Abstract
Study objectives: To determine the safety and efficacy of the use of oral anhydrous caffeine and intravenous aminophylline in the neonatal population using therapeutic drug levels and clinical effects as markers for determination.
Design: Prospective randomised study.
Patients: Thirty-one neonates admitted (aminophylline n = 16, caffeine n = 15) with a gestational age of less than or equal to 34 weeks for prevention of apnoea of prematurity (AOP) were enrolled.
Results: Oral anhydrous caffeine or intravenous aminophylline were administered using prescribed study regimens. One peak level was taken for the two drugs on day 4 of treatment 2 hours after the maintenance dose was administered. The two regimens were clinically monitored using cardiovascular, respiratory, gastro-intestinal and central nervous system parameters four hourly. The two groups did not differ significantly for gestational age (p = 0.782), birth weight (p = 1), gender (p = 0.722), and Apgar scores determined at 5 minutes (p = 0.068). Serum concentrations were within range (5–20 μg/ml) for both study groups. The median pulse rate (beats per minute) for two days; day 7: 160 vs. 148 (p = 0.019); day 9: 168 vs. 147 (p = 0.020) and median respiratory rate (breaths per minute) for five days; day 3: 68 vs. 61 (p = 0.039); day 4: 67 vs. 57 (p = 0.014); day 5: 64 vs. 58 (p = 0.045); day 7: 65 vs. 50 (p = 0.021); day 8: 66 vs. 56 p = 0.014) were significantly higher in the aminophylline study arm.
Conclusion: The findings of the study indicated that caffeine is an effective alternative for intravenous aminophylline in prevention of AOP. The oral administration of caffeine may also have an advantage in a resource-poor setting.
Introduction
Apnoea of prematurity (AOP), defined as cessation of breathing that lasts for more than 15 seconds accompanied by hypoxia or bradycardia, occurs in at least 85% of infants born at less than 34 weeks of gestation.Citation1 WongCitation2 extends the period which defines apnoea to 20 seconds and includes a birth weight of less than 2 500 g as an additional risk factor.
Therapy often includes supportive management. The primary pharmacologic agents used to treat apnoea of prematurity are the methylxanthines (aminophylline, theophylline, and caffeine).Citation3 The methylxanthines reduce the need for mechanical ventilation during the first seven days of therapy.Citation1 Methylxanthines reduce the incidence of neonatal apnoea by increasing the sensitivity of the medullary respiratory centre to carbon dioxide, and this in turn stimulates central inspiratory drive, improves skeletal muscle contraction and improves oxygenation via increased cardiac output. The result is a decrease in hypoxic episodes.Citation4 This is particularly beneficial in preterm infants, as the central problem in this population is the decrease in responsiveness to carbon dioxide due to a decreased sensitivity in the chemical centre.Citation4
Intravenous (IV) aminophylline has been used as standard of care in the neonatal intensive care unit (NICU) at Dr George Mukhari Hospital to treat AOP. Many neonates were seen to experience significant tachycardia on IV aminophylline which often required dosing changes. Intravenous access was also problematic in many neonates who were admitted only for kangaroo mother care (KMC). Caffeine occurs naturally in many plants and has been used for over 20 years to treat apnoea of prematurityCitation5 and may be a suitable alternative.
Based on these clinical observations it was decided to embark on a study that would monitor the efficacy and safety of intravenous aminophylline, using the standard treatment regimen in the unit, to oral anhydrous caffeine. The study aimed to assess the feasibility of using oral anhydrous caffeine in a real word resource-poor situation.
Method
Study design and patient population
This was an open label, prospective randomised controlled study performed at the NICU of Dr George Mukhari Academic Hospital, a public sector teaching hospital situated in Ga-Rankuwa, north of Pretoria, South Africa. The NICU is a 55-bed unit, which is part of the paediatric corridor. The NICU is subdivided into the following sections:
| • | Intensive care unit | ||||
| • | High care unit | ||||
| • | Kangaroo mother care unit (KMCU) | ||||
| • | Isolation | ||||
In this specific NICU they admit 99 patients per month on average, of which 42 are premature infants.Citation6 All infants born with a gestational age of less than or equal to 34 weeks admitted during the 5-week study period were included. Patients were enrolled randomly using a blocked randomisation schedule, to either anhydrous caffeine or aminophylline.
