause is sought and complications are assessed forCitation2,20 (Figure ). The advantage is that serial imaging can be performed due to lack of ionizing radiation. Dynamic cine observation with coronal imaging can be used to assess dynamic bowel movement and, thus, identify an akinetic distended loop from normal kinetic bowel, thus indicating ischaemia.Citation20
Figure 10: Reproduced with permission from: Amzallag-Bellenger E, Oudjit A, Ruiz A, et al. Effectiveness of MR enterography for the assessment of small-bowel diseases beyond Crohn Disease. RadioGraphics 2012;32:1423–1444. Adhesive ileal obstruction in a 30-year-old woman with a history of appendectomy and recurrent low-grade bowel obstruction. MR enterography was performed after the administration of 1 litre of an oral contrast agent. Coronal FISP image from MR enterography demonstrates ileal loop dilatation (curved arrow), a transition point (straight arrow), and normal distal caliber (arrowhead). No mass, bowel wall thickening, stricture, or other specific cause of obstruction was identified. These findings were suggestive of an obstruction due to bowel adhesion, which was later confirmed at laparotomy
Despite the impressive results documented in the literature by Beall et al.Citation19 and Matsuoka et al.,Citation20 which purport MRI to be superior to CT in the pre-operative diagnosis of SBO, MRI has not gained widespread clinical use given its cost and lack of availability, particularly in the public sector in South Africa.
Advantages: Compared to CT Enteroclysis, MR Enteroclysis is advantageous as it allows direct imaging in the coronal plane and provides real time acquisition of functional information.Citation4 There is excellent soft tissue characterization, allowing assessment of the bowel wall and lumen. MRI is especially useful in children and pregnant patients due to lack of ionizing radiation. It is not limited by previous barium investigations, and it may be performed without oral and IV contrast.
Limitations: Relatively poorer anatomic definition when compared to CT. Contraindications includes metallic implants, pacemakers, and claustrophobia. The main limiting factors are cost, availability in the acute setting, and the need for radiologists with the necessary experience in abdominal MRI.Citation19
From a pragmatic perspective, the use of MRI is promising, however further research and experience will help determine whether MRI ± enterography & MRE will become integral parts of the SBO diagnostic algorithm or be reserved as a problem-solving tool.Citation3,4
Contrast studies
Barium studies can be performed by intubation–infusion methods or non-intubation techniques.Citation4 Contrast studies have essentially been supplanted by cross-sectional imaging.
Non-intubation methods
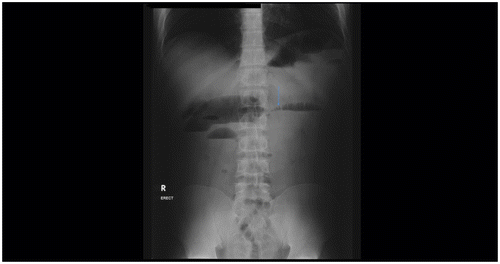
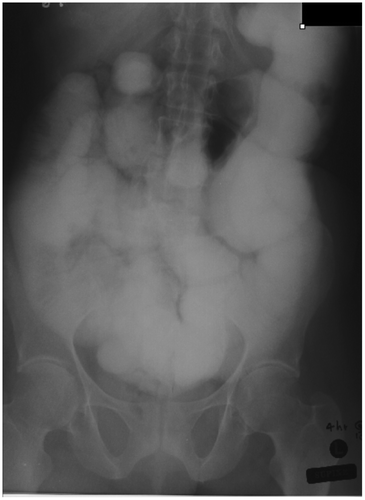
The non-intubation methods include the SB follow-through (Figure ), retrograde SB enema, the per enterostomy (ileostomy/colostomy) small bowel enema.Citation4 Non-intubation fluoroscopic studies are used where EC is unavailable.Citation5 Findings suggestive of obstruction include dilated SB loops and delayed transit time of Barium through the TP.Citation6 Orally ingested water-soluble contrast agents are hypertonic with poor mucosal coating and are, thus, not recommended for oral evaluation of SBO.Citation5
Figure 11: Small bowel follow-through study showing gross small bowel dilatation with dilution of barium and poor mucosal detail and poor delineation of the transition point due to overlapping bowel loops
Advantages: Non-invasive. Cheap and readily available.
Limitations: Limitations of using orally ingested contrast include the inability of patients to drink large volumes in an acute setting, problems in assessing distensibility and fixation of SB, flocculation, and dilution of Barium, leading to poor mucosal detail and incomplete bowel opacification.Citation5,6 Oral Barium studies are time-consuming, often fail to determine the TP, do not offer information on extra-enteric structures, have frequent blind spots due to overlapping bowel, and may delay subsequent CT due to retained barium.Citation5 This method only provides endoluminal information, as opposed to CT, which evaluates enteric and extra-enteric pathology simultaneously.