Treatment protocol
Patients were randomly assigned to one of two treatment protocols:
| • | Aminophylline: Loading dose 6 mg/kg/IV, followed by a maintenance dose of 2.5 mg/kg/dose/IV administered every 8 hours. The drug was infused over 30 minutes. | ||||
| • | Caffeine (anhydrous): The oral loading dose was 10 mg/kg/dose, with an oral maintenance dose 2.5 mg/kg/day. The maintenance dose was started 24 hours after the loading dose. The oral loading dose was given in two divided doses, one hour apart to prevent gastro-intestinal upset. | ||||
The research team prepared the loading and maintenance doses for all patients according to the treatment arm to which the patient has been enrolled (see Figure ).
Figure 1: Treatment protocol
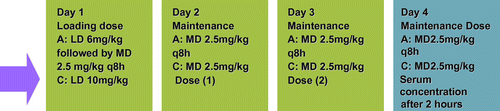
Neonates were weighed weekly and the doses were adjusted if there was a change in body weight of more than or equal to 10%.
Aminophylline and caffeine blood specimens were drawn two hours after administration of the respective drugs on Day 4 (refer to Figure ). The therapeutic ranges used for both the study drugs were 5–20 μg/ml.Citation4,7
Outcome measures
Patient demographic data were recorded on admission. Clinical parameters were used to monitor efficacy and safety for the two study drugs. Safety was measured by monitoring the cardiovascular system, respiratory system, gastrointestinal effects and the central nervous system. Efficacy was measured by recording the number of apnoeic attacks and serum concentrations. The following clinical parameters were monitored every four hours:
| • | Cardiovascular: pulse rate and mean arterial pressure (MAP), using an electronic oscillometer. | ||||
| • | Respiratory: respiratory rate, saturation and the type of ventilatory support. The number of hours on each method on ventilation was determined for the study patients from the second observation onwards, the duration since the previous monitoring was considered to have been spent on the method of ventilation observed. | ||||
| • | Gastro-intestinal support: feeding intolerance, volume aspirated, gastrointestinal irritation presenting as bloody aspirates, nutritional support and other (e.g. diarrhoea and vomiting). | ||||
| • | Central nervous system: irritability and jitteriness. | ||||
| • | The number of apnoeic attacks was determined by measuring saturation every 4 hours. Patients who were not monitored by a pulse oximeter (e.g. those patients in the kangaroo mother care unit), were diagnosed by the treating physician. | ||||
Patients were monitored until the end of the study period or discharged from the unit.
Ethics
Informed consent was obtained from the guardians of each of the patients prior to commencement of the therapy. Information leaflets were also given to the guardians to describe the study. The study was approved by the institutional review board/ethics committee.
Results
During the 5-week period, 31 study patients were enrolled; 16 were assigned to the aminophylline group and 15 to the caffeine group.
Demographics
There were no significant differences for gestational age, birth weight, gender or the Apgar scores determined at five minutes (see Table ).Citation8 Concurrent medicines included anti-infectives, metoclopramide and others, and were used similarly in both groups. Respiratory distress syndrome (RDS) was diagnosed on admission in 12 of 15 patients in the caffeine group and 6of the 16 patients in the aminophylline group.
Table 1: Comparative demographics between two study groups
Serum concentrations
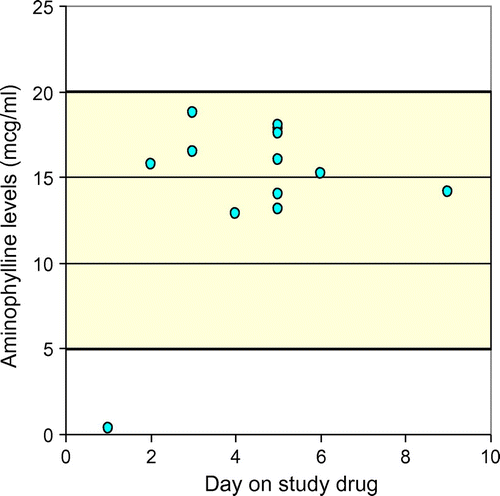
Figures and illustrate the serum levels of the study drugs in the two groups.