Intubation methods
Barium EC overcomes the limitations of oral techniques by challenging bowel distensibility and exaggerating the effect of subclinical SBO.Citation3,4 Barium enteroclysis entails naso-intestinal intubation with the continuous controlled infusion of contrast in conjunction with intermittent fluoroscopic screening. This method can successfully assess the presence, level, and cause of SBO with an accuracy of 86–100%Citation3,4, 100% sensitivity and 88% specificity sensitivity.Citation3 It detects subtle mucosal abnormalities, small intraluminal masses, and minimal adhesions, even in the absence of SB dilatation. It is contra-indicated when ischaemia/perforation or complete SBO is suspected.
Advantages: The ability to distinguish normality from low grade SBO and to assess multilevel obstruction,Citation4,5 which makes it an invaluable clinical weapon in patients who present with diagnostic dilemmas.
Limitations: Requires naso-intestinal intubation, near constant radiologist involvement.Citation4 High radiation dose. Limited imaging of luminal pathology only compared to CT.
Thus, the hybrid technique of CTE is now performed more frequently in the assessment of clinically stable patients with SBO.Citation3
Abdominal ultrasound (US)
Abdominal sonography has traditionally been regarded as the ugly step-sister in the diagnostic pathway for assessment of SBO; however, the literature shows US to demonstrate great potential. At sonography, SBO is diagnosed when SB loops are dilated to more than 3 cm; there is hyperperistalsis in the dilated segment and a whirling motion of bowel contents.Citation1 Worsening mechanical obstruction and the need for imminent surgery is suggested by the presence of free interloop fluid in the absence of other causes for ascites.Citation3 Evaluation by an expert sonologist can determine the presence, level, and cause of SBO. Adynamic ileus can be accurately differentiated from mechanical obstruction by observing bowel peristalsis on USCitation3 and by searching for a transition point. Aetiologies such as bezoars, tumours, and hernias can be easily identified.Citation1 Doppler sonography can be used to assess bowel wall perfusion.Citation6 Infarction may be suggested by mural thickening, aperistalsis in a dilated segment, and free fluid in an appropriate clinical setting.Citation1,6
Advantages: It is noninvasive, utilizes non-ionising radiation, and is portable; allowing bedside evaluation of critically ill patients who cannot be transported. Useful when cross-sectional imaging is unavailable.
Limitations: Increased inter-observer variability, large patient body habitus, and excessive intraluminal bowel gas decreasing accuracy and the lack of expert sonologists for emergency cases.Citation1,3 CT may still be required when US is equivocal.
Conclusion
SBO presents a diagnostic challenge. It is important for clinicians to understand the advantages and limitations of the various imaging modalities to guide clinical management. Ultimately in the South African context, the local availability of different services (e.g. on-site CT/MRI scanner) and local clinical expertise will guide the choice of investigation. From the above review it is apparent that plain radiographs remain at the forefront of the diagnostic imaging pathway, given their ready availability, cost-effectiveness, and ability to assess clinical progression on serial radiographs.
The documented literature advocates the use of MDCT with IV contrast, as the next step in the imaging pathway for both high grade and low grade partial obstruction. CT is highly sensitive and specific for high grade obstruction; however, that sensitivity decreases in cases of low grade obstruction, thus methods that improve bowel distention, e.g. enterography/enteroclysis should complement MDCT to improve the diagnostic yield. Practically, the invasive nature and high technical intricacy of enteroclysis methods restricts its utilization to highly specialized centres and problem cases, and, in the South African context, CT enterography may be a more feasible option.
According to the published literature, MRI shows marked promise in the diagnosis of SBO; however, it has limited clinical usefulness currently. Newer investigations showing marked potential in the diagnosis of SBO (MR Enterography/Enteroclysis) require more research, specifically in the South African context, and may play a role in problem-solving rather than as a first line investigation.
CT has ultimately proven useful in establishing the clinical diagnosis, determining the aetiology, and assessing for complications which assist the clinician in deciding on operative versus conservative management. Direct communication between clinicians regarding the most appropriate imaging pathway is imperative, as patients may not necessarily fit into the prescribed boxes in the diagnostic pathway.
Whilst there is a myriad of causes of SBO, it must be remembered that, in the developing world, HIV and TB infection have altered the landscape for the aetiology of SBO. Opportunistic infections coupled with neoplasms of the gastrointestinal tract have emerged as lead points for the development of SBO in immunocompromised patients. HIV and concomitant TB infection causing SBO have management implications which clinicians need to take cognisance of.
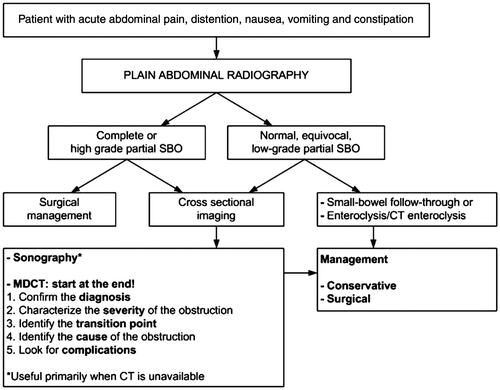
The following Figure shows a diagnostic algorithm provides a guideline for the investigation of SBO.Citation1 It is sincerely hoped that the above review has clarified the diagnostic imaging approach to SBO.