Figure 3: Serum concentrations of caffeine
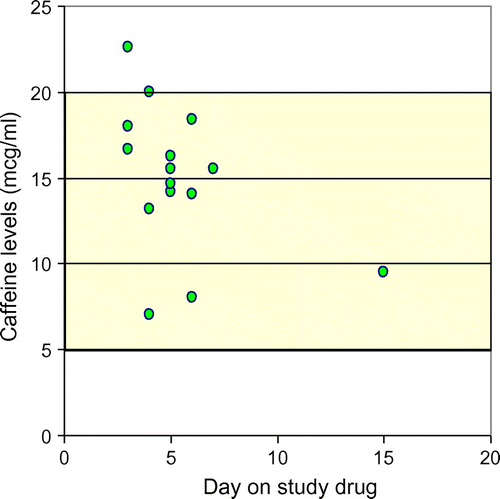
In the caffeine group (n = 15), one patient died before a level could be obtained. In each of the remaining patients, one level was determined except for one patient. In this patient the determination was repeated to confirm the first result. In the aminophylline group (n = 16) two patients died and one was discharged before a sample could be obtained. In one patient a second sample was taken to confirm the first result. This resulted in a total of 29 samples.
Cardiovascular system
Heart rate and blood pressure were measured as clinical parameters of the cardiovascular system.
Heart rate
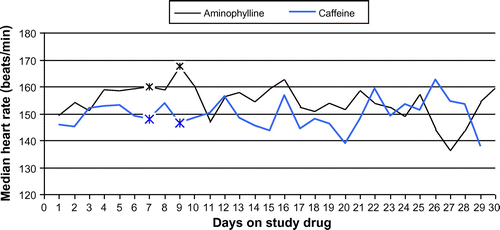
Figure illustrates the median heart rates for each day in the two study groups.
Measurements for each patient were averaged over each day. Differences of median heart rates (averaged per patient over each day) between the groups on each day were investigated using the Wilcoxon two-sample test (two-sided). A significant difference between the groups was detected on two days, i.e. Day 7 (p = 0.019) and Day 9 (p = 0.020) on therapy. The median heart rates for the two groups on Day 7 were 160 beats per minute (bpm) for aminophylline (n = 10) and 148 bpm for caffeine (n = 13) and on Day 9 168 bpm for aminophylline (n = 7) and 147 bpm for the patients receiving caffeine (n = 10) respectively.
Blood pressure
Figure illustrates the medians of mean arterial pressure (MAP) results (averaged per patient per day) in the two study groups on each study day.
Figure 5: Median mean arterial pressure in the two study groups
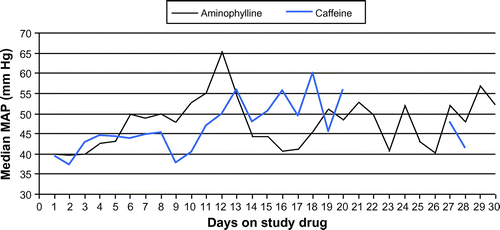
The Wilcoxon two-sample test was used to analyse and determine statistical significance, and no statistical difference could be observed at the 5% level.
Respiratory system
The patients spent most of their time on room air (80.7%), followed by the use of nasal cannulae (9.2%). The least amount of hours was spent on using intermittent positive pressure ventilation (1.2%). The groups did not differ significantly in terms of the number of hours spent on the different forms of ventilation (p = 0.347, chi square test), nor were there statistical differences in terms of numbers of patients with oxygen saturation levels below 92% or below 80% (Fisher Exact test).
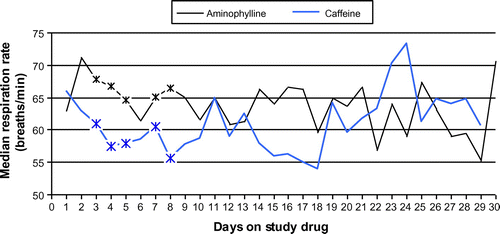
The median respiratory rate (see Figure ) was significantly higher (Wilcoxon two-sample test) in the aminophylline group than in the caffeine group on five study days, i.e. Day 3 (68 vs. 61 breaths per minute p = 0.039), Day 4 (67 vs. 57 breaths per minute p = 0.014), Day 5 (64 vs. 58 breaths per minute p = 0.045), Day 7 (65 vs. 50 breaths per minute p = 0.021) and Day 8 (66 vs. 56 breaths per minute p = 0.014).
Apnoeic attacks
Four out of the 31 patients developed apnoeic attacks. Of these four patients three belonged to the aminophylline study group, of these two died and one patient was transferred to a hospital closer to the family home. In the caffeine study group, the one patient that developed apnoeic attacks also died. Their data are summarised in Table .