References
- Silva AC, Pimenta M, Guimaraes LS. Small bowel obstruction: what to look for. Radiographics. 2009;29(2):423–9. Available from: http://dx.doi.org/10.1148/rg.292085514
- Cronin CG, Lohan DG, Browne AM, et al. MR enterography in the evaluation of small bowel dilation. Clin Radiol. 2009;64:1026–34. Available from: http://dx.doi.org/10.1016/j.crad.2009.05.007
- Maglinte DDT, Howard TJ, Lillemoe KD, et al. Small-bowel obstruction: State-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol. 2008;6(2):130–9. Available from: http://dx.doi.org/10.1016%2Fj.cgh.2007.11.025
- Maglinte DD, Heitkamp DE, Howard TJ, et al. Current concepts in imaging of small bowel obstruction. Radiol Clin North Am. 2003, Mar;41(2):263–83, vi p. Available from: http://dx.doi.org/10.1016%2FS0033-8389%2802%2900114-8
- Sandrasegaran K, Maglinte DDT, Howard JT, et al.The multifaceted role of radiology in small bowel obstruction. Semin Ultrasound CT MRI. 2003, Oct;29(5):319–35. Available from: http://dx.doi.org/10.1016%2FS0887-2171%2803%2900072-6
- Nicolaou S, Kai B, Ho S, et al. Imaging of acute small-bowel obstruction. Am J Roentgenol. 2005;185(4):1036–44. Available from: http://dx.doi.org/10.2214%2FAJR.04.0815
- Furukawa A, Yamasaki M, Takahashi M, et al. CT diagnosis of small bowel obstruction: scanning technique, interpretation and role in the diagnosis. Semin Ultrasound CT MRI. 2003, Oct;24(5):336–52. Available from: http://dx.doi.org/10.1016%2Fj.sult.2003.08.001
- Khurana B, Ledbetter S, McTavish J, et al. Bowel obstruction revealed by Multidector CT. AJR. 2002;178: 1139–44. Available from: http://dx.doi.org/10.2214%2Fajr.178.5.1781139
- Chalya PL, Mchembe MD, Mshana SE, et al. Tuberculous bowel obstruction at a university teaching hospital in Northwestern Tanznia: a surgical experience with 118 cases. World J Emer Surg. 2013;8:12. Available from: http://dx.doi.org/10.1186/1749-7922-8-12
- Sinha R, Verma R. Multidector row computed tomography in bowel obstruction. Part 1. Small bowel obstruction. Clin Radiol. 2005;60:1058–67. Available from: http://dx.doi.org/10.1016%2Fj.crad.2005.06.002
- Ali N, Hussain M, Israr M. Tuberculosis as a cause of small bowel obstruction in adults. Gomal J Med Sci. 2011, Jul–Dec;9(2):233–5.
- Sheikh RA, Yasmeen S, Prindiville TP, et al. Intestinal perforation and peritonitis in AIDS: case series and review of the literature. JK–Practioner. 2004;11(4):248–56.
- Nawabi DH, Ffolkes L, Bichere AO. Crytococcal small-bowel obstruction in an HIV-positive patient. J R Soc Med. 2005, Nov;98(11):513–4. Available from: http://dx.doi.org/10.1258/jrsm.98.11.513
- Kehagias I, Karamanakos SN, Panagiotopoulos S, et al. A rare case of intussusception leading to the diagnosis of acquired immune deficiency syndrome: a case report. J Med Case Reports. 2009;3:61. Available from: http://dx.doi.org/10.1186/1752-1947-3-61
- Farrier J, Dinerman C, Hoyt DB, et al. Intestinal lymphoma causing intussusception in HIV+ patient: a rare presentation. Curr Surg. 2004, Jul/Aug;61(4). Available from: http://dx.doi.org/10.1016/j.cursur.2004.01.010
- Mak YSKS, Roach SC, Sukumar SA. Small bowel obstruction: computed tomography features and pitfalls. CurrProbl Diag Radiol. 2006;35:65–74. PMID: 16517290.
- Gümüştaş, OG, Gümüştaş A, Yalçın R, et al. Unusual causes of small bowel obstruction and contemporary diagnostic algorithm. J Med Imaging Radiat Oncol. 2008;52(3):208–15. doi: 10.1111/j.1440-1673.2008.01949.x
- Federle MP. CT of the small intestine: enterography and angiography. Appl Radiol. 2007;36(11):55–62.
- Beall DP, Fortman BJ, Lawler BC, et al. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57:719–24. Available from: http://dx.doi.org/10.1053%2Fcrad.2001.0735
- Matsuoka H, Takahara T, Masaki T, et al. Preoperative evaluation by magnetic resonance imaging in patients with bowel obstruction. Am J Surg. 2002;183:614–17. Available from: http://dx.doi.org/10.1016/S0002-9610(02)00855-3.