Table 2: Background data of patients who developed apnoeic attacks
Gastrointestinal system
Nutritional support
There was no difference with the nutritional support (breast feeding, expressed breast milk, formula feeding and total nutritional support) given to the two groups.
Similar proportions of patient-days on a nil per os (NPO) regimen were observed in the two groups. This is illustrated by Table .
Table 3: Nutritional support given to the two study groups
Volume aspirated
Volumes aspirated were recorded on the intake and output charts. The volumes aspirated each day were expressed as a percentage of the feeds that the patient had taken in on that particular day.
Actual volumes aspirated ranged from 0.2–11 ml in the aminophylline group (n = 16) and from 0.1–9 ml in the caffeine group (n = 15).
The aminophylline group was followed for a total of 153 days; aspirates were recorded for 112 days (73%). Aspirates exceeding 30% of total daily intake occurred on 9 days (6%). The caffeine population was followed for 175 days. Aspirates were recorded on 136 days (77%), including 13 (7%) days on which aspirates totalled more than 30% of daily intake. There was no statistically significant difference between the groups in terms of the proportion of days with aspirates exceeding 30% of daily feeds (p = 0.577, chi square test). Bloody aspirates were recorded for two patients in each group.
Central nervous system
Irritability and/or jitteriness were not detected in both groups during clinical assessments.
Discussion
Eight patients receiving caffeine and seven receiving aminophylline were admitted to this study with a gestational age of 28–31 weeks. The rest were between 32 and 34 weeks. The incidence of apnoea increases with decreasing gestational age and is maximal at the gestational age of 30–31 weeks.Citation10 The median birth weight for all the patients treated with aminophylline was 1 400 g and those treated with caffeine was 1 600 g. The incidence of AOP in this patient population is 25%.Citation11 Both these parameters in the two study arms were comparable.
All serum concentrations (except for one in each group) were within range (5–20 μg/ml) for both study drugs. The median serum concentrations were 15.5 μg/ml in both groups. In the aminophylline group one patient had a sub-therapeutic serum concentration of 0.35 μg/ml. This patient received his medication as prescribed according to the protocol and to confirm the result, repeated determination done one day later was 14.13 μg/ml, thus within the therapeutic range. In the caffeine group one patient’s serum concentration (22.59 μg/ml) was above the therapeutic range. This patient did not show any clinical signs of toxicity.
Parameters monitored in which a significant difference was found include pulse rate and respiratory rate. The aminophylline study arm had higher pulse rate and respiratory rate than the caffeine. This confirmed the reason for performing the study. No co-morbidities were noted to account for the difference in pulse and respiratory rates.
The advantages of breast milk over formula feeding are well established and are strongly supported by government institutions and private institutions alike in South Africa. Hence, this was an important criterion to consider when feeding intolerance was evaluated, as the aspirates might be associated with the type of feeding that the neonate was receiving. Between the two study groups there was no significant difference in the type of feeding given to the study patients.
In patients receiving oral caffeine, intravenous aminophylline therapy could be discontinued, which facilitated breastfeeding. Patients receiving intravenous aminophylline could not be breastfed as easily and more often received manually expressed breast milk.
There was no significant difference between the groups in terms of the proportion of days with aspirates exceeding 30% of daily feeds. Various references mention the gastrointestinal side effects of the methylxanthines, including feeding intolerance, aspirates, and gastrointestinal irritation presenting as bloody aspirates.Citation4,5,9,10
Apnoea also has been found to be associated with low birth weights below 1 000 gCitation11 and has been observed in as many as 80% of infants under 1 000 g.Citation12 Four patients (n = 31) developed apnoeic attacks during the study period.
These patients developed apnoeic attacks between 2–3 weeks post-admission. The aminophylline serum concentrations for the three patients were within range, 16.5 μg/ml, 17.6 μg/ml and 14.1 μg/ml respectively. Two of the three patients developed fatal apnoeic attacks whilst the third patient was transferred to another hospital following the apnoeic attack. The patient who was treated with caffeine had a gestational age of 29 weeks, and developed the apnoeic attack two weeks post-admission. The caffeine level for this patient was within therapeutic range (18 μg/ml). This patient died of a fatal apnoeic attack. All four patients that developed apnoeic attacks were at risk according to literature as they had low birth weights. However, these patients were not at a higher risk than the rest of the population as their serum concentrations were within therapeutic range. Reasons for developing apnoeic attacks are unknown and could be due to other co-morbid conditions that influenced them to a larger extent than the rest of the population.
No neurological effects were seen in either of the treatment arms, and in other studies, it appears that infants receiving respiratory support derived greater neurological benefit from caffeine than those not receiving any support.Citation13,14 This study was not designed to study any long term effects of the study drugs but studies that have investigated the long term effects of caffeine when used for apnoea of prematurity did not indicate either a reduction in cognitive function or any long term effects on sleep duration or sleep apnoea.Citation14–17
Conclusion
The two study groups were comparable. There was a trend towards fewer cardiovascular and respiratory side-effects and apnoeic attacks occurred in the caffeine population. The oral administration of caffeine was also found to be more practical, convenient and also facilitates breast feeding. The findings of the study indicated that caffeine is an effective alternative to aminophylline in preventing apnoea of prematurity in the NICU. A longitudinal study investigating the effects of caffeine should be undertaken in a South African setting. Caffeine is now the treatment of choice for the prevention of apnoea of prematurity in this NICU.
Acknowledgements
The researcher was supported by an internship grant from the Medical Research Council (MRC); South Africa
Additional information
Funding
References
- Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–21.10.1056/NEJMoa054065
- Wong DL, editor. Whaley and Wong’s nursing care of infants and children. 5th ed. Louis: Mosby-Year Book; 1995. p. 1344, 1359.
- Kriter KE, Blanchard J. Management of apnoea in infants. Clin Pharm. 1989;8:577–87.
- Zenk KE, Sills JH, Koeppel RM. Neonatal medications and nutrition: a comprehensive guide. 3rd ed. Santa Rosa: NICU Ink Book; 2003.
- Comer AM, Perry CM, Figgitt DP. Caffeine citrate. Paediatr Drugs. 2001;3:61–79.10.2165/00128072-200103010-00005
- Statistical Document of the Department of Paediatrics (NICU). Dr George Mukhari Hospital; 2007.
- Brenner GM. Pharmacology. 3rd ed. Philadelphia, PA: W.B. Saunders; 2000. p. 294.
- Behrman RE, Kliegman RM, editor. Nelson essentials of pediatrics. 3rd ed. Philadelphia, PA: WB Saunders; 1998. p. 463.
- Bhatia J. Current options in the management of apnea of prematurity. Clin Pediatr (Phila). 2000;39:327–36.10.1177/000992280003900602
- Henderson-Smart DJ, Steer PA. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst Rev 1998;(2) Art. No.: CD00027. doi: 10.1002/14651858.CD000273
- Erenberg A, Leff RD, Haack DG, et al. Caffeine citrate for the treatment of apnea of prematurity: a double-blind, placebo-controlled study. Pharmacotherapy. 2000;20:644–52.10.1592/phco.20.7.644.35167
- Scanlon JEM, Chin KC, Morgan MEI, et al. Caffeine or theophylline for neonatal apnoea? Arch Dis Child. 1992;67:425–8.10.1136/adc.67.4_Spec_No.425
- Mueni E, Opiyo N, English M. Caffeine for the management of apnea in preterm infants. Int Health. 2009;1:190–5.10.1016/j.inhe.2009.09.005
- Schmidt B, Ohlsson A, Davis P. Caffeine therapy for apnoea of prematurity in very low-birthweight infants. Hamdan Med J. 2012 [cited 2014 Oct 08]. doi: 10.7707/hmj.v5i3.190. Available from http://hamdanjournal.org/journal/index.php?journal=HAMDAN&page=article&op=view&path%5B%5D=190&path%5B%5D=301.
- Schmidt B, Anderson P, Doyle L, et al. The caffeine for apnea of prematurity (CAP) trial: preliminary outcomes at 5 years. Pediatr Res. 2011;70:24–24. doi: 10.1038/pr.2011.249.10.1038/pr.2011.249
- Schmidt B, Anderson PJ, Doyle LW, et al. Caffeine for Apnea of Prematurity (CAP) Trial Investigators. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012;307(3):275–82. doi: 10.1001/jama.2011.2024.10.1001/jama.2011.2024
- Marcus CL, Meltzer LJ, Roberts RS, et al. Caffeine for apnea of prematurity–sleep study. Am J Respir Crit Care Med. 2014 Oct 1;190(7):791–9. doi: 10.1164/rccm.201406-1092OC.